Abstract
Insulin/IGF-like signalling (IIS) is an evolutionarily conserved pathway that has diverse functions in multi-cellular organisms. Mutations that reduce IIS can have pleiotropic effects on growth, development, metabolic homeostasis, fecundity, stress resistance and lifespan. IIS is also modified by extrinsic factors. For instance, in the fruitfly Drosophila melanogaster, both nutrition and stress can alter the activity of the pathway. Here, we test experimentally the hypothesis that a widespread endosymbiont of arthropods, Wolbachia pipientis, can alter the degree to which mutations in genes encoding IIS components affect IIS and its resultant phenotypes. Wolbachia infection, which is widespread in D. melanogaster in nature and has been estimated to infect 30 per cent of strains in the Bloomington stock centre, can affect broad aspects of insect physiology, particularly traits associated with reproduction. We measured a range of IIS-related phenotypes in flies ubiquitously mutant for IIS in the presence and absence of Wolbachia. We show that removal of Wolbachia further reduces IIS and hence enhances the mutant phenotypes, suggesting that Wolbachia normally acts to increase insulin signalling. This effect of Wolbachia infection on IIS could have an evolutionary explanation, and has some implications for studies of IIS in Drosophila and other organisms that harbour endosymbionts.
Keywords: Drosophila, insulin-like signalling, Wolbachia
1. Introduction
The insulin/IGF-like signalling (IIS) pathway is ubiquitous in multi-cellular animals and may have been involved in the evolution of multi-cellularity itself (Skorokhod et al. 1999). The pathway, via a conserved intracellular signalling cascade including phosphatidylinositol-3-kinase (PI3K) and protein kinase B (PKB or AKT), negatively regulates the activity of several transcription factors of the forkhead family, by their phosphorylation and subsequent exclusion from the nucleus. Mutations that reduce the activity of the IIS signalling cascade have pleiotropic effects on many traits, including growth (Brogiolo et al. 2001; Butler & Le Roith 2001; Ikeya et al. 2002; Rulifson et al. 2002), development (Kimura et al. 1997), metabolic homeostasis (Saltiel & Kahn 2001), adult lifespan, resistance to stress (Lithgow et al. 1995; Clancy et al. 2001; Holzenberger et al. 2003) and fecundity (Partridge & Gems 2002; Liang et al. 2003; Nelson & Padgett 2003; Tatar et al. 2003; Broughton et al. 2005; Piper et al. 2008) in the nematode worm Caenrhabditis elegans, the fruitfly Drosophila melanogaster and the mouse Mus musculus. Natural variation in some ecologically significant traits has also been found to be associated with IIS. For example, allelic variants of the PI3K are implicated in the latitudinal variation of reproductive diapause in Drosophila melanogaster (Williams et al. 2006).
A primary function of IIS is to match energetically demanding activities such as growth and reproduction to nutrition and other informative environmental inputs such as various stresses, and to ensure metabolic homeostasis. Thus, consistent with its role in nutrient and stress sensing, a variety of extrinsic factors can be important regulators of insulin sensitivity in animals and humans. For example, the stress-responsive Jun-N-terminal kinase (JNK) pathway regulates IIS, linking responses to environmental stresses with metabolic homeostasis (see Karpac & Jasper 2009 for review). In both C. elegans and Drosophila, genetically upregulating JNK signalling leads to decreased IIS, increased resistance to oxidative stress and extension of lifespan (Oh et al. 2005; Wang et al. 2005). The evolutionarily conserved nutrient-sensing TOR pathway regulates protein synthesis and growth in response to amino acids and growth factors (Wullschleger et al. 2006), and interacts with IIS to control lifespan in worms and flies (Vellai et al. 2003; Jia et al. 2004; Kapahi et al. 2004). In humans, diet (Romao & Roth 2008), stress (Depke et al. 2008) and exercise (Colberg 2007) have all been shown to affect insulin sensitivity. Acute (transient) insensitivity can also be experienced following infections and injuries in humans (Ikezu et al. 1997; Del Aguila et al. 2000; Van den Berghe et al. 2001), and, in Drosophila, infection by pathogenic Mycobacterium has been found to alter IIS (Dionne et al. 2006).
Another possible infectious extrinsic factor modifying IIS in Drosophila is Wolbachia pipientis, a micro-organism of the rickettsial family. Wolbachia is a widespread endosymbiont of arthropods and often causes infection-induced reproductive incompatibility in the host. This Wolbachia-induced trait has been found to occur in many Drosophila species, including D. melanogaster (Bourtzis et al. 1996; Veneti et al. 2003; McGraw & O'Neill 2004). Wolbachia infection can also affect other aspects of host reproduction, and feminization, male killing and parthenogenesis have all been reported (Stouthamer et al. 1999; Hurst et al. 2000). Although the relationship between Wolbachia and the host species is usually symbiotic, a virulent form of Wolbachia that shortens lifespan has been found in D. melanogaster (Min & Benzer 1997). In the Bloomington stock centre, 30 per cent of D. melanogaster strains have been estimated to be infected by endosymbionts (Clark et al. 2005). Removal of Wolbachia by tetracycline treatment showed that it was involved in the sterility of the Sxl4 mutant allele (Starr & Cline 2002) and the lifespan-extension phenotype of an Indy mutant stock (Toivonen et al. 2007). The mechanisms involved in these effects of Wolbachia are not yet understood.
In the course of specifying and standardizing the Wolbachia infection status of our D. melanogaster laboratory stocks, we observed that the removal of Wolbachia by tetracycline appeared to exacerbate IIS mutant phenotypes. We therefore made a systematic investigation by measuring the effect of Wolbachia infection status on a range of IIS-related phenotypes in control and IIS mutant flies. In the presence of Wolbachia, ubiquitous expression of a dominant negative form of the insulin receptor (InRDN) resulted in moderate dwarfism, reduced fecundity and extension of female lifespan, all typical phenotypes of reduced IIS. In the absence of Wolbachia, the previously moderate effects of InRDN expression were accentuated, resulting in flies that showed severe IIS-related phenotypes including extreme dwarfism, sterility, increased fat levels and shortened lifespan. The absence of Wolbachia in control genotypes resulted in a decrease in fecundity and adult weight compared with infected control genotypes, but no effect on other IIS-related phenotypes. These data suggest that Wolbachia can increase IIS in infected flies.
