Abstract
Wildlife on isolated oceanic islands is highly susceptible to the introduction of pathogens. The recent establishment in the Galápagos Islands of the mosquito Culex quinquefasciatus, a vector for diseases such as avian malaria and West Nile fever, is considered a serious risk factor for the archipelago's endemic fauna. Here we present evidence from the monitoring of aeroplanes and genetic analysis that C. quinquefasciatus is regularly introduced via aircraft into the Galápagos Archipelago. Genetic population structure and admixture analysis demonstrates that these mosquitoes breed with, and integrate successfully into, already-established populations of C. quinquefasciatus in the Galápagos, and that there is ongoing movement of mosquitoes between islands. Tourist cruise boats and inter-island boat services are the most likely mechanism for transporting Culex mosquitoes between islands. Such anthropogenic mosquito movements increase the risk of the introduction of mosquito-borne diseases novel to Galápagos and their subsequent widespread dissemination across the archipelago. Failure to implement and maintain measures to prevent the human-assisted transport of mosquitoes to and among the islands could have catastrophic consequences for the endemic wildlife of Galápagos.
Keywords: Galápagos, Culex quinquefasciatus, emerging infectious disease, West Nile virus, population genetics, aircraft monitoring
1. Introduction
Human-aided transport and human-induced environmental changes have dramatically increased the capacity of disease vectors, such as mosquitoes, to reach, establish and spread in previously inaccessible areas, triggering emergence and epidemics of vector-borne diseases across the globe (Lounibos 2002). Disease vector introductions in recent history are closely linked with the development of global transportation links such as the Worldwide Airline Network (Tatem et al. 2006). Recent outbreaks of West Nile fever in the USA (Kramer et al. 2008) and of Chikungunya disease throughout the Indian Ocean region (Chevillon et al. 2008) are striking examples of the impacts of globalization on human and animal health. Recently, emerging infectious diseases that have wildlife host reservoirs have been highlighted as threats to human health, the global economy and biodiversity (Daszak et al. 2000; Jones et al. 2008). Wildlife endemic to isolated oceanic islands is particularly susceptible to the introduction of infectious diseases (Matson 2006). For example, the co-introduction of the southern house mosquito Culex quinquefasciatus Say (Diptera: Culicidae) and avianpoxvirus in Hawaii, followed by a later introduction of avian malaria, precipitated dramatic declines and extinctions among Hawaiian endemic birds (Warner 1968; Van Riper et al. 1986, 2002). In contrast to Hawaii, Galápagos remains one of the most pristine archipelagos on Earth, with much of its endemic fauna intact (Tye et al. 2002; Watkins & Cruz 2007). The introduction of new pathogens and disease vectors could have devastating effects on the Galápagos biodiversity (Wikelski et al. 2004; Kilpatrick et al. 2006), and because of this, the establishment of C. quinquefasciatus in Galápagos in the mid-1980s is considered a serious threat to its endemic fauna (Whiteman et al. 2005; Causton et al. 2006).
Culex quinquefasciatus is a member of the Culex (pipiens) pipiens complex found in tropical and sub-tropical regions and is an important vector of wildlife diseases such as avian malaria, avianpox (Van Riper et al. 1986, 2002), filariasis (Labarthe & Guerrero 2005) and West Nile fever (Sardelis et al. 2001). Kilpatrick et al. (2006) estimated that West Nile virus (WNV) would most likely reach Galápagos by means of the anthropogenic introduction of mosquitoes from mainland South America. Further introduction of infectious C. quinquefasciatus mosquitoes was considered to be a particular risk for WNV introduction, with the risk from aeroplane transport of infectious mosquitoes to the islands calculated as being two orders of magnitude greater than the next most likely route of introduction by migratory birds (Kilpatrick et al. 2006). Once introduced, the native Galápagos mosquito Aedes taeniorhynchus has the potential to spread WNV (or other mosquito-borne pathogens) throughout the archipelago to a wide range of endemic vertebrate taxa (Bataille et al. 2009).
Given these risks, it is imperative to determine if there are ongoing introductions of C. quinquefasciatus and, if so, to assess how effectively newly introduced mosquitoes incorporate into established populations (as this can be taken as a proxy of survival for immigrants), in order to determine ongoing risks of mosquito-borne pathogen introductions to Galápagos. Also, the identification of introduction pathways is essential for the implementation of appropriate measures to mitigate the risk of mosquito-borne disease introductions. Recent reports indicate that aeroplanes (commercial and freight) and cargo boats from mainland Ecuador represent the main routes for insect introductions to Galápagos (Causton et al. 2006; Kilpatrick et al. 2006). Once introduced to an island, insects may invade the rest of the archipelago through natural dispersal or via human-aided transport such as aeroplanes and boats. It has been shown, for example, that lights on tourist boats attract flying insects, including C. quinquefasciatus (Roque Albelo et al. 2006), increasing the likelihood of spread of insects and vector-borne diseases among the islands.
