Abstract
There are two forms of ovulation: spontaneous and induced. As copulation triggers ovulation for induced ovulators, males can predict the timing of ovulation and may have greater paternity monopolization than spontaneous ovulators. However, this prediction has never, to my knowledge, been tested. Using a cross-species comparison I examined the percentage of offspring sired within a litter (single paternity) and in social species the percentage of offspring sired by the dominant male (alpha paternity). My results indicate that ovulation mode alters the ability of males to monopolize paternity, with males of induced ovulators having higher single paternity and greater alpha paternity where male–female association is intermittent.
Keywords: multiple paternity, male intrasexual competition, copulation, length of mating season
1. Introduction
Mammalian ovulation has two modes: spontaneous, where a female's ova are released without the need for copulation, and induced, where ovulation is stimulated by copulation (Conaway 1971). Spontaneous ovulators display a continuous cycling of reproductive hormones and ovulation, regardless of physical stimulation by a male. By contrast, induced ovulators do not show this constant cyclical ovulation, instead, the physical stimulation of coitus causes a cascade of hormones, culminating in ovulation and subsequent corpora lutea production (Bakker & Baum 2000). Induced ovulation has been demonstrated in several mammalian taxa, but not all species within these taxa are induced ovulators (Bakker & Baum 2000), suggesting that selective pressures may have differed for various species.
For induced ovulators, copulation triggers ovulation and therefore it is thought that the first male to copulate with a female will fertilize most of her ova (Lacey et al. 1997; Gomendio et al. 1998). By contrast, for spontaneous ovulators, the male who copulates with the female closest to the time of ovulation will sire most of her ova (Gomendio et al. 1998) but, as he cannot accurately predict the exact timing of ovulation, it is more likely that his ejaculate will undergo competition to fertilize her ova. Consequently, males of induced ovulators who copulate may be expected to sire a greater proportion of a litter than males of spontaneous ovulators.
The ability of male mammals to monopolize paternity has been linked to several factors including oestrous synchrony, family structure and male–female associations (Clutton-Brock & Isvaran 2006; Ostner et al. 2008; Cohas & Allainé 2009) but never, to my knowledge, to the mode of ovulation. To test this, I examined the effect of ovulation mode on two measures of paternity: the percentage of the litter sired by a single male (single paternity) and the percentage of offspring sired by the dominant male (sensu alpha paternity; Ostner et al. 2008) in group living species. Using genetic data from published sources, I carried a phylogenetically corrected cross-species comparison to investigate whether ovulation mode alters the degree of single and alpha paternity.
2. Material and methods
To quantify paternity monopolization, I selected studies in which individual paternity had been identified. Studies detecting the presence of multiple paternity through additional paternal alleles were excluded. Single paternity was the average percentage offspring sired by the male with the most paternity within a litter. Alpha paternity was the percentage of all paternities acquired by the dominant male within a social group. If only one male was present, he was assumed to be dominant. Data on all variables were collated from published sources and from the same population where possible (see the electronic supplementary material, appendix A).
To analyse the influence of ovulation mode (spontaneous, induced) on single paternity, I included as a covariate the percentage of litters sired by multiple males (multiple paternity). Single paternity and multiple paternity are negatively correlated (r = −0.86); adding multiple paternity is a short-hand method for attempting to control for a variety of other factors associated with paternity patterns (e.g. male intrasexual competition) that could be associated with ovulation mode. Only multiple paternity rates >0% were included. Also included in the model were length of mating season (months) and litter size as variables that potentially affect the ability of males to acquire single paternity. For group-living species, association patterns between males and females affect alpha paternity (continuously associated (CA); intermittently associated (IA) sensu; Clutton-Brock & Isvaran 2006). To control for this, I included the male–female association pattern along with ovulation mode as variables in the alpha paternity model. For both models a phylogenetically corrected general linear model (PGLM; for details Iossa et al. 2008) was used. Branch lengths were calculated from a mammalian phylogeny (Bininda-Emonds et al. 2007, 2008; see the electronic supplementary material, appendix B), except for Papio cynocephalus which was missing; divergence time was taken to be the same as Papio hamadryas. The phylogenetic signal (λ) was calculated directly from the model. Ovulation mode may not be phylogenetically independent and this may cause a phylogenetic signal in the dependent variable (e.g. McKechnie et al. 2006). To exclude that this was occurring, I ran a PGLM on single paternity for the Carnivora, as both modes of ovulation were represented in this dataset, and GLMs (i.e. without phylogenetic correction) for single paternity and alpha paternity. These results do not differ from the phylogenetically corrected results (see the electronic supplementary material, appendix C), indicating that any phylogenetic distribution of ovulation is not important in this dataset. All data were transformed to meet normality assumptions. Analyses were run on the statistical package ‘R’ v. 2.8.0 (R Foundation for Statistical Computing 2007) using a function written by R. Freckleton for the PGLM. I estimated effect sizes (correlation coefficient r, sensu Cohen 1988) and non-central confidence intervals (CI) from t-values obtained from PGLMs (Nakagawa & Cuthill 2007).
