Abstract
Physical exercise is known to stimulate the release of endorphins, creating a mild sense of euphoria that has rewarding properties. Using pain tolerance (a conventional non-invasive assay for endorphin release), we show that synchronized training in a college rowing crew creates a heightened endorphin surge compared with a similar training regime carried out alone. This heightened effect from synchronized activity may explain the sense of euphoria experienced during other social activities (such as laughter, music-making and dancing) that are involved in social bonding in humans and possibly other vertebrates.
Keywords: endorphins, rowing, synchronized performance, euphoria
1. Introduction
Physical exercise is known to stimulate the release of endogenous opioids (endorphins) (Howlett et al. 1984; Seeger et al. 1984; Harbach et al. 2000; Madsen et al. 2007; Boecker et al. 2008). Psychologically, endorphin release is experienced as a mild opiate ‘high’, a corresponding feeling of well-being, and light analgesia (Belluzi & Stein 1977; Stephano et al. 2000), reflecting the role that endorphins play as part of the pain control system. Endorphins have been explicitly implicated in the processes of social bonding, especially in primates and humans (Keverne et al. 1989; Nelson & Panksepp 1998; Depue & Morrone-Strupinsky 2005; Dunbar in press), although the mechanisms involved remain unclear. However, there is evidence to suggest that engaging in coordinated physical exercise with another individual gives rise to a heightened sense of social bonding compared to engaging in less energetic activities (Durkheim 1915/1965; McNeill 1995; Mueller et al. 2003; Ehrenreich 2006).
We tested for an enhanced opioidergic effect from behavioural synchrony in a group of rowers who trained and competed together as a squad in a world class sweep-oar racing ‘eight’. Competitive rowing is an ideal activity to test for such an effect because success is dependent on the extent to which the individual members of a crew can synchronize their strokes and not just on power output. Of particular importance for our study is the fact that the ergometer rowing machines on which crews train in the gym allow individuals to ‘row’ alone as well as in virtual boats, thus standardizing many of the contextual variables that might otherwise be difficult to control on the river.
Owing to the blood–brain barrier, direct measurement of brain endorphin levels is possible only through an invasive lumbar puncture (Dearman & Francis 1983; Boecker et al. 2008). We therefore followed conventional practice and assayed central endorphin release by measuring pain threshold, a widely used assay for central nervous system endorphin uptake (Zillman et al. 1993; Jamner & Leigh 1999). We take for granted the fact that physical exertion triggers endorphin release, thereby elevating pain thresholds, and compare the difference in pain threshold before and after individual training with the difference before and after group training in a within-subject design.
2. Material and methods
Twelve male athletes (mean age 24.25 ± 3.769 s.d.) were recruited from the University of Oxford Boat Club squad (a total of 16 athletes who provide the pool for the University's two principal eight-man rowing crews, the Blue and Goldie boats). All were non-smokers. Testing took place over a two-week period and was coordinated with the athletes' normal training schedule on the ergometers in the gym. Subjects were tested during the same week in an individual and a group training condition, with each subject repeating these test sessions again the following week (identified herein as sessions 1 and 2). In individual trials, subjects trained and were tested alone at different times/days from all others; in group trials, carried out on a different day from the individual trials, the 12 subjects were divided into two groups of six who worked out together as a virtual boat, working in synchrony. In each trial, athletes completed 45 min of continuous rowing on the ergometers. Power output, measured as an average 500 m split (time taken to row 500 m), was recorded in the first session and athletes were asked to replicate this in each of the subsequent trials (something that can be controlled relatively easily by the athlete because it is largely determined by stroke frequency, this normally being set by the rower in ‘stroke’ position in a boat).
We measured pain threshold using a Medisave Littman Classic II sphygmomanometer (blood pressure cuff). Following convention, the cuff was inflated on the participants' non-dominant arm, above the elbow, to induce ischaemic pain. All testing took place between 5 and 10 min following training and was conducted out of view of any other athletes. Pressure was increased at a rate of 10 mm Hg s−1 by gentle pumping and participants were instructed to indicate the point at which they felt discomfort by saying ‘now’. The level of pressure where pain was reported was recorded to the nearest 5 mm Hg and the cuff deflated immediately. In group sessions, two experimenters were used to reduce the delay on testing after the end of rowing sessions.