In this paper we integrate the results of an aeroplane-monitoring programme in Galápagos with a population genetics study of C. quinquefasciatus. The former directly assesses the rates of transport of mosquitoes to Galápagos by air from mainland Ecuador, and the latter determines the population structure of C. quinquefasciatus, the pathways and frequency of introduction from the mainland to the archipelago and its spread among the islands.
2. Material and methods
(a). Aeroplane monitoring for mosquito introductions
Monitoring was conducted twice a month between October 2006 and September 2007 in Baltra airport and from October 2006 until March 2007 in San Cristobal airport, these being the only two airports in Galápagos that regularly receive flights from mainland Ecuador. On each monitoring occasion, the luggage compartments and the cabin areas of two to five aeroplanes were visited within an hour of arrival from mainland Ecuador, after the passengers had left the aeroplane, and once approximately half the cargo had been removed to allow access to the hold. Any live or dead invertebrates found were collected with hand-held aspirators and butterfly nets, and transported to the Charles Darwin Research Station for storage and identification. Mosquitoes were then sent to the Galápagos National Park's Genetics, Epidemiology and Pathology Laboratory (GGEPL) for further study.
(b). Mosquito sample collection, DNA extraction and microsatellite genotyping
For the population genetics study, adult C. quinquefasciatus mosquitoes were collected across the five inhabited Galápagos Islands (Baltra, Floreana, Isabela, San Cristobal, Santa Cruz; 302 specimens) and from mainland Ecuador (36 specimens) (figure 1) using miniature ultraviolet light traps or miniature incandescent light traps with a photoswitch-controlled CO2 release system (John W. Hock Company, Gainesville, FL). Light trap contents were examined either at GGEPL or at a laboratory on the mainland (Concepto Azul, Guayaquil, Ecuador). Culex mosquitoes were identified using morphological features, separated from other insects and collected and stored at −20°C. Additional C. quinquefasciatus specimens were collected as larvae from oviposition traps following the method described by Whiteman et al. (2005), reared to adulthood and then stored at −20°C. Six Culex specimens collected from the aeroplane monitoring performed in Baltra airport were also included in the study.
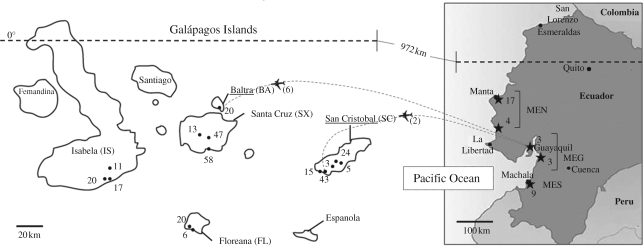
Figure 1.
Mosquito sampling sites in the Galápagos Islands (dots) and in mainland Ecuador (stars). The numbers beside the sampling sites correspond to the number of Culex quinquefasciatus mosquitoes collected. The aeroplane images indicate flight paths to Galápagos from mainland Ecuador. The numbers in parentheses beside aeroplane images indicate the number of C. quinquefasciatus mosquitoes caught in aeroplanes arriving on those flight paths. MEN, mainland Ecuador North; MEG, mainland Ecuador Guayaquil; MES, mainland Ecuador South.
The abdomen was removed from each female specimen before DNA extraction and the whole body was used for male specimens. DNA was extracted using a salting-out extraction method with ammonium acetate (Nicholls et al. 2000). We identified the Culex specimens collected as C. quinquefasciatus by using a polymerase chain reaction (PCR)-based method (Smith & Fonseca 2004). We genotyped each mosquito individually using 11 microsatellites originally isolated from C. quinquefasciatus or C. pipiens (Fonseca et al. 1998; Keyghobadi et al. 2004; Smith et al. 2005) in two fluorescently labelled multiplexes (table A1 in the electronic supplementary material). Multiplex PCR was performed in a 2 µl volume containing approximately 10 ng of desiccated DNA, 0.2–0.3 µM of each primer and 1 µl of QIAGEN four-dye system Multiplex PCR Master Mix (Kenta et al. 2008). The PCR program used was 95°C for 15 min, followed by 35 cycles of 94°C for 30 s, 54°C for 90 s, 72°C for 60 s, and finally 60°C for 30 min. Allele sizes were assigned using an ABI3730 DNA Analyser and Genemapper v. 2.0 software (Applied Biosystems).