2. Material and methods
(a). Fly stocks and husbandry
yw;UAS-dInRA1409K (chr. II) was obtained from the Bloomington Drosophila Stock Centre (BDSC; ref. number. FBal0156359). The UAS-InRDN transgene causes an amino acid substitution in the kinase domain (R1409A) of the Drosophila insulin receptor (dInR), resulting in its dominant negative activity (Wu et al. 2005). w1118; Daughterless-GAL4 (chr. III) (Fly Base ID FBti0013991) was obtained from the BDSC. UAS-PTEN was provided by Prof. Ernst Hafen (ETH, Switzerland), and w1118;;S1,106-GeneSwitch (chr. III) is described in Giannakou et al. (2004). The whiteDahomey (wDah) background stock is described in Broughton et al. (2005). The parental GAL4 and UAS strains used to generate experimental and control genotypes were backcrossed to our laboratory control strain wDah (Wolbachia-infected) to standardize genetic background, beginning with an initial cross between wDah females and transgenic males, followed by five subsequent backcrosses between transgenic females and wDah males. All stocks were maintained and experiments performed on standard sugar/yeast food as described in Bass et al. (2007), except food containing 160 g l−1 yeast was used for GeneSwitch experiments using Mifepristone (RU486, Sigma).
(b). Generation of Wolbachia-infected and -uninfected flies
PCR to detect the wsp gene (Zhou et al. 1998) confirmed that, after backcrossing, all stocks were infected with Wolbachia (Wol+; data not shown). To create genetically identical Wolbachia-uninfected stocks (Wol−), the backcrossed Wol+ GAL4, UAS and wDah strains were treated with 30 µg ml−1 of tetracycline contained in standard food for two generations and retested by PCR to confirm that Wolbachia had been removed (figure 1a). All stocks were then cultured under normal conditions for at least five generations to allow full recovery from the effects of tetracycline treatment and to restore intestinal flora (Holden et al. 1993; Toivonen et al. 2007) prior to the generation of Wolbachia-infected and -uninfected daGAL4/UAS-InRDN, daGAL4/+, UAS-InRDN/+ and wDah genotypes for further analysis.
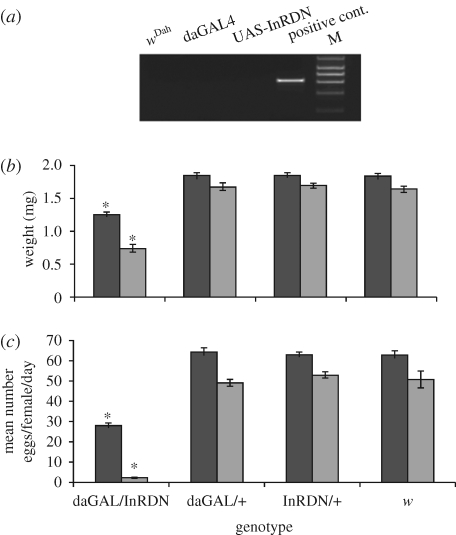
Figure 1.
The effect of Wolbachia infection status on weight and fecundity of IIS mutant and control female flies. (a) PCR to the wsp gene demonstrating the removal of Wolbachia from parental strains. Positive control was genomic DNA obtained from tetracycline-untreated wDah strain. (b) The body weight of 7-day-old adult females was examined (n = 12): Wol+ wDah = 1.819 mg; Wol− wDah = 1.628 mg; Wol+ InRDN/+ = 1.829 mg; Wol− InRDN/+ = 1.683 mg; Wol+ daGAL4/dInRDN = 1.239 mg; Wol− daGAL4/dInRDN = 0.727 mg; Wol+ daGAL4/+ = 1.83 mg; and Wol− daGAL4/+ = 1.66 mg. Black bars, Wolbachia-infected; grey bars, -uninfected. (c) Average number of eggs laid per female per day at two weeks of age (n = 8 vials of 10 flies): Wol+ wDah = 62.76 eggs; Wol− wDah = 50.65 eggs; Wol+ InRDN/+ = 62.72 eggs; Wol− InRDN/+ = 52.53 eggs; Wol+ daGAL4/InRDN = 27.7 eggs; Wol− daGAL4/InRDN = 1.98 eggs; Wol+ daGAL/+ = 63.73 eggs; and Wol− daGAL/+ = 49.1 eggs. (b,c) Weight and fecundity data were subjected to two-way ANOVAs. Genotype (p < 0.0001) and Wolbachia status (p < 0.0001) had significant effects on both phenotypes, and there was a significant interaction effect on weight (p = 0.0238) and fecundity (p < 0.0001). Planned comparisons of weight and fecundity by genotype in Wol+ or Wol− backgrounds were performed using the Tukey HSD, p < 0.05. Error bars are s.e.m. and asterisk indicates significant difference from genetic controls. The statistical analyses of planned comparisons of weight and fecundity by Wolbachia status for each genotype are shown in table 1.
(c). Lifespan experiments
Procedures for lifespan studies were as described in Clancy et al. (2001) and Mair et al. (2003). The experimental flies were allowed to mate for 48 h before collection and sorting. Lifespans were measured in flies kept at 10 per vial on standard food medium and transferred to new food three times a week. Deaths were scored five to six times in every 7 days.
(d). Fecundity and body weight
Females for measuring body weight were prepared as for lifespan experiments and weighed individually when 7 days old. For fecundity, the number of eggs laid per vial of 10 females per day in lifespan experiments was counted.
(e). Triacylglycerol assay
Triacylglyceride (TAG) content quantification was performed as described in Grönke et al. (2003), except that data were normalized to whole fly weight.
(f). Oil red O staining
Lipid droplets were stained with Oil Red O (Sigma; Gutierrez et al. 2007). Seven-day-old (adult) female abdomens were attached to a glass slide using double-sided tape and dissected to expose the adult fat body attached to the dorsal abdomen. Samples were embedded in Vectashield with DAPI (Vector). Images were taken using an ORKA-ER CCD camera (Hamamatsu Photonics) and an Axioskop 2 microscope (Zeiss), and subsequently analysed using ImageJ.