3. Results
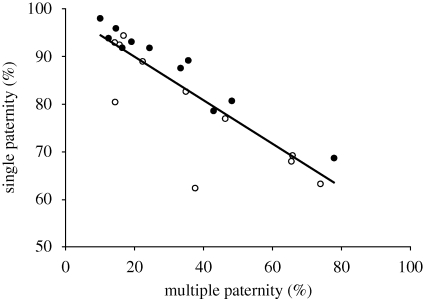
Single paternity was calculated for 23 species; six additional species had no multiple paternity and were not used in the analysis. Mean (±s.e.) single paternity was 84.0 ± 2.0% (range 63.1–98.0%). Single paternity was negatively correlated with the frequency of multiple paternity (table 1). In addition, single paternity was significantly higher in induced ovulators (table 1); induced ovulators sired a greater percentage of offspring in a litter (figure 1). Single paternity was not significantly related to the length of the mating season or litter size (table 1).
Table 1.
Results of PGLM models comparing (a) single paternity, multiple paternity, ovulation mode, length of mating season and litter size (n = 23 species; full model: adjusted R2 = 0.86, F5,18 = 35.24, p < 0.001) and (b) alpha paternity, association pattern between males and females (M–F association) and ovulation mode (n = 36 species; full model: adjusted R2 = 0.46, F4,32 = 11.32, p < 0.001). (Conventions for effect sizes: small r = 0.10, medium r = 0.30, large r = 0.50 (Cohen 1988). The non-central 95% CIs associated with r are presented; relationships are significant where CI exclude zero. λ = 0: trait has evolved independently of phylogeny, 0< λ <1: trait has evolved according to a process in which the effect of phylogeny is weaker than in the Brownian model, λ = 1: trait has evolved under Brownian evolution (Freckleton et al. 2002). SPO, spontaneous ovulation; IND, induced ovulation; CA, continuous association; IA, intermittent association.)
| analysis | λ | variable | slope | t | p | r | CI |
|---|---|---|---|---|---|---|---|
| (a) | 0.89a,b | multiple paternityc | −0.54 | −9.42 | <0.001 | −0.91 | −0.80/−0.95 |
| ovulation: IND | 0 | ||||||
| ovulation: SPOc | −3.75 | −2.47 | 0.024 | −0.51 | −0.73/−0.08 | ||
| length of mating season | −0.54 | −0.49 | 0.487 | −0.11 | −0.49/0.33 | ||
| litter size | 0.58 | 0.67 | 0.507 | 0.16 | −0.30/0.53 | ||
| (b) | 0.51a | M–F association: CA | 0 | ||||
| M–F association: IAc | −8.69 | −1.08 | 0.285 | −0.24 | −0.58/0.21 | ||
| ovulation: IND | 0 | ||||||
| ovulation: SPO | 7.20 | 0.87 | 0.389 | 0.20 | −0.25/0.55 | ||
| M–F association: CA × ovulation: IND | 0 | ||||||
| M–F association: CA × ovulation: SPO | 0 | ||||||
| M–F association: IA × ovulation: IND | 0 | ||||||
| M–F association: IA × ovulation: SPOc | 29.75 | −2.74 | 0.001 | −0.54 | −0.75/−0.15 |
aSignificantly different from 0.
bSignificantly different from 1.
cSignificant overall (PGLM ANOVA p < 0.05).
Figure 1.
The relationship between single and multiple paternity, with induced (filled circles) and spontaneous (empty circles) ovulators shown. The regression line (y =−0.4591x + 99.006) is shown through all the data.