(c). Microsatellite data analysis
For all analyses we initially grouped mosquitoes collected from different locations within each island together, and considered each grouping as a single-island population. Samples from mainland Ecuador were pooled in three groups according to their geographical origin (figure 1): a northern population (MEN), a population of the region of Guayaquil (MEG) and a southern population (MES). The six mosquitoes collected from aeroplanes in Baltra airport were considered to be of unknown origin, and were used only in individual assignment tests and tests for the detection of migrants (see following text).
Heterozygosity values and estimates of the frequency of null alleles were calculated using the program CERVUS v. 3.0 (Kalinowski et al. 2007). Conformity to Hardy–Weinberg equilibrium and linkage disequilibrium were tested with GenePop 4.0 (Raymond & Rousset 1995). Genetic differentiation between populations was quantified by calculating pairwise FST (Weir & Cockerham 1984) in Fstat v. 2.9.3 (Goudet 1995). Permutation tests with 10 000 randomizations were used to determine if the observed values were significantly different from zero, and the p-value adjusted using a sequential Bonferroni correction (Rice 1989). We also examined the population structure in our dataset with the Bayesian individual clustering method implemented in the program Structure v. 2.2 (Pritchard et al. 2000). We used the admixture model and tested for K potential genetic clusters contributing to the sample, with values of K ranging from 1 to 14. The program was run five times for each K for 300 000 generations with 100 000 burn-in steps (after checking for convergence of the Markov chain Monte Carlo chains), and the most likely K was identified according to the log-likelihood of the data ([ln P(D)] values), by estimation of P(K|X). In addition, we performed a principal component analysis (PCA) on the gene frequency data using the program PCA-GEN v. 1.2 (http://www2.unil.ch/popgen/softwares/pcagen.htm) with 10 000 randomizations to test if total inertia as well as inertia for individual axes were significantly different from zero.
The program Structure v. 2.2 was used to detect migrants in populations by supplying prior information on the origin of each individual genotyped. Here we specified the individuals as coming from one of the five Galápagos islands sampled or from one of the three mainland populations (total of eight populations), and the program was run as described above for K = 8. The six mosquitoes caught in aeroplanes were described as originating from Baltra to test if the program could identify them as migrants from mainland Ecuador. We estimated, in terms of admixture proportions, the contribution of introductions from the mainland population to the genetic diversity of each island population with LeadMix (Wang 2003). The effective number of migrants between each population (4Nm, where N is the effective population size and m the migration rate) was calculated with a Bayesian approach (Beerli 2006) implemented in the program Migrate v. 3.0 (Beerli 2008). The program was run initially with default setting for 10 000 000 generations with 2 000 000 generations burn-in. The outputs from the initial run were then used to parameterize three further runs of the same length, and congruence of the results verified.
3. Results
(a). Aeroplane monitoring
Ninety-three aeroplanes were assessed for the presence of dead or live invertebrates at Baltra airport over a period of 12 months (October 2006 to September 2007) and 33 aeroplanes were assessed at San Cristobal airport over a 6 month period (October 2006 to March 2007). We collected 105 invertebrates, 74 of which were alive (table A2 in the electronic supplementary material). Eleven of the live invertebrates collected were mosquitoes; no dead mosquitoes were found. Three specimens of A. taeniorhynchus and six specimens of C. quinquefasciatus mosquitoes were caught in aeroplanes landing at Baltra airport, and two C. quinquefasciatus were caught in aeroplanes landing at San Cristobal airport (these two specimens were not available for further genetic analysis). All A. taeniorhynchus and five C. quinquefasciatus mosquitoes were caught during the mainland wet season (January to March 2007; table A2 in the electronic supplementary material).
(b). Microsatellite variation, linkage disequilibrium and Hardy–Weinberg equilibrium
The number of alleles per locus ranged from 4 to 15 and the expected heterozygosity ranged from 0.30 to 0.75 (table A1 in the electronic supplementary material). Tests for linkage disequilibrium between all pairs of loci across populations found no significant disequilibrium after a sequential Bonferroni correction. Exact tests showed that locus pGT46 deviated significantly from the Hardy–Weinberg expectations in four of eight populations analysed (table A3 in the electronic supplementary material). These deviations were associated with positive FIS values ranging from 0.194 to 0.417, reflecting heterozygosity deficiency, probably owing to a high frequency of null alleles in pGT46 (estimated with CERVUS to range from 0.18 to 0.24). Subsequent analyses were performed without the locus pGT46 to ensure that results would not be biased by the presence of null alleles.
