Abstract
Evolutionary researchers have recently suggested that pre-modern human societies habitually practised cooperative breeding and that this feature helps explain human prosocial tendencies. Despite circumstantial evidence that post-reproductive females and extra-pair males both provide resources required for successful reproduction by mated pairs, no study has yet provided details about the flow of food resources by different age and sex categories to breeders and offspring, nor documented the ratio of helpers to breeders. Here, we show in two hunter–gatherer societies of South America that each breeding pair with dependent offspring on average obtained help from approximately 1.3 non-reproductive adults. Young married males and unmarried males of all ages were the main food providers, accounting for 93–100% of all excess food production available to breeding pairs and their offspring. Thus, each breeding pair with dependants was provisioned on average by 0.8 adult male helpers. The data provide no support for the hypothesis that post-reproductive females are the main provisioners of younger reproductive-aged kin in hunter–gatherer societies. Demographic and food acquisition data show that most breeding pairs can expect food deficits owing to foraging luck, health disabilities and accumulating dependency ratio of offspring in middle age, and that extra-pair provisioning may be essential to the evolved human life history.
Keywords: cooperative breeding, hunter–gatherers, life history
1. Introduction
Evolutionary researchers have recently suggested that our species has been organized, throughout much of its natural history, into partially kin-based resource acquisition and consumption units that engage in ‘cooperative breeding’ (e.g. Emlen 1995; Hrdy 1999, 2005, 2009; Kaplan et al. 2000; Wiessner 2002; Kaplan & Gurven 2005; Mace & Sear 2005). By cooperative breeding we mean that some adults exhibit costly behaviours that can usually be expected to increase the successful reproductive output of other adults. If true, the recognition that our human ancestors were cooperative breeders can potentially unite a large set of independent observations concerning the ‘human-evolved adaptive complex’ (Lancaster & Kaplan 2009) and may help explain important differences in prosocial behaviour, ‘shared intentionality’, social intelligence and cumulative cultural capacity that distinguish humans from other great apes (Hermann et al. 2007; Hrdy 2009; Hill et al. in press). However, the details of human alloparental helping patterns remain to be determined, and these patterns must be considered in the context of a more general theory about cooperative breeding in vertebrates (e.g. Koenig & Dickinson 2004; Clutton-Brock 2006) in order to account for variation across human societies. Does most help come from other breeders, or are non-breeding helpers common? What age and sex classes provide most help, and are there patterns in the different types of help provided by different classes of helpers? Do these patterns affect parental investment in different sexed offspring? Are helpers critical for successful reproduction? And, finally, why do helpers help?
Despite variation, juveniles in all hunter–gatherer societies require food provisioning to survive (Kaplan 1994). Likewise, hunter–gatherer women often acquire less food than they themselves require (Kaplan et al. 2000). These observations imply provisioning by someone other than reproductive females. The male-provisioning hypothesis (Kaplan et al. 2000) suggests that most of the food resources used to subsidize reproductive females and their offspring are provided by males (husbands and others), who acquire the majority of food energy and nearly all the protein–lipid macronutrients in foraging societies, and share those resources for a variety of reasons (Gurven & Hill 2009). Males often form long-term pair bonds with reproducing females and emotional bonds with presumed offspring that they provision and sometimes raise to adulthood, even in the absence of a female partner (Hill & Hurtado 1996). But males may also share food with females and juveniles as a form of phenotypic signalling, mating investment, contingent reciprocity with other adults or as alloparental investors with a variety of potential fitness payoffs.
An alternative view of cooperative provisioning is termed the ‘grandmother hypothesis’ (Hawkes et al. 1998). Based on observations that post-reproductive females sometimes acquire more food than reproductive-aged females (e.g. Hawkes et al. 1989; Hurtado et al. 1992), and that juveniles with surviving grandmothers show higher age-specific survival in many societies (Sear & Mace 2008), proponents of this view suggest that post-reproductive females are the major food providers to breeding females and their young, and that male provisioning is an incidental side effect of mating strategies, nutritionally less important than the food obtained by women and their mothers.
