Abstract
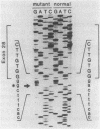
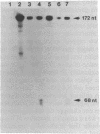
Previous observations demonstrated that a lethal variant of osteogenesis imperfecta had two altered alleles for pro alpha 2(I) chains of type I procollagen. One mutation produced a nonfunctioning allele in that there was synthesis of mRNA but no detectable synthesis of pro alpha 2(I) chains from the allele. The mutation in the other allele caused synthesis of shortened pro alpha 2(I) chains that lacked most or all of the 18 amino acids encoded by exon 28. Subclones of the pro alpha 2(I) gene were prepared from the proband's DNA and the DNA sequence was determined for a 582-base-pair (bp) region that extended from the last 30 bp of intervening sequence 26 to the first 26 bp of intervening sequence 29. Data from six independent subclones demonstrated that all had the same sequence as a previously isolated normal clone for the pro alpha 2(I) gene except that four subclones had a single base mutation at the 3' end of intervening sequence 27. The mutation was a substitution of guanine for adenine that changed the universal consensus sequence for the 3' splicing site of RNA from -AG- to -GG-. S1 nuclease experiments demonstrated that about half the pro alpha 2(I) mRNA in the proband's fibroblasts was abnormally spliced and that the major species of abnormal pro alpha 2(I) mRNA was completely spliced from the last codon of exon 27 to the first codon of exon 29. The mutation is apparently unique among RNA splicing mutations of mammalian systems in producing a shortened polypeptide chain that is in-frame in terms of coding sequences, that is used in the subunit assembly of a protein, and that contributes to a lethal phenotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Guglielmi P., Siebenlist U., Ravetch J. V., Jensen J. P., Korsmeyer S. J. A DNA insertion/deletion necessitates an aberrant RNA splice accounting for a mu heavy chain disease protein. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2689–2693. doi: 10.1073/pnas.83.8.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Bonadio J., Byers P. H., Gelinas R. E. Intron-mediated recombination may cause a deletion in an alpha 1 type I collagen chain in a lethal form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1985 May;82(9):2870–2874. doi: 10.1073/pnas.82.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Beier G., Engel J. The renaturation of soluble collagen. Products formed at different temperatures. Biochemistry. 1966 Aug;5(8):2744–2755. doi: 10.1021/bi00872a035. [DOI] [PubMed] [Google Scholar]
- Boedtker H., Finer M., Aho S. The structure of the chicken alpha 2 collagen gene. Ann N Y Acad Sci. 1985;460:85–116. doi: 10.1111/j.1749-6632.1985.tb51159.x. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Shapiro J. R., Rowe D. W., David K. E., Holbrook K. A. Abnormal alpha 2-chain in type I collagen from a patient with a form of osteogenesis imperfecta. J Clin Invest. 1983 Mar;71(3):689–697. doi: 10.1172/JCI110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Gargiulo V., Williams C. J., Ramirez F. Multiexon deletion in an osteogenesis imperfecta variant with increased type III collagen mRNA. J Biol Chem. 1985 Jan 25;260(2):691–694. [PubMed] [Google Scholar]
- Cladaras C., Hadzopoulou-Cladaras M., Felber B. K., Pavlakis G., Zannis V. I. The molecular basis of a familial apoE deficiency. An acceptor splice site mutation in the third intron of the deficient apoE gene. J Biol Chem. 1987 Feb 15;262(5):2310–2315. [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H., Steinmann B., Gelinas R. E. Lethal osteogenesis imperfecta resulting from a single nucleotide change in one human pro alpha 1(I) collagen allele. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6045–6047. doi: 10.1073/pnas.83.16.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chan D., Chambers G. W., Walker I. D., Bateman J. F. Deletion of 24 amino acids from the pro-alpha 1(I) chain of type I procollagen in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1986 Apr 25;261(12):5496–5503. [PubMed] [Google Scholar]