(c). Population structure
Of 28 FST values for pairwise comparisons between locations, 26 were significantly different from zero (range 0.023–0.35, p < 0.001; table A4 in the electronic supplementary material) and all remained significant after sequential Bonferroni correction. FST values showed that there was no significant differentiation between mosquito populations sampled in Baltra and San Cristobal Islands, or between the northern and southern mainland populations. FST values between Baltra and San Cristobal Islands and the northern and southern mainland populations were small but significant (table A4 in the electronic supplementary material).
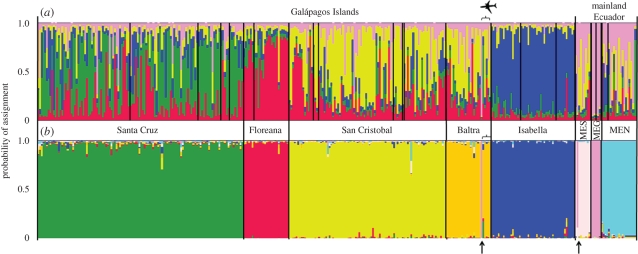
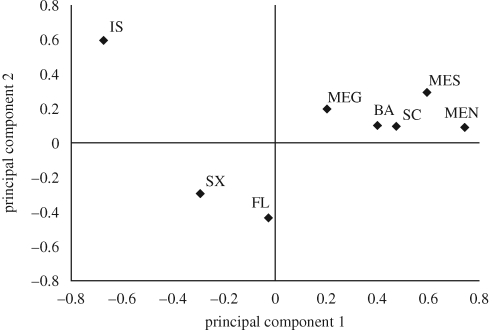
The Bayesian clustering analysis in Structure 2.2 determined K = 5 to have the highest probability ((for K = 5, P(K|X) = 1, for all other values of K, P(K|X) = 0); figure A1 and table A5 in the electronic supplementary material). Individuals of Santa Cruz, Isabela and Floreana Islands were predominantly assigned to three clusters, each specific for each island, whereas C. quinquefasciatus individuals from Baltra and San Cristobal Islands were clustered together and mainland individuals were grouped in a fifth cluster (figure 2a). Only two mosquitoes out of six caught in aeroplanes in Baltra airport were assigned to the mainland genetic cluster. Three of the remaining individuals were assigned to the Baltra–San Cristobal cluster, and the last one was assigned to the Santa Cruz cluster (figure 2a). In the PCA, the first principal component accounted for 41.6 per cent of the inertia in the data and separated Santa Cruz, Isabela and Floreana Islands from Baltra and San Cristobal Islands, which clustered with the three mainland populations (figure 3). The second principal component accounted for 22.8 per cent of the inertia in the data and separated Isabela Island from Santa Cruz and Floreana Islands (figure 3).
Figure 2.
Clustering of Culex quinquefasciatus individuals from the Galápagos Islands and mainland Ecuador based on the Structure 2.2 algorithm (Pritchard et al. 2000). Each of 343 individuals is represented by a vertical bar partitioned in colours segmented according to the probability of belonging to one of the K genetic clusters. (a) No prior information on population is given and K is defined as the number of genetic clusters that best fit with the data (here K = 5, identified by the five colours in the graph). (b) Prior information on population is given (K = 8, according to geographical locations) to detect potential migrants. Mosquitoes caught in aeroplanes are grouped and indicated by an aeroplane image. Potential migrants are identified by an arrow. MEN, mainland Ecuador North; MEG, mainland Ecuador Guayaquil; MES, mainland Ecuador South.
Figure 3.
Plot of the Eigen-values for the two first components of the principal component analysis (PCA) performed on Culex quinquefasciatus populations from Galápagos Islands and mainland Ecuador. IS, Isabela; SX, Santa Cruz; SC, San Cristobal; BA, Baltra; FL, Floreana; MEN, mainland Ecuador North; MES, mainland Ecuador South; MEG, mainland Ecuador Guayaquil.
(d). Detection of potential migrants
Analyses in Structure 2.2 incorporating prior information on the origin of each individual identified two potential migrants (figure 2b). Both individuals, one collected in an aeroplane that landed in Baltra Island and another from the southern mainland population, were identified as migrants from the Guayaquil region in mainland Ecuador (figure 2b). The other five mosquitoes caught in aeroplanes were assigned to the Baltra genetic cluster (figure 2b).