Human hunter–gatherers are described as highly cooperative in both food acquisition and juvenile care. They are characterized by high rates of resource transfer between most members of a residential unit (Gurven 2004), which potentially includes non-breeders, dependent juveniles and breeders with both low and high juvenile-dependency ratios. Most hunter–gatherer social units contain adults with few or no dependants, and those individuals often produce more food than individuals with multiple dependants (e.g. Hawkes et al. 1989; Hurtado et al. 1992). Thus, some adults behave as alloparental provisioners. While we focus on food provisioning in this paper, many other types of alloparental care are also commonly reported in foraging societies (e.g. Hames & Draper 2004; Hewlett & Lamb 2005), including a wide array of ‘altruistic’ services provided for both juveniles and reproducing adults (e.g. clearing trails and camps, building shelters and bridges, lending tools, providing firewood and water, carrying children and possessions, caring for debilitated individuals, and supplying information about edible resources; Hill 2002).
In this paper, we consider breeding pairs as social, economic and reproductive units, and examine the extent to which they are assisted by non-reproductive helpers or other breeding pairs. All food-sharing studies in foraging societies show that fathers provide a significant fraction of the food consumed by their offspring (Gurven 2004). Marriage is a human universal (universally accompanied by ritual and/or social regulation), and most hunter–gatherer females experience long-term co-residence with one or only a few males, producing most or all of their offspring together (see below and Wiessner 2009). A long natural history of pair bonding in Homo sapiens is indicated by the small relative size of human testes despite living in multi-male, multi-female social groups, and the extensive hormonal regulation of emotional bonding between males and their mates and offspring (Ellison & Gray 2009).
Thus, our goal is to determine whether food deficits are typical for hunter–gatherer nuclear families and, if so, who provisions the needy families. We provide analyses to test between the male-provisioning and the grandmother hypotheses of nourishing helpers, and we analyse demographic and production data in order to determine whether hunter–gatherers have developed a life history that obligates extra-pair provisioning. Finally, we discuss the relationship between the evolved post-reproductive phase, and the obligate provisioning required by reproducing females.
2. Study populations
Ache foragers inhabited the tropical forests of Eastern Paraguay, making first peaceful contact with outsiders in the mid-1970s, shortly before we began our study. During the past 30 years they have lived part-time on reservation settlements, returning frequently to the forest for extended time periods (McMillan 2001; Hill & Kintigh 2009). Foraging-dependent Ache live mainly on mammalian game, honey and extracted palm starch, with fruits and other collected resources accounting for less than 5 per cent of the diet (Kaplan et al. 2000). Sexual division of labour is pronounced, with men foraging over 6 h daily while women care for children and transport household items, foraging less than 2 h daily (Hill et al. 1985; Hurtado et al. 1985). Women forage even less when married to a high-producing husband (Hurtado et al. 1992). Cooperation in all realms of food acquisition and daily life is extensive (Hill 2002). Analyses of quantitative data on food sharing demonstrates band-wide division of game with no kin bias, and extensive, but slightly kin-biased, sharing of vegetable and invertebrate foods (Kaplan et al. 1984; Kaplan & Hill 1985a,b). Both contingent reciprocity and need-based provisioning are typical of collected foods and meat sharing at reservations (Gurven et al. 2002; Allen-Arave et al. 2008). Ache demographic patterns include high fertility, long lifespans and measured positive effects on survivorship and fertility associated with the presence of some kin categories and for some age and sex classes (Hill & Hurtado 1996). Women in the Ache population show the highest rates of pair-bond dissolution of any foraging group reported, yet post-reproductive women still produced children with fewer than two men, on average, in a lifetime (Hill & Hurtado 1996, pp. 219–237). Recent research with the Ache has documented age patterns of food acquisition and growth (e.g. Kaplan et al. 2000; Walker et al. 2002; Walker & Hill 2003) that are the basis for some of the calculations presented here.