- Eyre D. R., Shapiro F. D., Aldridge J. F. A heterozygous collagen defect in a variant of the Ehlers-Danlos syndrome type VII. Evidence for a deleted amino-telopeptide domain in the pro-alpha 2(I) chain. J Biol Chem. 1985 Sep 15;260(20):11322–11329. [PubMed] [Google Scholar]
- Hauschka P. V., Harrington W. F. Collagen structure in solution. 3. Effec of ross-links on thermal stability and refolding kinetics. Biochemistry. 1970 Sep 15;9(19):3734–3745. doi: 10.1021/bi00821a012. [DOI] [PubMed] [Google Scholar]
- Hidaka Y., Palella T. D., O'Toole T. E., Tarlé S. A., Kelley W. N. Human adenine phosphoribosyltransferase. Identification of allelic mutations at the nucleotide level as a cause of complete deficiency of the enzyme. J Clin Invest. 1987 Nov;80(5):1409–1415. doi: 10.1172/JCI113219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Bradshaw R. A. Strand separation of DNA fragments and their isolation from nondenaturing polyacrylamide gels. Anal Biochem. 1984 Aug 1;140(2):456–458. doi: 10.1016/0003-2697(84)90193-3. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Chu M. L., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988 Jun 15;252(3):633–640. doi: 10.1042/bj2520633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvit J., DiLella A. G., Brayton K., Ledley F. D., Robson K. J., Woo S. L. GT to AT transition at a splice donor site causes skipping of the preceding exon in phenylketonuria. Nucleic Acids Res. 1987 Jul 24;15(14):5613–5628. doi: 10.1093/nar/15.14.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Urlaub G., Chasin L. Spontaneous splicing mutations at the dihydrofolate reductase locus in Chinese hamster ovary cells. Mol Cell Biol. 1986 Jun;6(6):1926–1935. doi: 10.1128/mcb.6.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Dickson L. A., Pope F. M., Korhonen V. R., Nicholls A., Prockop D. J., Myers J. C. Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem. 1984 Nov 10;259(21):12941–12944. [PubMed] [Google Scholar]
- Ramirez F., Bernard M., Chu M. L., Dickson L., Sangiorgi F., Weil D., De Wet W., Junien C., Sobel M. Isolation and characterization of the human fibrillar collagen genes. Ann N Y Acad Sci. 1985;460:117–129. doi: 10.1111/j.1749-6632.1985.tb51160.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Harvey Lect. 1985 1986;81:1–31. [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippola M., Kaffe S., Prockop D. J. A heterozygous defect for structurally altered pro-alpha 2 chain of type I procollagen in a mild variant of osteogenesis imperfecta. The altered structure decreases the thermal stability of procollagen and makes it resistant to procollagen N-proteinase. J Biol Chem. 1984 Nov 25;259(22):14094–14100. [PubMed] [Google Scholar]
- Steinmann B., Nicholls A., Pope F. M. Clinical variability of osteogenesis imperfecta reflecting molecular heterogeneity: cysteine substitutions in the alpha 1(I) collagen chain producing lethal and mild forms. J Biol Chem. 1986 Jul 5;261(19):8958–8964. [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Wirtz M. K., Glanville R. W., Steinmann B., Rao V. H., Hollister D. W. Ehlers-Danlos syndrome type VIIB. Deletion of 18 amino acids comprising the N-telopeptide region of a pro-alpha 2(I) chain. J Biol Chem. 1987 Dec 5;262(34):16376–16385. [PubMed] [Google Scholar]
- de Vries W. N., de Wet W. J. The molecular defect in an autosomal dominant form of osteogenesis imperfecta. Synthesis of type I procollagen containing cysteine in the triple-helical domain of pro-alpha 1(I) chains. J Biol Chem. 1986 Jul 5;261(19):9056–9064. [PubMed] [Google Scholar]
- de Wet W. J., Pihlajaniemi T., Myers J., Kelly T. E., Prockop D. J. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem. 1983 Jun 25;258(12):7721–7728. [PubMed] [Google Scholar]
- de Wett W., Sippola M., Tromp G., Prockop D., Chu M. L., Ramirez F. Use of R-loop mapping for the assessment of human collagen mutations. J Biol Chem. 1986 Mar 15;261(8):3857–3862. [PubMed] [Google Scholar]