(e). Admixture proportions and migration patterns
Admixture analyses with LeadMix showed that the Baltra and San Cristobal populations (the sites of the airports) have a mixture of alleles from both the established Galápagos populations and the mainland populations, with an input from the mainland population of 50.7 per cent (confidence intervals (CI) 28–77%) in Baltra and of 33.6 per cent (CI 11.5–56.4%) in San Cristobal. The estimated admixture between the mainland populations and Isabela and Floreana had CIs overlapping zero, and so were not significant. The mainland input in the Santa Cruz population was significantly smaller than in Baltra and San Cristobal populations (3.2%; CI 0.7–10.6%). We then assessed the input from Baltra and San Cristobal Islands separately into each of the three remaining islands. The admixture levels obtained had large confidence intervals often overlapping zero, making the results hard to compare. However, there is some suggestion that input from both Baltra and San Cristobal may be important in Floreana Island (43.6 and 43.7%; CI 0.4–87%) and in Isabela (24.6 and 25.7%; CI 0–73.9%). In the Santa Cruz population, some allelic input may come from San Cristobal (12.6%; CI 0–66%), but probably not from Baltra (0.01%; CI 0–12.2%).
Precise estimation of migration rates between populations using Migrate was problematic for our data, because the estimates had large CIs (table A6 in the electronic supplementary material). Nevertheless, results indicated that the highest level of migration was from mainland Ecuador towards Baltra Island (4Nm = 5.23; CI 95%, 3–9.9) and San Cristobal Island (5.22; CI 1.6–9.95; table A6 in the electronic supplementary material). Our results also showed that there is some gene flow from Santa Cruz Island population towards Isabela (0.92; CI 0.17–1.68), San Cristobal (1.22; CI 0.6–2.62) and Baltra Islands (1.66; CI 0.46–4.62). Some gene flow was also detected from Isabela towards San Cristobal Island (2.33; CI 0.1–4.35) and from Baltra towards Floreana (2.24; CI 0.33–5.27). The results also indicated some gene flow between Floreana Island and mainland Ecuador (2.17; CI 0.55–4.86) and high numbers of migrants between Baltra and San Cristobal (5.88; CI 2.15–10; table A6 in the electronic supplementary material); the latter is probably owing to the influence of the continental mosquito populations on the mosquito populations of Baltra and San Cristobal Islands.
4. Discussion
Many invertebrate specimens from a wide diversity of orders were collected during the aeroplane monitoring, indicating that the introduction of invasive species by aeroplane represents a continuing and serious threat to the conservation of the endemic biodiversity of Galápagos, especially during the rainy season (Causton et al. 2006). The presence of C. quinquefasciatus specimens in aeroplanes combined with our genetic data suggests that this species has been introduced frequently to the Galápagos Archipelago by air transportation since it was first identified in Galápagos (on Santa Cruz Island) in 1985 (Whiteman et al. 2005). The genetic similarity between Baltra, San Cristobal and mainland C. quinquefasciatus populations (figures 2 and 3) suggests that arrival rates, survival and subsequent breeding with established island populations are high, and the Galápagos population is not therefore the result of a single colonization event.
Most aircraft landing in Galápagos transit through the international airport of Guayaquil (Cruz Martinez & Causton 2007), Ecuador's largest city, which is situated on the edge of the Guayas river delta. Within the airport perimeter are a large number of drainage ditches, potentially providing a good breeding habitat for C. quinquefasciatus. Therefore, it is most likely that mosquitoes introduced to Galápagos originate from Guayaquil airport, rather than from Quito, the origin for most Galápagos passenger flights, but which is at a high altitude (approx. 2800 m) and has much lower mosquito populations. Our results indicate that the Baltra and San Cristobal populations might be more closely related to the southern and northern Ecuadorian mainland populations than to the Guayaquil population. However, few samples were available from the Guayaquil region and southern Ecuador (6 and 9 individuals, respectively; figure 1), which might not be sufficient to give a true representation of the genotype frequencies in these areas. Alternatively, some mosquitoes might have survived on aircraft that had travelled to Guayaquil from other cities in the Ecuadorian domestic airline network, and that were subsequently routed to Galápagos via Guayaquil. Another option is that mosquitoes might have been introduced directly from elsewhere in Ecuador prior to the current restrictions on the routing of Galápagos-bound flights via Guayaquil.
Cargo boats transporting food, agricultural supplies and other goods to Galápagos have also been recognized as an important route for the introduction of invasive insect species (Causton et al. 2006). Santa Cruz, San Cristobal and Baltra islands are the locations for the main sea ports of Galápagos. At Santa Cruz, cargo boats unload in Puerto Ayora on the southern side of the island, approximately 45 km from Baltra airport, whereas on San Cristobal both the port and the airport are situated in the town of Puerto Baquerizo Moreno. Baltra has a small deep-water port next to the airport. Admixture analysis showed that the direct continental input into the C. quinquefasciatus population of Santa Cruz was small (3.2%), and the PCA and FST values indicated significant differentiation of the Santa Cruz population from the Baltra, San Cristobal and mainland populations (figure 3). This suggests that introductions of C. quinquefasciatus to Santa Cruz Island by boat are much less important than introductions by aeroplane (or boat) to Baltra and San Cristobal.