(g). AKT and FOXO protein detection
Anti-phospho-Drosophila Akt (no. 4054) and Akt (no. 9272) antibodies were purchased from New England BioLab (UK distributor for The Cell Signalling). Anti-dFOXO is described in Giannakou et al. (2007). Five flies were homogenized with 200 µl of 1× sample buffer containing proteinase inhibitor and phosphatase inhibitor cocktail I (Sigma). SDS–PAGE analysis was performed using a BioRad mini gel system and the proteins were transferred to a membrane by semidry blotter. Primary AKT antibodies were used at 1∶1000 dilution and the dFOXO primary antibody at a dilution of 1∶000. A secondary antibody conjugated with HRP was used at 1∶10 000, and the signal developed using an ECL detection kit (GE Healthcare).
(h). Statistical analyses
Statistical analyses were performed using JMP (v. 7) software (SAS Institute). Lifespan data were subjected to log-rank tests and are presented as survival curves. Weight, fecundity, TAG levels and fat-cell number data were tested for normality using the Shapiro–Wilk W-test on studentized residuals (Sokal & Rohlf 1998). Two-way (genotype, Wolbachia status) analyses of variance (ANOVA) were performed and planned comparisons of means were made using the Tukey HSD test or Student's t, as appropriate. Data are presented as means of raw values ± s.e.m., and asterisk denotes significant difference from controls.
3. Results
(a). Removal of Wolbachia enhances IGF-like signalling mutant phenotypes
To determine how Wolbachia infection status affects IIS, we characterized typical IIS-related phenotypes in Wolbachia-infected and -uninfected IIS mutant flies and their controls in parallel. IIS mutant flies were created by expression of a dominant negative form of the insulin receptor (UAS-InRDN) under the control of the ubiquitous and constitutive daughterless-GAL4 (daGAL4) driver.
Reduced growth and fecundity are typical phenotypes of lowered IIS and, as expected, daGAL4/InRDN females weighed significantly less and laid fewer eggs than their daGAL/+, InRDN/+ and w control genotypes, irrespective of Wolbachia status (figure 1b,c; and electronic supplementary material, figure 1a–d). However, removal of Wolbachia greatly accentuated the reduction in weight and fecundity of the daGAL4/InRDN genotype and moderately reduced these phenotypes in control genotypes (figure 1b,c; and table 1 for statistical analysis by Wolbachia status).
Table 1.
Statistical comparisons of mean weight and fecundity data from figure 1b,c by Wolbachia status. Two-way ANOVAs were performed, which found genotype and Wolbachia status to be the main effects. Planned comparisons of mean weight and fecundity of Wolbachia-infected (Wol+) and -uninfected (Wol−) flies for each genotype were performed using the Tukey HSD.
| genotype | weight |
fecundity |
||||
|---|---|---|---|---|---|---|
| Wol+ | Wol− | p-value | Wol+ | Wol− | p-value | |
| wDah | 1.819 | 1.628 | <0.05 | 62.76 | 50.65 | <0.01 |
| InRDN/+ | 1.829 | 1.683 | <0.05 | 62.75 | 52.52 | <0.01 |
| daGAL4/+ | 1.83 | 1.661 | <0.05 | 63.72 | 49.08 | <0.01 |
| daGAL4/InRDN | 1.239 | 0.728 | <0.05 | 27.7 | 1.98 | <0.01 |
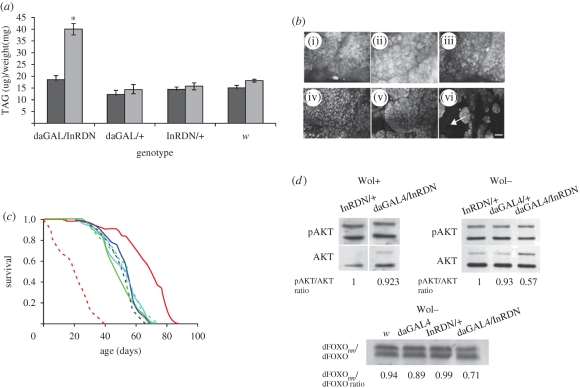
Increases in TAG levels and alterations in adult fat tissue morphology are often associated with reduced IIS (Bohni et al. 1999; Tatar et al. 2001). Wol+ daGAL4/InRDN females had normal TAG levels (figure 2a) and adult fat body morphology (figure 2b) compared to Wol+ controls. In contrast, Wol− daGAL4/InRDN females had significantly higher TAG levels (figure 2a; Tukey HSD, p < 0.05) and alterations in fat body morphology (figure 2b) compared to all other genotypes. Unlike the morphologically normal abdominal fat tissue of Wolbachia-infected daGAL4/InRDN and control flies (figure 2b(i–iii)), this tissue was misshapen and shrunken in Wol− daGAL4/InRDN flies (figure 2b(vi)), similar to that seen under starvation conditions (Scott et al. 2004). For these IIS-related phenotypes, Wolbachia status had no effect on control genotypes (figure 2a,b).
Figure 2.
The effect of Wolbachia infection status on fat, survival and AKT phosphorylation. (a) TAG concentration of individual whole virgin females (n = 12). Wol+ wDah = 15.15 µg mg−1 of fly weight; Wol− wDah = 18.15 µg mg−1; Wol+ InRDN/+ = 14.38 µg mg−1; Wol− InRDN/+ = 15.74 µg mg−1; Wol+ daGAL4/dInRDN = 18.29 µg mg−1; Wol− daGAL4/dInRDN = 40.07 µg mg−1; Wol+ daGAL4/+ = 12.25 µg mg−1; and Wol− daGAL4/+ = 14.37 µg mg−1. Data were subjected to two-way ANOVA, with genotype (p < 0.0001) and Wolbachia status (p < 0.0001) having significant effects. There was a significant interaction effect of genotype and Wolbachia status (p < 0.0001). Planned comparisons of the mean TAG level by genotype/Wolbachia status were made using the Tukey HSD. Error bars are s.e.m. and asterisk indicates significant difference from all groups, p < 0.05. Black bars, Wolbachia-infected; grey bars, -uninfected. (b) Adult abdominal fat body morphology for each genotype: (i) Wol+ InRDN/+; (ii) Wol+ daGAL4/+; (iii) Wol+ daGAL4/InRDN; (iv) Wol− InRDN/+; (v) Wol− daGAL4/+; (vi) Wol− daGAL4/InRDN. Arrow indicates area of missing tissue and scale bar is 20 µm. (c) Survival of Wolbachia-infected and -uninfected daGAL4/InRDN once-mated females and controls. Median lifespans and statistical analyses are given in table 2. Red solid line, daGAL4/InRDN Wol+; red dashed line, daGAL4/InRDN Wol−; dark blue solid line, daGAL4 Wol+; dark blue dashed line, daGAL4 Wol−; green solid line, InRDN Wol+; green dashed line, InRDN Wol−; light blue solid line, w Wol+; light blue dashed line, w Wol−. (d) Levels of AKT and dFOXO phosphorylation were detected by Western blotting. For AKT phosphorylation, representative blots of two independent experiments each for Wol+ and Wol− backgrounds are shown. The upper two bands of each panel are phospho-AKT (pAKT) and the bottom two bands are tAKT. The presence of two bands is due to expression of two isoforms of AKT. pAKT/tAKT relative ratio was estimated from the signal intensity of the lower bands of both pAKT and AKT, which was averaged by ROI analysis in ImageJ. For dFOXO phosphorylation in the Wol− background, a single experiment was performed. The ratio of phosphorylated to unphosphorylated dFOXO (dFOXOppp/dFOXO ratio) was estimated from the intensity of the upper (dFOXOppp) to the lower (dFOXO) band in ImageJ.