One subject was dropped from the main analyses because his pain threshold differences was 5.12 s.d. below the mean for all subjects and sessions combined in session 2, and 3.11 s.d. below the mean in session 1. He also recorded lower than all other subjects on a motivation scale on all four trials and lowest on a readiness scale in three of four trials (in each case, a 1–7 Likert scale that each subject completed before the start of each training session), suggesting that he was below psychological and/or physical par during the testing period.
3. Results
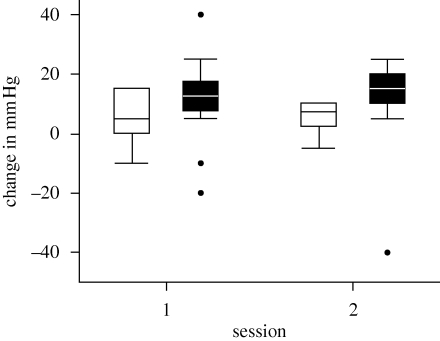
We first test power outputs across the individual and group conditions to ensure that they do not vary across trials. Differences between the individual and group conditions were not significant (ANOVA: F3,44 = 0.005, p ≈ 1.0; session 1: mean solo–group difference = −0.07, t11 = −1.19, p = 0.087; session 2: mean difference = −0.08, t11 = −1.92, p = 0.082). Differences in pain threshold between trials could not, therefore, be owing to differential work effort. In addition, as expected from the endorphin hypothesis, there was a significant increase in pain threshold following exercise in all conditions (one sample t-tests against null hypothesis that δ = 0 mm Hg: individual trials, mean change δ = 5.63 mm Hg, t23 = 3.87, p = 0.001; group trials, mean change δ = 11.04 mm Hg, t23 = 3.41, p = 0.002; the results are also significant for each session analysed separately). Finally, to check that there was no confound or carry-over effect owing to trial order, we compared the changes in pain threshold in the individual condition between sessions 1 and 2: they were not significantly different (figure 1: matched pairs t11 = 0.13, p = 0.903).
Figure 1.
The median (with 50 and 95% ranges) change in pain threshold (pre- to post-exercise, in mm Hg) as measured by the blood pressure cuff test in the individual and group training conditions for each of the two sessions. Circles indicate outliers.
Figure 1 plots the distribution of pre- to post-pain threshold changes for individual and group trials for each session. To test for an increase in pain threshold from the individual to the group trials while controlling for individual subjects, we plotted the change in pre- to post-pain threshold for group trials against the equivalent values separately for individual sessions. For the two sessions combined, the slope parameter bobs = 0.31 does not differ from the null hypothesis of b = 0 (r = 0.138, t22 = 0.66, p = 0.519) but the intercept aobs = 9.31 mm Hg is significantly greater than a = 0 (t22 = 2.21, p = 0.038): group trial pain threshold changes are significantly elevated above those for individual trials. To check that this was not owing to pseudoreplication, we repeated the analysis for each session separately and combined the results using Fisher's log-likelihood method (Sokal & Rolf 1969). This confirms that, taken together, the slope parameters do not differ significantly from b = 0 (using two-tailed p-values: χ2 = 5.50, d.f. = 2 × number of tests = 4, p = 0.240) but the intercepts are significantly greater than 0 (using one-tailed p-values to test a directional hypothesis: χ2 = 11.24, d.f. = 4, p = 0.024). To check these results, we ran a paired-sample t-test comparing the difference in pain threshold between individual and group trials for each session separately: in both cases, the difference was significant (session 1: t = 2.52, p = 0.016; session 2: t = 4.08, p = 0.001; d.f. = 10 in each case, with one-tailed tests for a directional hypothesis).