Our results also provide evidence for movements of mosquitoes between the islands of the archipelago. Culex quinquefasciatus has poor flying capacity and would not normally disperse successfully from human settlements because of the scarcity of fresh water on Galápagos. Therefore any movements of Culex mosquitoes among islands are most probably human-aided. Our results support this hypothesis, as movements detected with Migrate seem to originate from Santa Cruz, which is the most populated island of the archipelago. The port of Santa Cruz Island, Puerto Ayora, is a local maritime transport hub for the other islands, and a stop off for most tourist cruises. Gene flow was also detected between Baltra and Floreana Islands, which are not linked by local transport. This suggests that tourist cruises, most of which start from Baltra, may play a significant role in the movement of mosquitoes between islands (Roque Albelo et al. 2006).
Our results emphasize that the transport of mosquitoes by aeroplane remains one of the most important risk factors for vector-borne disease introduction to Galápagos, as previously proposed (Kilpatrick et al. 2006). Kilpatrick et al. (2006) used data on mosquito transport on aircraft from other parts of the world to parameterize a risk assessment model for WNV introduction to Galápagos. Using direct observations for flights to Galápagos, we calculated that an average of 0.098 live mosquitoes arrived per aeroplane at Baltra airport and 0.061 mosquitoes per aeroplane at San Cristobal airport. When estimations were made on results from the wet season only (January to March 2007), the average number of mosquitoes per aeroplane increased to 0.19 in Baltra airport. These numbers are lower than previously estimated (Kilpatrick et al. 2006), but we may have missed mosquitoes escaping from aeroplanes before we were allowed to enter them. The number of commercial flights has almost doubled from the 2004 values used by Kilpatrick et al. (2006) (Cruz Martinez & Causton 2007). Using these updated data along with our Galápagos-specific mosquito arrival rates and following the methods of Kilpatrick et al. (2006), we can estimate a minimum risk of 4.33 (CI 1.09–10.89) WNV-infectious mosquito days being introduced to Galápagos per year if WNV becomes established in the mainland Ecuador mosquito population. This risk increases to 13.48 (CI 3.41–33.92) infectious mosquito days per year during the rainy season. These compare with an overall air transportation risk of 101.7 (CI 8.3–272.9) infectious mosquito days originally calculated by Kilpatrick et al. (2006). Although the absolute values using the updated data are lower, the CIs overlap with those of the original estimate.
Proof of regular introduction of mosquitoes by aeroplane is a great concern and the risks of introduction and spread within the archipelago scale directly with the increase in flights and in visitor numbers (Cruz Martinez & Causton 2007). Moreover, there is continuing pressure to allow flights from other Ecuadorian airports to Galápagos and to open a newly extended airport at Puerto Villamil on Isabela Island to flights from the mainland. Aeroplane disinsection, according to standard World Health Organization protocols, have been mandatory for commercial flights from mainland Ecuador to Galápagos since August 2007. This should greatly reduce the threat of live mosquito introductions, but monitoring of the success of the disinsection is required to ensure its effectiveness. Also, disinsection must be an enforced requirement for all aircraft (commercial passenger, freight, military or private) landing in the Galápagos Islands to minimize the risk of transporting any invasive invertebrates to the archipelago.
Disinsection protocols have recently been implemented for inter-island aeroplane services, which should help reduce risks of transport via this route. For sea routes, a quarantine and inspection system is in place to control the movement of goods between islands (Causton et al. 2006), but presently it does not have the means to effectively prevent the transport of stowaway invertebrates on tourist or local boats. Ideally, fumigation or disinsection protocols for tourist and local boats should be implemented, with strict measures especially taken for boats departing from Baltra, San Cristobal and Santa Cruz. Roque Albelo et al. (2006) recommended measures such as using insect traps and permitting only yellow lights to be used at night by boats to limit the transportation of insects. Ideally, these would be made standard procedure in Galápagos.
This study is the first, to our knowledge, to present genetic evidence showing the role of the Worldwide Airline transportation Network (Tatem et al. 2006) in the invasion of isolated oceanic islands by disease-carrying mosquitoes. Oceanic islands such as the Galápagos are losing this isolation and it might represent one of the most important threats to the endemic biodiversity and the economies of these environments. With steadily growing transport links between the Galápagos Islands and the continent, strict mitigation measures must be implemented and maintained, otherwise invasive species and pathogens, such as WNV, are certain to reach and spread among the Galápagos Islands, with a potentially devastating effect on the endemic wildlife.