Lifespan is another trait that responds to changes in IIS and the effect of reducing the activity of the pathway can be either positive or negative. Some mutations or genetic manipulations of IIS, such as strong, ubiquitous downregulations, are often detrimental to lifespan (Clancy et al. 2001; Tatar et al. 2001; Taguchi et al. 2007; Selman et al. 2008), but moderate or tissue-specific reductions in IIS can extend it (Clancy et al. 2001; Tatar et al. 2001; Giannakou et al. 2004; Hwangbo et al. 2004; Broughton et al. 2005; Selman et al. 2008). It appears that alterations of specific IIS components at particular times or in specific tissues may be important, or that lifespan may peak at an intermediate level of IIS activity. Interestingly, Wol+ daGAL4/UAS-InRDN once-mated females showed a large extension of median and maximum lifespan (figure 2c; table 2 for statistical analysis; electronic supplementary material, figure 2a), whereas Wol− daGAL4/InRDN females were very short-lived (figure 2c; table 2 for statistical analysis; electronic supplementary material, figure 2b). For control genotypes, Wolbachia infection status had no effect on lifespan (figure 2c; table 2 for statistical analysis; electronic supplementary material, figure 2a–c). This effect of Wolbachia removal to drastically shorten the lifespan of daGAL4/UAS-InRDN females is consistent with an accentuation of the inhibitory effect of InRDN expression, resulting in a reduction in IIS to levels detrimental to survival. The effect of over-expression of InRDN on lifespan of males in the Wol+ strain was also measured, and a slight reduction in median lifespan was found (electronic supplementary material, figure 2d). This detrimental effect of InRDN expression to male lifespan appeared to be accentuated in the absence of Wolbachia as only very few Wol− daGAL4/UAS-InRDN males enclosed, and those that did died within a few days. This sex difference in the effect on survival is typical for IIS mutants in Drosophila (Clancy et al. 2001; Tatar et al. 2001; Giannakou et al. 2004; Broughton et al. 2005) and in mice (Holzenberger et al. 2003; Selman et al. 2008).
Table 2.
Median and maximum lifespans and statistical comparisons of survivorship data from figure 2c. Each pairwise comparison of survivorship data by genotype and Wolbachia infection status was made using the log-rank test, and χ2 p-values are given.
| genotype/Wolbachia status | daGAL/InRDN+ | daGAL/InRDN− | daGAL+ | InRDN− | daGAL− | InRDN+ | w− | w+ |
|---|---|---|---|---|---|---|---|---|
| median lifespan (days) | 70.5 | 21.4 | 54.0 | 54.0 | 49.0 | 45.5 | 54.0 | 54.0 |
| maximum lifespan (days) | 82 | 36.0 | 66.0 | 65.0 | 63.0 | 66.0 | 69.0 | 66.0 |
| daGAL/InRDN+ | 2.197×10−36 | 1.632×10−16 | 3.861×10−17 | 1.853×10−19 | 3.707×10−18 | 2.923×10−13 | 1.488×10−13 | |
| daGAL/InRDN− | 3.695×10−35 | 5.675×10−29 | 2.102×10−32 | 2.073×10−31 | 6.635×10−27 | 1.417E×10−25 | ||
| daGAL+ | 0.2081 | 0.00976 | 0.08486 | 0.46632 | 0.75395 | |||
| InRDN− | 0.20105 | 0.52295 | 0.12628 | 0.20625 | ||||
| daGAL− | 0.88930 | 0.01548 | 0.03060 | |||||
| InRDN+ | 0.06816 | 0.19695 | ||||||
| w− | 0.67115 | |||||||
| w+ |
The previous data suggested that Wolbachia acts to enhance IIS. As a further indicator of a change in the magnitude of IIS downregulation in daGAL4/UAS-InRDN flies owing to Wolbachia infection, the activation of two key downstream effectors of IIS—AKT and the fly forkhead box O transcription factor, dFOXO—were measured. The Ser505 of AKT is a target of the TOR/RICTOR complex, the phosphorylation of which is required for the full activation of the AKT protein (Sarbassov et al. 2005) and which is used as an indicator of AKT activity (Hietakangas & Cohen 2007). We measured the phosphorylation state of this serine using an antibody specific to the phosphoS505-AKT form of the protein (pAKT) and an antibody to total AKT (tAKT) (which recognizes both phosphorylated and unphosphorylated forms of AKT) in Wol+ and Wol− flies in Western blots. The level of AKT activation was then estimated by normalizing the pAKT signal to the tAKT signal. Only Wol− daGAL4/InRDN females showed a reduced pAKT/tAKT ratio compared with controls in two independent experiments (figure 2d), suggesting that the downregulation of IIS owing to daGAL4-driven expression InRDN was stronger in the absence of Wolbachia than in its presence. Active AKT phosphorylates dFOXO, and this phosphorylation retards the migration of dFOXO on SDS–PAGE (Puig et al. 2003). To confirm the reduction in AKT activity in Wol− daGAL4/InRDN females, we examined levels of phosphorylated dFOXO. Indeed, consistent with a reduction in AKT activity in the Wol− daGAL4/InRDN females, we observed a decrease in the ratio of slower-migrating/phosphorylated to faster-migrating/unphosphorylated dFOXO in these flies (figure 2d).