The Hiwi of Venezuela live in seasonally flooded grassland savannahs, and primarily forage for riverine resources and species that inhabit gallery forests of the region. Hiwi foragers were first pacified in 1959 and studied by us from 1984–1990. Unlike the Ache, the Hiwi did not partially adopt agriculture prior to our study period. Quantitative data on Hiwi foraging show they are mainly dependent on mammalian game, fish and roots (Hurtado & Hill 1987, 1990). As reported for the Ache, Hiwi women also work fewer hours daily when their husband is a high food producer (Hurtado et al. 1992). While food sharing is extensive, transfers to other families statistically favour kin, neighbours and reciprocity partners, and meat is typically shared more than non-meat resources (Gurven et al. 2000a). Relative to the Ache, the Hiwi show medium fertility, high mortality owing to violence, strong preferential female infanticide and rapid childhood growth with earlier age at maturity (Kaplan et al. 2000; Walker et al. 2006; Hill et al. 2007). Pair-bond stability is considerably higher among the Hiwi, with post-reproductive women reporting a mean of only 1.7 husbands in a reproductive career (Hurtado & Hill 1992).
3. Food transfers to breeding pairs ameliorate unpredictable shortfalls
Regardless of their causal motivator, food transfers in foraging societies buffer critical resource shortfalls for breeding pairs on three time scales. These correspond to food deficits caused by acquisition luck, health disabilities and cumulative dependency load. First, daily fluctuations in foraging success and short-term stochasticity (see electronic supplementary material) create conditions in which food transfers are required for optimal health and survival. Among the Ache, who hunt mainly small game, daily variability owing to hunting luck is moderate, and men obtain game on 50 per cent of all days (Hill & Kintigh 2009). Among the Hiwi, men obtained some meat on only 24 per cent of the days that they foraged, and the frequency of successful prey acquisition among other low-latitude hunter–gatherers appears similar to the Hiwi or lower—for example, 27 per cent of all hunting days for !Kung men (Hill & Kintigh 2009); 21 per cent of hunting days for Agta men (Headland 1986), 11 to 30 per cent on individual hunts for Efe men (Bailey 1991, pp. 84–85); and 3.4 per cent on individual hunts for Hadza big-game hunters (Hawkes et al. 2001, p. 684).
Although healthy modern adults can survive for more than two months without food, small children in hunter–gatherer societies, because of lower fat reserves, parasite infestation and allometrically higher metabolic rates, can probably only survive for approximately one week without food (see electronic supplementary material). A tabulation of the actual amount of meat obtained by Ache hunters on all days when they hunted (n = 14 364 total days on 148 hunters over a 27-year period; Hill & Kintigh 2009) shows that 4 per cent of all 7-day runs of monitored hunting for individual men resulted in no meat at all. Because hunted game provides approximately 78 per cent of the food energy consumed in Ache society (Kaplan et al. 2000), we might guess that for about two weeks per year (0.04 × 52), Ache breeding pairs require extra-pair food transfers for their juvenile dependants to remain healthy.
Second, hunters often experience longer runs of hunting failure owing to injury or illness. Recent analyses of Ache health records from 1997 to 2000 show that men in one study settlement were sick or injured in ways that would significantly limit hunting effort on 21 per cent of all days (n = 27 470 man days; see electronic supplementary material). Runs of debilitating health ranged in length from 3 to 360 days for morbidity the onset of which began during our sample period, and 11 of 29 men experienced a run of poor health lasting at least 30 days. One man in the sample was permanently disabled after falling from a tree prior to the start of the study period, and another (not in the sample) was permanently disabled from tuberculosis.