Acknowledgment
We thank the Dirección de Aviación Civil (DAC) of Ecuador, TAME and AeroGal airlines for providing us with the necessary permits to carry out the aircraft monitoring, and the personnel of the Sistema de Inspección y Cuarentena de Galápagos (SESA-SICGAL) for helping and facilitating aircraft monitoring in Baltra and San Cristobal airports. Piedad Lincango (Department of Invertebrates, Charles Darwin Research Station) helped in the organization and implementation of the aircraft monitoring. D. Dawson (NERC Molecular Genetics Facility, Sheffield) provided microsatellite genotyping advice and project support. A. Krupa (Molecular Ecology Laboratory, University of Sheffield) kindly provided technical support. We are very grateful to P. Martinez, the Galápagos Genetics, Epidemiology and Pathology Laboratory, el Servicio del Parque Nacional Galápagos (in particular the personnel of the Control Santa Rosa), and the Charles Darwin Research Station (Isla Santa Cruz, Galápagos, Ecuador) for logistical support and research permits. This study was supported by the Marie Curie Early Stage Training programme of the European Union, the Darwin Initiative, DEFRA (162-12-17, EIDPO15), UK, and the Natural Environment Research Council (NERC), UK.
References
- Bataille A., Cunningham A. A., Cedeno V., Patino L., Constantinou A., Kramer L. D., Goodman S. J.2009Natural colonization and adaptation of a mosquito species in Galápagos and its implications for disease threats to endemic wildlife. Proc. Natl Acad. Sci. USA (doi:10.1073/pnas.0901308106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerli P.2006Comparison of Bayesian and maximum likelihood inference of population genetic parameters. Bioinformatics 22, 341–345 (doi:10.1093/bioinformatics/bti803) [DOI] [PubMed] [Google Scholar]
- Beerli P.2008Migrate version 3.0: a maximum likelihood and Bayesian estimator of gene flow using the coalescent. See http://popgen.scs.edu/migrate.html. [Google Scholar]
- Causton C. E., Peck S. B., Sinclair B. J., Roque-Albelo L., Hodgson C. J., Landry B.2006Alien insects: threats and implications for conservation of Galápagos Islands. Ann. Entomol. Soc. Am. 99, 121–143 (doi:10.1603/0013-8746(2006)099[0121:AITAIF]2.0.CO;2) [Google Scholar]
- Chevillon C., Briant L., Renaud F., Devaux C.2008The Chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 16, 80–88 (doi:10.1016/j.tim.2007.12.003) [DOI] [PubMed] [Google Scholar]
- Cruz Martinez J. D., Causton C. E.2007Análisis del riesgo asociado a las operaciones y rutas aéreas al Archipiélago de Galápagos Puerto Ayora, Galápagos Islands, Ecuador: Charles Darwin Foundation [Google Scholar]
- Daszak P., Cunningham A. A., Hyatt A. D.2000Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443–449 (doi:10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- Fonseca D. M., Atkinson C. T., Fleischer R. C.1998Microsatellite primers for Culex pipiens quinquefasciatus, the vector of avian malaria in Hawaii. Mol. Ecol. 7, 1617–1619 [PubMed] [Google Scholar]
- Goudet J.1995Fstat (version 1.2): a computer program to calculate F-statistics. J. Hered. 86, 485–486 [Google Scholar]
- Jones K. E., Patel N. G., Levy M. A., Storeygard A., Balk D., Gittleman J. L., Daszak P.2008Global trends in emerging infectious diseases. Nature 451, 990–993 (doi:10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski S. T., Taper M. L., Marshall T. C.2007Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106 (doi:10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- Kenta T., Gratten J., Haigh N. S., Hinten G. N., Slate J., Butlin R. K., Burke T.2008Multiplex SNP-SCALE: a cost-effective medium-throughput single nucleotide polymorphism genotyping method. Mol. Ecol. Resources 8, 1230–1238 [DOI] [PubMed] [Google Scholar]
- Keyghobadi N., Matrone M. A., Ebel G. D., Kramer L. D., Fonseca D. M.2004Microsatellite loci from the northern house mosquito (Culex pipiens), a principal vector of West Nile virus in North America. Mol. Ecol. Notes 4, 20–22 (doi:10.1046/j.1471-8286.2003.00557.x) [Google Scholar]
- Kilpatrick A. M., Daszak P., Goodman S. J., Roog H., Kramer L. D., Cedeno V., Cunningham A. A.2006Predicting pathogen introduction: West Nile virus spread in Galápagos. Cons. Biol. 20, 1224–1231 (doi:10.1111/j.1523-1739.2006.00423.x) [DOI] [PubMed] [Google Scholar]