Interestingly, the enhanced IIS-related phenotypes of the Wol− daGAL4/InRDN females resembled those due to a strong downregulation of the pathway obtained using daGAL-driven expression of UAS-PTEN. The essential function of the phosphoinositide phosphatase PTEN is to antagonize the effects of PI3K by dephosphorylation of the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3; Stocker et al. 2002). UAS-PTEN expression thus downregulates IIS by converting PIP3 to PIP2 (Goberdhan et al. 1999; Maehama et al. 2004). Even in the presence of Wolbachia, daGAL/UAS-PTEN flies showed severe IIS-related phenotypes including growth, fecundity, fat and AKT phosphorylation, and they were very short-lived (electronic supplementary material, figures 1–3).
Taken together, these data suggest that the removal of the endosymbiont Wolbachia from daGAL4/InRDN females caused a greater inhibitory effect of InRDN expression on IIS, resulting in enhanced IIS-related phenotypes. The similar but less pronounced reduction in weight and fecundity seen upon removal of Wolbachia from control genotypes suggests that these two phenotypes may be the most sensitive to changes in IIS and demonstrates that Wolbachia's effect is not specific to the daGAL4/InRDN genotype.
(b). Removal of Wolbachia enhances the effect of expression of InRDN in adult fat body
The quantitatively different effects of InRDN expression on IIS-related phenotypes in the presence and absence of Wolbachia, described above, suggested a dose dependency of IIS downregulation. To begin to determine if these effects are general, we used the RU486 GeneSwitch-inducible GAL/UAS system (Osterwalder et al. 2001; Roman et al. 2001) to express InRDN in adult fat body. Manipulation of IIS by over-expression of the key IIS effector forkhead transcription factor, dFOXO, in this tissue using the inducible S1106-GS driver has previously been shown to be sufficient to extend lifespan (Giannakou et al. 2004). This driver was initially reported as expressed in adult fat body (Roman et al. 2001) and has since been shown also to express in the digestive system and testes (Poirier et al. 2008).
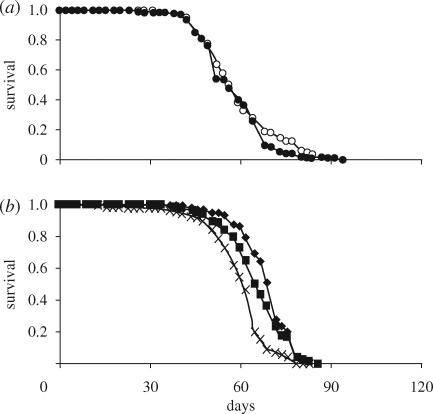
Expression of InRDN using the S1106-GS driver under the same induction conditions as in Giannakou et al. (2004) had no effect on lifespan in the presence of Wolbachia (figure 3a). However, removal of Wolbachia from S1106-GS/UAS-InRDN female flies resulted in extension of lifespan and revealed a dose-dependent effect of expression of InRDN (figure 3b). At 200 µM, RU486 inducer Wol− S1106-GS/UAS-InRDN females showed a 6.5 per cent increase in median lifespan (χ2 p = 1.16×10−7) and at 300 µM RU486 a 13 per cent increase (χ2 p = 3.65×10−15) compared with the same genotype at 0 µM RU486. There was no effect of RU486 on survival of driver control female flies (electronic supplementary material, figure 4). S1106-GS/InRDN expression had no effect on fecundity or lipid levels (electronic supplementary material, figure 5), confirming the previous study modulating IIS in this tissue (Giannakou et al. 2004, 2007). pAKT levels in the long-lived Wol− S1106-GS/InR-DN females were found to be indistinguishable from controls (electronic supplementary material, figure 5).
Figure 3.
Expression of InRDN in the abdominal adult fat body extends lifespan only in the absence of Wolbachia. Lifespan experiments were done in (a) Wolbachia-infected and (b) Wolbachia-uninfected flies. (a) Survival of Wol+ S1,106-GS/InRDN females was not affected by administration of 200 µM of RU486 inducer. The median lifespan was 56 days (n = 185) in the absence of the drug and 56 days (n = 188) in the presence of the drug. Open circles, S106/InRDN RU−; filled circles, S106/InRDN RU+. (b) Survival of Wol-S1,106-GS/InRDN on 200 and 300 µM RU486 concentrations compared with uninduced genetically identical flies (0 µM RU486). Individual median lifespans were 61 days on 0 µM (n = 141), 65 days on 200 µM (n = 137, χ2 p = 1.16 × 10−7) and 69 days on 300 µM (n = 130, χ2 p = 3.65 × 10−15). Crosses, S106/InRDN RU−; filled squares, S106/InRDN 200; filled diamonds, S106/InRDN 300.
These data suggest that removal of Wolbachia from Wol+ normal-lived S1106-GS/InRDN females acted to increase the inhibitory effect of InRDN in this tissue to a threshold sufficient for lifespan extension. The data further suggest that ubiquitous expression of InRDN by daGAL4 may have affected fecundity, growth and fat phenotypes via expression during development and/or in other tissues.
5. Discussion
The data presented here demonstrate that mutant IIS and its consequent phenotypes in Drosophila can be enhanced by removal of Wolbachia, suggesting that one of the normal consequences of Wolbachia infection is enhanced IIS. The data are consistent with a general effect on IIS, and not a specific interaction with mutants such as the daGAL4/InRDN genotype, because removal of Wolbachia from control strains had similar, although weaker, effects. More work will be needed both to determine the generality of these kinds of effects and exactly how Wolbachia modifies IIS-related phenotypes.
Evolutionary considerations suggest that the mechanisms by which Wolbachia interacts with IIS may have its origin in the way that these endosymbionts are transmitted. Their habitat is inside the cytoplasm of cells, and they are transmitted maternally, via infection of eggs (Stouthamer et al. 1999). Wolbachia are thought to persist in host populations by providing females with a reproductive advantage via mechanisms that can include male killing, feminization of genetic males and cytoplasmic incompatibility (see Stouthamer et al. 1999 for review). In D. melanogaster, an effect of Wolbachia infection is to confer reproductive advantage through cytoplasmic incompatibility (Hoffmann et al. 1998). Wolbachia-infected females can successfully mate with infected or uninfected males, but uninfected females are incompatible with infected males. Thus, Wolbachia infection can spread and persist in the host population, although other mechanisms must also be involved (Olsen et al. 2001; Fry et al. 2004; Weeks et al. 2007; Brownlie et al. 2008).