Although this health sample is drawn from reservation-based hunter–gatherers exposed to modern infectious disease as well as traditional health insults, interviews with older men suggest that similar or worse rates of compromised health plagued the Ache in the pre-contact period (rates of trauma were apparently higher, while rates of some infectious diseases appear lower). This problem is ubiquitous in all foraging populations. Bailey (1991) showed that Efe men from 1981 to 1982 were injured or sick on 21 per cent of all days sampled, and Sugiyama (2004a,b) documented frequent serious injury and long runs of disability among Yora (8% of all days) and Shiwar hunters of the Amazon, with a large impact on economic productivity (Sugiyama & Chacon 2000). Likewise, M. Gurven (personal communication, 2008) found in interviews that 75 per cent of Tsimane adults (n = 570) had been incapacitated by illness or injury in the previous three months (10% of all days).
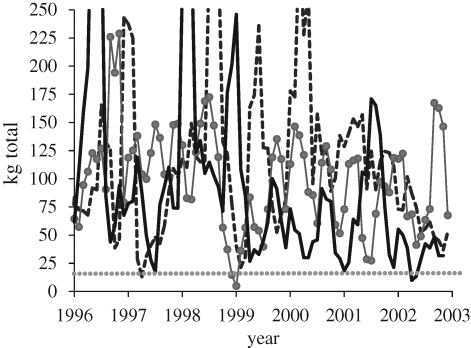
Significant fluctuations in mean hunting return rates caused by bouts of poor health can be detected by continuous monitoring of Ache men over a multi-year period. From 1996 to 2003, 9 of the 12 best hunters in our sample experienced at least one three-month period of hunting success of less than 10 per cent of their long-term average rate (figure 1; electronic supplementary material). Health-related production failure is even more pronounced among lower-return hunters. Data from the Arroyo Bandera Ache community shows that 42 of the 49 men who resided in that study community during these 7 years experienced at least one 90-day period with 0 kg meat acquisition. Interviews suggest that most of these periods were associated with poor health (A. M. Hurtado 2007, unpublished data). Not surprisingly, Ache hunters cite the fear of disability as an important reason why they willingly conform to the conventions of band-wide and need-based food sharing. Importantly, systematic observations on the reservation confirm that Ache men who are more generous in food sharing obtain more help from others when they are sick or injured (Gurven et al. 2000b).
Figure 1.
Tri-monthly total meat acquisition for the top three Ache hunters from 1996 to 2003 at the Arroyo Bandera settlement. Each point represents the 90-day total meat acquisition. The dotted horizontal line represents approximately 10 per cent of the mean 90-day meat acquisition. See electronic supplementary material for more hunters.
4. Food transfers are required to buffer predictable life-history shortfalls
The third time scale of energy shortfall experienced by breeding pairs is associated with the human life history. Because energy harvest rates and accumulating offspring dependency loads do not follow the same age curve in hunter–gatherers (Walker et al. 2002; Gurven & Walker 2006), breeding pairs can generally expect to be net producers of food during some periods and net consumers at other times. Most importantly, because of the age structure of need, we hypothesize that those who provide food to families with energetic deficits at one point in time may not receive shares back later from the prior recipients. If true, this would imply that food providers are often ‘helpers’ rather than reciprocity partners. In order to test this hypothesis, and determine the age–sex structure of food provisioning, we have estimated production and consumption patterns for the Ache and Hiwi during time periods when both were dependent on foraging and displayed population age structure and family composition of full-time foragers.