- Kramer L. D., Styer L. M., Ebel G. D.2008A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 53 (doi:10.1146/annurev.ento.53.103106.093258) [DOI] [PubMed] [Google Scholar]
- Labarthe N., Guerrero J.2005Epidemiology of heartworm: what is happening in South America and Mexico? Vet. Parasitol. 133, 149–156 (doi:10.1016/j.vetpar.2005.04.006) [DOI] [PubMed] [Google Scholar]
- Lounibos L. P.2002Invasions by insect vectors of human disease. Annu. Rev. Entomol. 47, 233–266 (doi:10.1146/annurev.ento.47.091201.145206) [DOI] [PubMed] [Google Scholar]
- Matson K. D.2006Are there differences in immune function between continental and insular birds? Proc. R. Soc. B 273, 2267–2274 (doi:10.1098/rspb.2006.3590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J. A., Double M. C., Rowell D. M., Magrath D.2000The evolution of cooperative and pair breeding in thornbills Acanthiza (Pardalotidae). J. Avian Biol. 31, 165–176 (doi:10.1034/j.1600-048X.2000.310208.x) [Google Scholar]
- Pritchard J. K., Stephens M., Donnelly P.2000Inference of population structure using multilocus genotype data. Genetics 155, 945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M., Rousset F.1995GenePop (version 1.2): population-genetics software for exact test and ecumenicism. J. Hered. 86, 248–249 [Google Scholar]
- Rice W. R.1989Analysing tables of statistical tests. Evolution 43, 223–225 (doi:10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- Roque Albelo L., Berg M., Galarza M.2006‘Polizontes peligrosos’, dispersion de insectos entre las Islas Galapagos en barcos de turismo. Research report.Puerto Ayora, Galapagos: Charles Darwin Foundation [Google Scholar]
- Sardelis M. R., Turell M. J., Dohm D. J., O'Guinn M. L.2001Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 7, 1018–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Fonseca D. M.2004Rapid assays for identification of members of the Culex (culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 70, 339–345 [PubMed] [Google Scholar]
- Smith J. L., Keyghobadi N., Matrone M. A., Escher R. L., Fonseca D. M.2005Cross-species comparison of microsatellite loci in the Culex pipiens complex and beyond. Mol. Ecol. Notes 5, 697–700 (doi:10.1111/j.1471-8286.2005.01034.x) [Google Scholar]
- Tatem A. J., Hay S. I., Rogers D. J.2006Global traffic and disease vector dispersal. Proc. Natl Acad. Sci. USA 103, 6242–6247 (doi:10.1073/pnas.0508391103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye A., Snell H. L., Peck S. B., Adsersen H.2002Oustanding terrestrial features of the Galápagos archipelago. In A biodiversity vision for the Galápagos Islands (ed. Bensted-Smith R.), pp. 12–23 Puerto Ayora, Galápagos: Charles Darwin Foundation & World Wide Fund [Google Scholar]
- Van Riper C., Van Riper S. G., Hansen W. R.2002Epizootiology and effect of avian pox on Hawaiian forest birds. Auk 119, 929–942 (doi:10.1642/0004-8038(2002)119[0929:EAEOAP]2.0.CO;2) [Google Scholar]
- Van Riper C., Van Riper S. G., Lee Goff M., Laird M.1986The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 56, 327–344 (doi:10.2307/1942550) [Google Scholar]
- Wang J.2003Maximum likelihood estimation of admixture proportions from genetic data. Genetics 164, 747–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner R. E.1968The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 70, 101–120 (doi:10.2307/1365954) [Google Scholar]
- Watkins G., Cruz F.2007Galapagos at risk: a socioeconomic analysis of the situation in the archipelago Puerto Ayora, Galápagos Islands, Ecuador: Charles Darwin Foundation [Google Scholar]
- Weir B. S., Cockerham C. C.1984Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (doi:10.2307/2408641) [DOI] [PubMed] [Google Scholar]
- Whiteman N. K., Goodman S. J., Sinclair B. J., Walsh T. I. M., Cunningham A. A., Kramer L. D., Parker P. G.2005Establishment of the avian disease vector Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) on the Galápagos Islands, Ecuador. Ibis 147, 844–847 (doi:10.1111/j.1474-919X.2005.00468.x) [Google Scholar]
- Wikelski M., Foufopoulos J., Vargas H., Snell H.2004Galápagos birds and diseases: invasive pathogens as threats for island species. Ecol. Soc. 9 See http://www.ecologyandsociety.org/vol9/iss1/art5. [Google Scholar]