In evolutionary terms, the interests of the female host and of the symbiont in host egg production are identical. Both host and symbiont are selected to maximize host fitness through this route. In populations of flies that are infected with Wolbachia, part of the response to selection for an optimal regime of host egg production through the life history may thus be attributable to evolution of the effects of the symbiont on host reproduction. However, this alone would not explain why Wolbachia would act as seen in this study, solely to promote high host fecundity. A possible explanation could come from events during adaptation to life in the laboratory. The life history of Drosophila evolves so as to optimize fitness under the prevailing culture regime (Sgro & Partridge 2000; Spencer & Promislow 2002). The usual regime consists of the transfer of young adults to fresh culture vials, with consequent selection for rapid maturation and copious early reproduction. This produces evolutionary increases in the speed of pre-adult development and in female fecundity relative both to the levels seen in wild flies and in laboratory stocks maintained with overlapping generations. These traits that are favoured in laboratory culture are also promoted by increased IIS, although it has not yet been determined whether laboratory adaptation is achieved in whole or in part by increased activity of this pathway. In stocks that are infected with Wolbachia, part of the adaptation to laboratory conditions may be achieved by the evolution of the symbiont so as to alter host physiology in ways that promote an optimal regime of egg production through copious, early female reproduction. One prediction of the hypothesis is that these effects of Wolbachia would be expected to be particularly obvious in strains that have undergone laboratory adaptation, in traits promoting fitness through the regime of fecundity and in females. Alternatively, interactions of IIS with the Wolbachia infection itself could play a role.
The tetracycline treatment used for the removal of Wolbachia has itself been found to result in a decline of mitochondrial activity and increase in mitochondrial DNA density, which may increase insulin insensitivity regardless of the removal of the endosymbionts (Ballard & Melvin 2007). However, this study measured the effects only two generations after treatment and, because the effect is based on damage to the mitochondria, it is expected to decline rapidly. The parental strains used in the current study were reared on normal food for at least five generations following tetracycline treatment to restore flora in their intestine prior to the generation of experimental flies for analysis. That such a regime does not produce ill-effects as a result of the tetracycline treatment itself is supported by the finding that tetracycline treatment of already-Wolbachia-free flies had no effect on lifespan when tested several generations after treatment (Toivonen et al. 2007).
The variability of the phenotypes owing to IIS reduction in the fly is probably due to the complex nature of the pathway itself, as well as the developmental stage and tissue affected by the genetic manipulation in question. However, the dose dependency of IIS downregulation identified here supports the idea that some of the variability could be related to differing strengths of IIS downregulation. Over-expression of the FOXO transcription factor in the fly eye imaginal disks induced cell death (Junger et al. 2003), whereas the same gene expressed in the adult fat body extended lifespan (Giannakou et al. 2004; Hwangbo et al. 2004). It is not known what intracellular differences exist between the adult fat body and imaginal disks that separate FOXO's longevity function from its apoptotic function, but a likely explanation could be differences in the level of FOXO activation in the two systems. Interestingly, a measurable reduction of AKT phosphorylation correlated with malformation of the adult fat body, sterility and shortened lifespan, which, given the role of AKT activity in preventing apoptosis, could suggest that the short lifespan of the pkb3 mutant was due to its reduced AKT activity level (Stocker et al. 2002).
Despite the uncertainties as to the mechanism by which IIS is modified, the Wolbachia effect has implications for studies of IIS in Drosophila. It is desirable to ensure that the Wolbachia status of all strains being compared is the same, to rule out any possibility that traits apparently attributable to altered IIS are in fact owing to differences in Wolbachia status. However, provided this stricture is observed, alteration in Wolbachia status could be a convenient way of altering the intensity of IIS. The initial state of IIS as well as the stage and tissue specificity of the IIS manipulation may determine how a change in Wolbachia status affects IIS phenotypes.
6. Conclusion
The data presented here show that the effects of mutant IIS are dose-dependent, and that Wolbachia acts to enhance IIS, thus modifying the extent to which IIS mutants affect signalling and the consequent pleiotropic phenotypes of the pathway. Furthermore, for some of these phenotypes, such as lifespan, the phenotype peaks at an intermediate level of IIS downregulation.
Acknowledgements
We acknowledge funding by the Wellcome Trust (L.P. and S.B.), Marie Curie and EMBO Fellowships (N.A.), a Marie Curie Fellowship (T.I.) and the Royal Society (T.I.). We thank G. Hurst for helpful advice and M. Piper for a critical reading of the manuscript.
References
- Ballard J. W. O., Melvin R. G.2007Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol. Biol. 16, 799–802 [DOI] [PubMed] [Google Scholar]
- Bass T. M., Grandison R. C., Wong R., Martinez P., Partridge L., Piper M. D. W.2007Optimization of dietary restriction protocols in Drosophila. J. Gerontol. 62, 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni R., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., Andruss B. F., Beckingham K., Hafen E.1999Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, 865–875 (doi:10.1016/S0092-8674(00)80799-0) [DOI] [PubMed] [Google Scholar]
- Bourtzis K., Nirgianaki A., Markakis G., Savakis C.1996Wolbachia infection and cytoplasm incompatibility in Drosophila species. Genetics 144, 1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E.2001An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, 213–221 (doi:10.1016/S0960-9822(01)00068-9) [DOI] [PubMed] [Google Scholar]