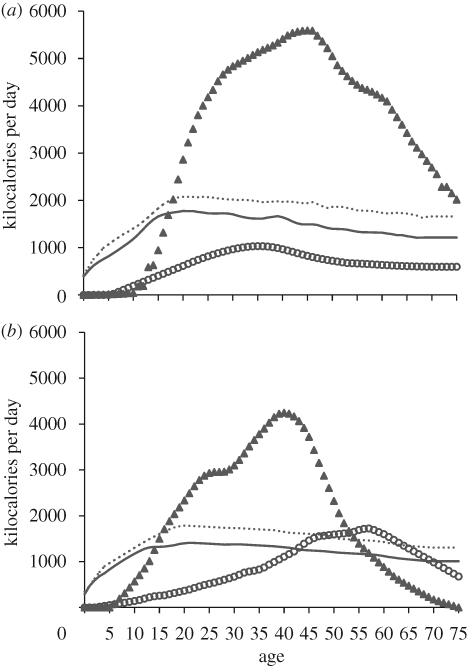
The 1970 pre-contact, forest-dwelling Ache population contained 142 adult females, 167 adult males and 236 dependent children (15 years or less; Hill & Hurtado 1996). Despite high fertility and long offspring dependency, only 69 per cent of women and 54 per cent of men had any surviving dependants during that year (1970). The men and women without dependent offspring in 1970 constituted the pool of potential helpers critical to the success of the active breeders in that year. Some of the helping adults were temporarily non-reproductive, others post-reproductive and others lifelong non-reproducers (two women were sterile, two men were disfigured from Leshmaniasis, two were mildly handicapped, three were homosexual and several had personality traits that reportedly precluded their chance of mating). Ache age–sex energy acquisition and consumption patterns measured from 1980 to 2007 (Kaplan 1994; Kaplan et al. 2000; Walker et al. 2002; Hill & Kintigh 2009; electronic supplementary material) can be combined with the pre-contact population structure and demographic parameters (Hill & Hurtado 1996) in order to estimate which age groups would have been net producers or net consumers of energy in the pre-contact population (figure 2).
Figure 2.
Measured daily energy acquisition and consumption for (a) Ache and (b) Hiwi hunter–gatherers based on observed foraging success, and weight and height of potential consumers. Male consumption, dotted line; female consumption, solid line; male production, triangles; female production, circles.
Given the observed mean rates of fertility, mortality and spousal age difference (Hill & Hurtado 1996), we can estimate the mean expected energy acquisition and consumption for nuclear families that experienced average demographic parameters throughout the lifespan, and mean production and consumption rates (figure 3). This net energy profile reflects the expected dependency ratio (Gurven & Walker 2006), but also includes expected net food production by family members (e.g. Kaplan & Gurven 2005). The analysis indicates that hypothetical forest-dwelling Ache families with the mean number of children and age-specific food acquisition and consumption patterns should experience net energy shortfalls across much of the female reproductive span from the age of about 30 to the late 50s. This expectation, based on population average demographic rates, can be confirmed with data on real family composition.
Figure 3.
Expected food acquisition and consumption for Ache nuclear families when the reproductive female of the family is of specified age. Parental consumption, solid line; total family consumption, dotted line; total family production, circles.
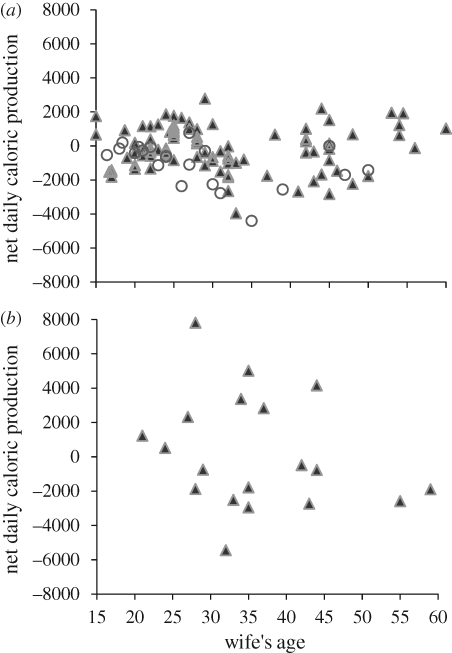
Table 1 shows the actual family composition of the Northern Ache in 1970 and the net summed energy production by all families in specific reproductive and age categories. The data show that real married couples should indeed have experienced energy deficits in middle age, with polygynous families requiring extensive provisioning (figure 4). Younger and older monogamous pairs with lower dependency loads could partially help subsidize the middle-aged breeders; however, unmarried males were by far the most important helpers. Post-reproductive females with no dependants and the spouses of post-reproductive females (about one-third were married) did not contribute food to ease the caloric deficit of middle-aged breeding pairs among the Ache. The population census shows that each breeding pair with dependants had on average 1.27 adult helpers or 0.82 male provisioners (80 men and 44 women without dependants, divided by 98 married women with dependants) to assist with care and food acquisition. There were also six unmarried adult males caring for dependent children with no surviving mother.