4. Discussion
These results indicate that, compared with training alone, group training significantly increases pain threshold, suggesting that synchronized activity somehow heightens opioidergic activity. We can rule out the possibility that this effect might have been owing to elevated work rates in the group condition because the rowers' power output was not significantly different in the two conditions in either session. We can also rule out any experiential or order confounds because pain thresholds did not differ between the two sessions. Thus, the heightened effect in the group condition appears to have been owing in some way to the effect of working together as a highly coordinated team. Positron emission tomography scanning has shown that, in the equivalent ‘runners' high’, opioid uptake is specific to the limbic/paralimbic areas and the prefrontal cortex, areas associated explicitly with affect (mood), rather than the reward system (Boecker et al. 2008).
The exact features of group activity that generate this effect are unknown. While it is possible that the effect on pain threshold of being in a group is independent of (and additive with) the opioid-mediated effect of exercise, we favour the simpler explanation that group exercise stimulates greater opioid production. Because coordination and tight behavioural synchrony are crucial in the national-level competitions in which these crews compete, it seems likely that feedback from specific behavioural aspects such as synchronicity from coordinated rowing-action timing and/or the achievement of overall group effort goals is involved. In humans, synchronized physical activity elevates mood and enhances a sense of social bonding (Mueller et al. 2003). Laughter, music and many religious rituals are also strongly synchronized activities associated with physical effort and a similarly strong sense of euphoria (Durkheim 1915/1965; McNeill 1995; Dunbar 2003, 2004, 2008; Ehrenreich 2006). There is some experimental evidence to suggest that at least laughter and music also trigger the release of opioids (Dunbar 2004; Kastastis 2006; MacDonald 2007). Synchronized activity (including both singing and walking) has been shown to enhance cooperativeness and generosity in public good games (Wiltermuth & Heath 2009); enhanced endorphin surges in the context of synchronized performance might explain the positive effect commonly associated with these activities (Provine 2000; Ehrenreich 2006) and, in turn, the greater willingness to behave altruistically towards those with whom one performs such activities. Group-activity-generated endorphin release may thus play a similar role in bonding human social groups (Dunbar 2008) as grooming plays in dyadic bonding in primates (Keverne et al. 1989).
Endorphins are thought to underpin social bonding in primates (Dunbar in press; Keverne et al. 1989; Broad et al. 2006). Although there are reasons for believing that the endorphin bonding mechanism might be specific to primates (Dunbar in press; Broad et al. 2006), the possibility that these effects might be more widespread among the higher vertebrates, or that similar effects might be found with other neurochemicals involved in social bonding in these taxa (notably oxytocin), remains to be explored.
Acknowledgements
Ethical approval for the study reported was granted by the Central University Research Ethics Committee at the University of Oxford.
We thank the Oxford University Boat Club for providing access to the University rowing squad and Nicholas Brodie (cox for the 2007 and 2008 Oxford University Blue boats) for assisting with the experiment.
References
- Belluzi J. D., Stein L.1977Enkephalin may mediate euphoria and drive-reduction reward. Nature 266, 556–558 (doi:10.1038/266556a0) [DOI] [PubMed] [Google Scholar]
- Boecker H., Sprenger T., Spilker M. E., Henriksen G., Koppenhoeffer M., Wagner K. J., Valet M., Berthele A., Tolle T. R.2008The runners’ high: opioidergic mechanisms in the human brain. Cereb. Cort. 18, 2523–2531 (doi:10.1093/cercor/bhn013) [DOI] [PubMed] [Google Scholar]