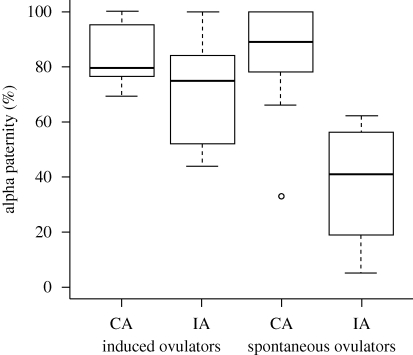
Alpha paternity was calculated for 36 species (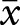 ± s.e. = 69.8 ± 4.4%, range 5.3–100%). Alpha paternity differed between association type, with continuously associated males (82.8 ± 3.9% of offspring sired) having higher paternity than intermittently associated males (49.5 ± 4.4%; table 1). Association type also interacted with ovulation mode; post hoc testing indicated differences between association types of spontaneous ovulators (PGLM ANOVA: F = 30.92, p < 0.001) but not induced ovulators (PGLM ANOVA: F = 0.30, p = 0.597; figure 2). In addition, intermittently associated induced ovulators had higher alpha paternity than spontaneous ovulators (PGLM ANOVA F = 6.33, p = 0.026), but no difference was found for continuously associated males (PGLM ANOVA F = 1.64, p = 0.215; figure 2).
± s.e. = 69.8 ± 4.4%, range 5.3–100%). Alpha paternity differed between association type, with continuously associated males (82.8 ± 3.9% of offspring sired) having higher paternity than intermittently associated males (49.5 ± 4.4%; table 1). Association type also interacted with ovulation mode; post hoc testing indicated differences between association types of spontaneous ovulators (PGLM ANOVA: F = 30.92, p < 0.001) but not induced ovulators (PGLM ANOVA: F = 0.30, p = 0.597; figure 2). In addition, intermittently associated induced ovulators had higher alpha paternity than spontaneous ovulators (PGLM ANOVA F = 6.33, p = 0.026), but no difference was found for continuously associated males (PGLM ANOVA F = 1.64, p = 0.215; figure 2).
Figure 2.
Box-plot of alpha paternity in relation to male–female association (CA, continuous association; IA, intermittent association) and ovulation mode.
4. Discussion
In accordance with predictions, there was a significant difference in the ability of males of induced and spontaneous ovulators to monopolize paternity. Males of induced ovulators sired a greater percentage of the litter than males of spontaneous ovulators, even in systems with high intrasexual competition. Ovulation mode is therefore an important variable modifying individual paternity across mammals.
Single paternity would be predicted to be higher in induced ovulators because the predictability of timing of the ova release for the first copulating male means that he would be expected to sire the majority of the litter. However, empirical evidence is mixed; for example, first-male precedence patterns in induced ovulating rodents vary between families (Storey et al. 1995; Ratkiewicz & Borkowska 2000; Waterman 2007). It is unclear why this variation should occur, but the copulatory mechanisms involved in inducing ovulation are species-specific, including the stimulation required to induce ovulation, e.g. intromission duration, number of intromissions (Bakker & Baum 2000). In addition, the time between copulation and ovulation varies considerably (Bakker & Baum 2000). So the ability of the first copulating male to stimulate ovulation or the opportunity for subsequent males to copulate successfully may vary. Even so, a single male generally sires a greater proportion of the offspring in a litter.
The pattern of association between males and females significantly affected alpha paternity (Clutton-Brock & Isvaran 2006; Cohas & Allainé 2009). Intermittently associated males were less able to monopolize paternity, but this pattern occurred for males of spontaneous, not induced, ovulators. An important determinant of alpha paternity is the ability of males to monopolize fertile females, typically through mate-guarding or by controlling male competitors (Brotherton & Komers 2003; Westneat & Stewart 2003). Tactics such as mate-guarding appear to be important for paternity monopolization in spontaneous ovulators, but less so in induced ovulators. Again this may be because, following copulation, the males of induced ovulators may be more certain of paternity than spontaneous ovulators.
It is unclear why females have evolved different modes of ovulation. It is thought that induced ovulation allows optimal gamete synchronization and assurance of fertilization, something that is important for solitary species (Conaway 1971; Bakker & Baum 2000). However, induced ovulation occurs frequently in group-living species, suggesting this is unlikely to have driven its evolution. Induced ovulation decreases male intrasexual competition (Bakker & Baum 2000; Iossa et al. 2008) and high levels of male competition may hinder female mate choice (Wong & Candolin 2005). Induced ovulation may have evolved as one way of decreasing male intrasexual competition, while at the same time acting as a post-copulatory selective mechanism, based on the level of stimulation (Larivière & Ferguson 2003). Despite these various hypotheses, the exact evolutionary selective forces determining ovulation mode are unclear. This represents an important aspect for future study, especially as ovulation mode has important downstream consequences for the reproductive patterns of both males and females.