Table 1.
Total net energy production by age and reproductive class, Ache population.
| agea |
|||
|---|---|---|---|
| <30 | 30–54 | ≥55 | |
| Ache population census (individuals aged more than 15 years) | |||
| monogamous | 51 | 40 | 8 |
| polygynous | 14 | 6 | 0 |
| single males | 38 | 17 | 3 |
| single females | 5 | 5 | 13 |
| total | 108 | 68 | 24 |
| <30 | 30–55 | >55 | |
| sum net daily caloric production (all nuclear families or individuals) | |||
| monogamous | 7872 | −22 261 | 5907 |
| polygynous | −6629 | −15 109 | |
| single males | 19 389 | 54 613 | 2427 |
| single females | −7505 | −8659 | −9756 |
| maternal orphans | −17 448 | ||
aRefers to wife's age for married couples.
Figure 4.
(a) Estimated mean net daily energy production for 119 monogamously or polygynously married Ache women, their mates and their dependent offspring (15 years or less), based on reported family composition in 1970 (Hill & Hurtado 1996) and age–sex-specific food production patterns measured between 1980 and 2007. Triangles, monogamous pairs; circles, polygynous pairs. (b) Mean net daily energy production for 19 Hiwi monogamously married women, their mates and their dependent offspring from 1985 to 1988 based on actual family composition and measured individual food acquisition rates.
The Ache example is based on actual pre-contact family compositions but food production averages that were measured during the post-contact period. In our second example, family composition and food acquisition were measured simultaneously in a population that had always been dependent on foraging over a 3-year period. From 1985 to 1988, we observed Hiwi hunter–gatherers of the Venezuelan savannah and directly recorded age–sex patterns of food acquisition along with population structure, fertility and mortality (Hurtado & Hill 1987, 1990; Hill et al. 2007). The Mahenemuthu band of Hiwi consisted of 34 men, 28 women and 39 dependent children, who lived by hunting capybara, turtles and fish, and collecting roots and fruits in the gallery forests of the Venezuelan llanos. Approximately 95 per cent of all food energy obtained during the sample period came from hunting and gathering (Hurtado & Hill 1990). The ratio of helpers to breeders was similar to that of the pre-contact Ache. Only 68 per cent of the Hiwi women had any dependent offspring in 1985, and 56 per cent of the adult males had surviving dependent offspring. Thus, 9 women and 15 men acted as non-reproductive helpers for 19 breeding couples (1.26 adult helpers, or 0.79 male provisioners per breeding pair with dependants). Age–sex-specific energy acquisition patterns (figure 2b) are similar in shape to the Ache, but post-reproductive Hiwi women acquired more energy than they consumed, and hunting return rates declined more steeply with age in Hiwi men.
Table 2 shows the family composition of the Hiwi study group and the net summed energy production of all families by reproductive and age category. The data show that married couples experienced an energy deficit in middle age (figure 4b), and this continued into old age because of the steep declines in male hunting returns with age. Younger monogamous pairs with lower dependency loads and middle-aged unmarried males were the most important helpers. Post-reproductive females and their partners (one-third were married) did not contribute food to ease the caloric deficit because females were either married to low-producing men, or the women themselves were very old and unproductive.
Table 2.