- Broughton S. J., et al. 2005Longer lifespan, altered metabolism and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl Acad. Sci. USA 102, 3105–3110 (doi:10.1073/pnas.0405775102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie J. C., Cass B. N., Riegler M., Witsenburg J. J., Iturbe-Ormaetxe I., McGraw E. A., O'Neill S. L.2008Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 5, e1000368 (doi:10.1371/journal.ppat.1000368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. A., Le Roith D.2001Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu. Rev. Physiol. 63, 141–164 (doi:10.1146/annurev.physiol.63.1.141) [DOI] [PubMed] [Google Scholar]
- Clancy D. J., Gems D., Harshman L. G., Oldham S., Stocker H., Hafen E., Leevers S. J., Partridge L.2001Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106 (doi:10.1126/science.1057991) [DOI] [PubMed] [Google Scholar]
- Clark M. E., Anderson C. L., Cande J., Karr T. L.2005Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics 170, 1667–1675 (doi:10.1534/genetics.104.038901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg S.2007Physical activity, insulin action, and diabetes prevention and control. Curr. Diabetes Rev. 3, 176–184 (doi:10.2174/157339907781368986) [DOI] [PubMed] [Google Scholar]
- Del Aguila L. F., Krishnan R. K., Ulbrecht J. S., Farrell P. A., Correll P. H., Lang C. H., Zierath J. R., Kirwan J. P.2000Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 279, E206–E212 [DOI] [PubMed] [Google Scholar]
- Depke M., Fusch G., Domanska G., Geffers R., Völker U., Schuett C., Kiank C.2008Hypermetabolic syndrome as a consequence of repeated psychological stress in mice. Endocrinology 149, 2714–2723 (doi:10.1210/en.2008-0038) [DOI] [PubMed] [Google Scholar]
- Dionne M. S., Pham L. N., Shirazu-Hiza M. M., Schneider D. S.2006Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 16, 1977–1985 (doi:10.1016/j.cub.2006.08.052) [DOI] [PubMed] [Google Scholar]
- Fry A. J., Palmer M. R., Rand D. M.2004Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 93, 379–389 (doi:10.1038/sj.hdy.6800514) [DOI] [PubMed] [Google Scholar]
- Giannakou M. E., Goss M., Junger M. A., Hafen E., Leevers S. J., Partridge L.2004Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 305, 361 (doi:10.1126/science.1098219) [DOI] [PubMed] [Google Scholar]
- Giannakou M. E., Goss M., Jacobson J., Vinti G., Leevers S. J., Partridge L.2007Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell 6, 429–438 (doi:10.1111/j.1474-9726.2007.00290.x) [DOI] [PubMed] [Google Scholar]
- Goberdhan D. C. I., Paricio N., Goodman E. C., Mlodzik M., Wilson C.1999Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 13, 3244–3258 (doi:10.1101/gad.13.24.3244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S., Beller M., Fellert S., Ramakrishnan H., Jäckle H., Kühnlein R. P.2003Control of fat storage by a Drosophila PAT domain protein. Curr. Biol. 13, 603–606 (doi:10.1016/S0960-9822(03)00175-1) [DOI] [PubMed] [Google Scholar]
- Gutierrez E., Wiggins D., Fielding B., Gould A. P.2007Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445, 275–280 (doi:10.1038/nature05382) [DOI] [PubMed] [Google Scholar]
- Hietakangas, Cohen2007Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 21, 632–637 (doi:10.1101/gad.416307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Hercus M., Dagher H.1998Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148, 221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden P. R., Jones P., Brookfield J. F.1993Evidence for a Wolbachia symbiont in Drosophila melanogaster. Genet. Res. 62, 23–29 [DOI] [PubMed] [Google Scholar]
- Holzenberger M., Dupont J., Ducos B., Leneuve P., Geloen A., Even P. C., Cervera P., Le Bouc Y.2003IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421, 182–187 (doi:10.1038/nature01298) [DOI] [PubMed] [Google Scholar]
- Hurst G. D., Johnson A. P., Schulenburg J. H., Fuyama Y.2000Male-killing Wolbachia in Drosophila: a temperature sensitive trait with a threshold bacterial density. Genetics 156, 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo D. S., Gershman B., Tu M. P., Palmer M., Tatar M.2004Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562–566 (doi:10.1038/nature02549) [DOI] [PubMed] [Google Scholar]
- Ikeya T., Galic M., Belawat P., Nairz K., Hafen E.2002Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 12, 1293–1300 (doi:10.1016/S0960-9822(02)01043-6) [DOI] [PubMed] [Google Scholar]
- Ikezu T., Okamoto T., Yonezawa K., Tompkins R. G., Martyn J. A.1997Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J. Biol. Chem. 272, 25 289–25 295 (doi:10.1074/jbc.272.40.25289) [DOI] [PubMed] [Google Scholar]
- Jia K., Chen D., Riddle D. L.2004The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131, 3897–3906 (doi:10.1242/dev.01255) [DOI] [PubMed] [Google Scholar]
- Junger M. A., Rintelen F., Stocker H., Wasserman J. D., Vegh M., Radimerski T., Greenberg M. E., Hafen E.2003The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2, 20 (doi:10.1186/1475-4924-2-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P., Zid B. M., Harper T., Koslover D., Sapin V., Benzer S.2004Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14, 885–890 (doi:10.1016/j.cub.2004.03.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J., Jasper H.2009Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol. Metab. 20, 100–106 (doi:10.1016/j.tem.2008.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G.1997daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (doi:10.1126/science.277.5328.942) [DOI] [PubMed] [Google Scholar]
- Liang H., Masoro E. J., Nelson J. F., Strong R., McMahan C. A., Richardson A.2003Genetic mouse models of extended lifespan. Exp. Gerontol. 38, 1353–1364 (doi:10.1016/j.exger.2003.10.019) [DOI] [PubMed] [Google Scholar]
- Lithgow G. J., White T. M., Melov S., Johnson T. E.1995Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl Acad. Sci. USA 92, 7540–7544 (doi:10.1073/pnas.92.16.7540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T., Kosaka N., Okahara F., Takeuchi K., Umeda M., Dixon J. E., Kanaho Y.2004Suppression of a phosphatidylinositol-3-kinase signal by a specific spliced variant of Drosophila PTEN. FEBS Lett. 565, 43–47 (doi:10.1016/j.febslet.2004.03.074) [DOI] [PubMed] [Google Scholar]
- Mair W., Goymer P., Pletcher S. D., Partridge L.2003Demography of dietary restriction and death in Drosophila. Science 301, 1731–1733 (doi:10.1126/science.1086016) [DOI] [PubMed] [Google Scholar]
- McGraw E. A., O'Neill S. L.2004Wolbachia pipientis: intracellular infection and pathogenesis in Drosophila. Curr. Opin. Microbiol. 7, 67–70 (doi:10.1016/j.mib.2003.12.003) [DOI] [PubMed] [Google Scholar]
- Min K.-T., Benzer S.1997Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl Acad. Sci. USA 94, 10 792–10 796 (doi:10.1073/pnas.94.20.10792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. W., Padgett R. W.2003Insulin worms its way into the spotlight. Genes Dev. 17, 813–818 (doi:10.1101/gad.1090203) [DOI] [PubMed] [Google Scholar]