Acknowledgements
I thank Rob Freckleton and Shinichi Nakagawa for providing unpublished functions for ‘R’ and Eva Bellemain for providing additional data. I also thank three referees and a board member for their extremely useful comments.
References
- Bakker J., Baum M. J.2000Neuroendocrine regulation of GnRH release in induced ovulators. Front. Neuroendocrinol. 21, 220–262 (doi:10.1006/frne.2000.0198) [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds O. R. P., et al. 2007The delayed rise of present-day mammals. Nature 446, 507–512 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds O. R. P., et al. 2008Corrigendum. Nature 456, 274 (doi:10.1038/nature07347) [Google Scholar]
- Brotherton P. N. M., Komers P. E.2003Mate guarding and the evolution of social monogamy in mammals. In Monogamy: mating strategies and partnerships in birds, humans and other mammals (eds Reichard U. H., Boesch C.), pp. 42–58 Cambridge, UK: Cambridge University Press [Google Scholar]
- Clutton-Brock T. H., Isvaran K.2006Paternity loss in contrasting mammalian societies. Biol. Lett. 22, 513–516 (doi:10.1098/rsbl.2006.0531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohas A., Allainé D.2009Social structure influences extra-pair paternity in socially monogamous mammals. Biol. Lett. 5, 313–316 (doi:10.1098/rsbl.2008.0760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.1988Statistical power analysis for the behavioral sciences, 2nd edn.Hillsdale, MI: Erlbaum [Google Scholar]
- Conaway C. H.1971Ecological adaptation and mammalian reproduction. Biol. Reprod. 4, 239–247 [DOI] [PubMed] [Google Scholar]
- Freckleton R. P., Harvey P. H., Pagel M.2002Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–716 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- Gomendio M., Harcourt A. H., Roldán E. R. S.1998Sperm competition in mammals. In Sperm competition and sexual selection (eds Birkhead T., Møller A. P.), pp. 667–755 London, UK: Academic Press [Google Scholar]
- Iossa G., Soulsbury C. D., Baker P. J., Harris S.2008Sperm competition and the evolution of testes size in terrestrial mammalian carnivores. Funct. Ecol. 22, 655–662 (doi:10.1111/j.1365-2435.2008.01409.x) [Google Scholar]
- Lacey E. A., Wieczorek J. R., Tucker P. K.1997Male mating behaviour and patterns of sperm precedence in Arctic ground squirrels. Anim. Behav. 53, 767–779 (doi:10.1006/anbe.1996.0342) [Google Scholar]
- Larivière S., Ferguson S. H.2003Evolution of induced ovulation in North American carnivores. J. Mammal. 84, 937–947 [Google Scholar]
- McKechnie A. E., Freckleton R. P., Jetz W.2006Phenotypic plasticity in the scaling of avian basal metabolic rate. Proc. R. Soc. B 273, 931–937 (doi:10.1098/rspb.2005.3415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Cuthill I. C.2007Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605 (doi:10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- Ostner J., Nunn C. L., Schülke O.2008Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150–1158 (doi:10.1093/beheco/arn093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratkiewicz M., Borkowska A.2000Multiple paternity in the bank vole (Clethrionomys glareolus): field and experimental data. Mamm. Biol. 65, 6–14 [Google Scholar]
- Storey A. E., French R. J., Payne R.1995Sperm competition and mate guarding in meadow voles. Ethology 101, 265–279 [Google Scholar]
- Waterman J. M.2007Male mating strategies in rodents. In Rodent societies: an ecological and evolutionary perspective (eds Wolff J. O., Sherman P. W.), pp. 27–41 Chicago, IL: Academic Press [Google Scholar]
- Westneat D. F., Stewart I. R. K.2003Extra-pair paternity in birds: causes, correlates and conflict. Annu. Rev. Ecol. Syst. 34, 365–396 (doi:10.1146/annurev.ecolsys.34.011802.132439) [Google Scholar]
- Wong B. B. M., Candolin U.2005How is female mate choice affected by male competition? Biol. Rev. 80, 559–571 (doi:10.1017/S1464793105006809) [DOI] [PubMed] [Google Scholar]