- Broad K. D., Curley J. P., Keverne E. B.2006Mother–infant bonding and the evolution of mammalian social relationships. Phil. Trans. R. Soc. B 361, 2199–2214 (doi:10.1098/rstb.2006.1940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearman J., Francis K. T.1983Plasma levels of catecholamines, cortisol, and beta-endorphins in male athletes after running 26.2, 6, and 2 miles. J. Sports Med. Phys. Fitness 23, 30–38 [PubMed] [Google Scholar]
- Depue R. A., Morrone-Strupinsky J. V.2005A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behav. Brain Sci. 28, 313–395 [DOI] [PubMed] [Google Scholar]
- Dunbar R. I. M.2003The social brain: mind, language and society in evolutionary perspective. Ann. Rev. Anthropol. 32, 163–181 (doi:10.1146/annurev.anthro.32.061002.093158) [Google Scholar]
- Dunbar R. I. M.2004Language, music and laughter in evolutionary perspective. In Evolution of communication systems: a comparative approach (ed. Oller D. K.), pp. 257–274 Cambridge, MA: MIT Press [Google Scholar]
- Dunbar R. I. M.2008Mind the gap: or why humans aren't just great apes. Proc. Br. Acad. 154, 403–423 [Google Scholar]
- Dunbar R. I. M.In press.The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. [DOI] [PubMed] [Google Scholar]
- Durkheim E.1915/1965The elementary forms of religious life New York, NY: Free Press [Google Scholar]
- Ehrenreich B.2006Dancing in the streets New York, NY: Metropolitan [Google Scholar]
- Harbach H., Hell K., Gramsch C., Katz N., Hempelmann G., Teschemacher H.2000β-endorphin (1–31) in the plasma of male volunteers undergoing physical exercise. Psychoneuroendocrinology 25, 551–562 (doi:10.1006/anbe.1993.1089) [DOI] [PubMed] [Google Scholar]
- Howlett T. A., Tomlin S., Ngahfoong L., Rees L. H., Bullen B. A., Skrinar G. S., MacArthur J. W.1984Release of β-endorphin and met-enkephalin during exercise in normal women in response to training. Brit. Med. J. 288, 295–307 (doi:10.1136/bmj.288.6435.1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamner L. D., Leigh H.1999Repressive/defensive coping, endogenous opioids and health: how a life so perfect can make you sick. Psychiatry Res. 8, 17–31 [DOI] [PubMed] [Google Scholar]
- Kastastis K.2006Searching for the evolutionary origins of music. PhD thesis, University of Liverpool, UK [Google Scholar]
- Keverne E. B., Martensz N. D., Tuite B.1989Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14, 155–161 (doi:10.1016/0306-4530(89)90065-6) [DOI] [PubMed] [Google Scholar]
- MacDonald I.2007Endorphin release following group drumming: a potential mechanism for social bonding. MSc thesis, University of Liverpool, UK [Google Scholar]
- Madsen E., Tunney R., Fieldman G., Plotkin H., Dunbar R. I. M., Richardson J., McFarland D.2007Altruism and kinship: a cross-cultural experimental study. Br. J. Psychol. 98, 339–359 (doi:10.1348/000712606X129213) [DOI] [PubMed] [Google Scholar]
- McNeill W. H.1995Keeping together in time: dance and drill in human history Cambridge, MA: Harvard University Press [Google Scholar]
- Mueller F., Agamanolis S., Picard R.2003Exertion interfaces: sports over a distance for social bonding and fun. Proc. of the SIGCHI Conf. on Human Factors in Computing Systems, pp. 561–568 Ft Lauderdale, FL: ACM [Google Scholar]
- Nelson E. E., Panksepp J.1998Brain structures of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev. 22, 437–452 (doi:10.1016/S0149-7634(97)00052-3) [DOI] [PubMed] [Google Scholar]
- Provine R. R.2000Laughter: a scientific investigation London, UK: Faber & Faber [Google Scholar]
- Seeger T. F., Sforzo G. A., Pert C. A., Pert A.1984In vivo autoradiography: visualization of stress-induced changes in opiate receptor occupancy in the rat brain. Brain Res. 305, 303–311 (doi:10.1016/0006-8993(84)90436-0) [DOI] [PubMed] [Google Scholar]
- Sokal R. R., Rolf F. J.1969Biometry San Francisco, CA: W. H. Freeman [Google Scholar]
- Stephano G., et al. 2000Endogenous morphine. Trends Neurosci. 23, 436–442 (doi:10.1016/S0166-2236(00)01611-8) [DOI] [PubMed] [Google Scholar]
- Wiltermuth S. S., Heath C.2009Synchrony and cooperation. Psychiatry Sci. 20, 1–5 (doi:10.1111/j.1467-9280.2008.02253.x) [DOI] [PubMed] [Google Scholar]
- Zillman D., Rockwell S., Schweitzer K., Sundar S.1993Does humor facilitate coping with physical discomfort? Motiv. Emot. 17, 1–21 (doi:10.1007/BF00995204) [Google Scholar]