- Oh S. W., Mukhopadhyay A., Svrzikapa N., Jiang F., Davis R. J., Tissenbaum H. A.2005JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. PNAS 102, 4494–4499 (doi:10.1073/pnas.0500749102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen K., Reynolds K. T., Hoffman A. A.2001A field cage test of the effects of the endosymbiont Wolbachia on Drosophila melanogaster. Heredity 86, 731–737 (doi:10.1046/j.1365-2540.2001.00892.x) [DOI] [PubMed] [Google Scholar]
- Osterwalder T., Yoon K. S., White B. H., Keshishian H.2001A conditional tissue-specific transgene expression system using inducible GAL4. PNAS 98, 12 596–12 601 (doi:10.1073/pnas.221303298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L., Gems D.2002Mechanisms of ageing: public or private? Nat. Rev. Genet. 3, 165–175 (doi:10.1038/nrg753) [DOI] [PubMed] [Google Scholar]
- Piper M. D., Selman C., McElwee J. J., Partridge L.2008Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J. Intern. Med. 263, 179–191 [DOI] [PubMed] [Google Scholar]
- Poirier L., Shane A., Zheng J., Seroude L.2008Characterization of the Drosophila gene-switch system in aging studies: a cautionary tale. Aging Cell 7, 758–770 (doi:10.1111/j.1474-9726.2008.00421.x) [DOI] [PubMed] [Google Scholar]
- Puig O., Marr M. T., Ruhf M. L., Tjian R.2003Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 17, 2006–2020 (doi:10.1101/gad.1098703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G., Endo K., Zong L., Davis R. L.2001P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 12 602–12 607 (doi:10.1073/pnas.221303998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romao I., Roth J.2008Genetic and environmental interactions in obesity and type 2 diabetes. J. Am. Diet Assoc. 108(Suppl. 41), S24–S28 (doi:10.1016/j.jada.2008.01.022) [DOI] [PubMed] [Google Scholar]
- Rulifson E. J., Kim S. K., Nusse R.2002Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120 (doi:10.1126/science.1070058) [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Kahn C. R.2001Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (doi:10.1038/414799a) [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M.2005Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (doi:10.1126/science.1106148) [DOI] [PubMed] [Google Scholar]
- Scott R. C., Schuldiner O., Neufeld T. P.2004Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell. 7, 167–178 (doi:10.1016/j.devcel.2004.07.009) [DOI] [PubMed] [Google Scholar]
- Selman C., et al. 2008Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 22, 807–818 (doi:10.1096/fj.07-9261com) [DOI] [PubMed] [Google Scholar]
- Sgro C. M., Partridge L.2000Evolutionary responses of the life history of wild caught Drosophila melanogaster to two standard methods of laboratory culture. Am. Nat. 156, 341–353 (doi:10.1086/303394) [Google Scholar]
- Skorokhod A., Gamulin V., Gundacker D., Kavsan V., Muller I. M., Muller W. E.1999Origin of insulin receptor-like tyrosine kinases in marine sponges. Biol. Bull. 197, 198–206 (doi:10.2307/1542615) [DOI] [PubMed] [Google Scholar]
- Sokal R. R., Rohlf F. J.1998Biometry New York, NY: W.H. Freeman [Google Scholar]
- Spencer C. C., Promislow D. E.2002Genes, culture, and aging flies—what the lab can and cannot tell us about natural genetic variation for senescence. Sci. Aging Knowl. Environ. 20, e6. [DOI] [PubMed] [Google Scholar]
- Starr D. J., Cline T. W.2002A host–parasite interaction rescues Drosophila oogenesis defects. Nature 418, 76–79 (doi:10.1038/nature00843) [DOI] [PubMed] [Google Scholar]
- Stocker H., Andjelkovic M., Oldham S., Laffargue M., Wymann M. P., Hemmings B. A., Hafen E.2002Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 295, 2088–2091 (doi:10.1126/science.1068094) [DOI] [PubMed] [Google Scholar]
- Stouthamer R., Breeuwer J. A., Hurst G. D.1999Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53, 71–102 (doi:10.1146/annurev.micro.53.1.71) [DOI] [PubMed] [Google Scholar]
- Taguchi A., Wartschow L. M., White M. F.2007Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317, 369–372 (doi:10.1126/science.1142179) [DOI] [PubMed] [Google Scholar]
- Tatar M., Kopelman A., Epstein D., Tu M.-P., Yin C.-M., Garofalo R. S.2001A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107 (doi:10.1126/science.1057987) [DOI] [PubMed] [Google Scholar]
- Tatar M., Bartke A., Antebi A.2003The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351 (doi:10.1126/science.1081447) [DOI] [PubMed] [Google Scholar]
- Toivonen J. M., Walker G. A., Martinez-Diaz P., Bjedov I., Driege Y., Jacobs H. T., Gems D., Partridge L.2007No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS Genet. 3, e95 (doi:10.1371/journal.pgen.0030095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe G., et al. 2001Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 345, 1359–1367 (doi:10.1056/NEJMoa011300) [DOI] [PubMed] [Google Scholar]
- Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A. L., Orosz L., Müller F.2003Influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 (doi:10.1038/426620a) [DOI] [PubMed] [Google Scholar]
- Veneti Z., Clark M. E., Zabalou S., Karr T. L., Savakis C., Bourtzis K.2003Cytoplasmic incompatibility and sperm cyst infection in different Drosophila–Wolbachia associations. Genetics. 164, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. C., Bohmann D., Jasper H.2005JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121, 115–125 (doi:10.1016/j.cell.2005.02.030) [DOI] [PubMed] [Google Scholar]
- Weeks A. R., Turelli M., Harcombe W. R., Reynolds K. T., Hoffman A. A.2007From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 5, e114 (doi:10.1371/journal.pbio.0050114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. D., Busto M., Suster M. L., So A. K.-C., Ben-Shahar Y., Leevers S. J., Sokolowski M. B.2006Natural variation in Drosophila melanogaster diapauses due to the insulin-regulated PI3-Kinase. Proc. Natl Acad. Sci. USA 103, 15 911–15 915 (doi:10.1073/pnas.0604592103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Zhang Y., Xu J., Shen P.2005Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc. Natl Acad. Sci. USA 102, 13 289–13 294 (doi:10.1073/pnas.0501914102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M. N.2006TOR signaling in growth and metabolism. Cell 124, 471–484 (doi:10.1016/j.cell.2006.01.016) [DOI] [PubMed] [Google Scholar]
- Zhou W., Rousset F., O'Neil S.1998Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 265, 509–515 (doi:10.1098/rspb.1998.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]