Total net energy production by age and reproductive class, Hiwi population.
| age |
|||
|---|---|---|---|
| <30 | 30–54 | ≥55 | |
| Hiwi population census (individuals aged more than 15 years) | |||
| monogamous | 7 | 13 | 4 |
| single males | 3 | 3 | 4 |
| single females | 0 | 2 | 2 |
| total | 10 | 18 | 10 |
| <30 | 30–55 | >55 | |
| sum net daily caloric production (all nuclear families or individuals) | |||
| monogamous | 11 511 | −2934 | −5801 |
| single males | 585 | 2676 | −3433 |
| single females | −1010 | −1517 | |
Anecdotal observations on food transfers to Hiwi families with large food deficits provide additional insight into food sharing in this group. For example, the nuclear family of one 33-year-old pregnant woman consisted of the woman, her husband (who was a poor hunter), a 10-year-old boy from a previous marriage and the 3-year-old daughter of the married couple, with a total net energy deficit of over 5000 calories per day. The 10-year-old boy was fed almost entirely by his genetic father's kin group, and the husband's close kin included four other adult non-reproducers who covered most of the remaining family energy deficit.
5. Discussion
Extra-pair provisioning of breeders and their offspring is extensive in these two foraging societies. The detailed patterns reported here confirm the rough calculation by Kaplan & Gurven (2005) in which they suggested that young males probably subsidize the reproduction of older breeders. We should note that at any point in time some of the provisioners are not single males, but simply other mated males with a low number of needy offspring. Thus, some portion of the provisioning we observed might be termed communal breeding (and perhaps reciprocal altruism), but this depends on the payoffs to the provisioning males. Because of phenotypic variation, polygyny and male-biased adult sex ratios, males are especially likely to fail in direct reproduction and resort to helping as a form of indirect reproduction. Preliminary data analyses (unpublished) suggest that Ache residential camps are statistically biased in favour of coresidence with male kin. For unrelated males, provisioning may represent a tactic to remain as tolerated members of a social group until reproductive opportunities arise. Group augmentation may also be important for males intending to remain resident (Wiessner 2002). Recent modelling suggests that cooperative group augmentation may be favoured through between-group competition in hunter–gatherer societies (e.g. Bowles 2009). Finally, some males probably help because of their belief that they might be the genetic fathers of some offspring through extra-pair copulation (Kaplan & Hill 1985a; Hill & Hurtado 1996, pp. 438–439). But it is important to emphasize that sharing of the major staples is nearly communal among the Ache (Kaplan & Hill 1985b; Gurven et al. 2002); thus, most provisioning is kin-directed only to the extent that residential group composition is kin-biased. Most importantly, recent work has shown that a large portion of the food sharing observed among the Ache and other traditional groups is ‘need-based’. Allen-Arave et al. (2008) measured the ‘need’ of Ache families as total family food production minus total family food consumption, and showed that difference in need alone was associated with 16 per cent of the variance in food flows between families. This need effect was strong among non-kin as well as kin. In addition, the multiple regression intercept implied that over 2000 calories were transferred on average to unrelated families who did not share with the donor family and achieved the same net food production level during the study period (100 h of observation). In other words some sharing takes place with others simply because they are members of the residential group. These observations confirm that the food provisioning is ubiquitous, generally biased in favour of helping families with large dependency loads and not limited to kin assistance. Similar patterns are reported in a half dozen other groups (Allen-Arave et al. 2008).
Although recent discussions have emphasized allomaternal and maternal grandmother roles as the main component of cooperative breeding in humans (e.g. Hawkes et al. 1998; Mace & Sear 2005), our research indicates that males are more important provisioners in the Ache and Hiwi. Hames & Draper (2004) recently suggested that the notable helper effect of female kin in previous studies may be limited to agricultural societies. If the provisioning patterns we report here are more typical of other foraging economies, it suggests that post-reproductive females contribute to increased grandoffspring survival in foragers through activities other than provisioning, or that the grandmother demographic effect may be limited mainly to farmers (e.g. Sear & Mace 2008). Post-reproductive Ache and Hiwi women contributed very little to meeting the food deficits of high-dependency families because older females made up a small portion of the population, and they not were very productive relative to younger males.
Simple life-history calculations also contradict the grandmother hypothesis of provisioning. The probability of an average reproductive-aged woman having a surviving mother to help is simply Lx+T/Lx, where Lx is survival to age x and T is mean generation time (the average age that any child is born). With a mean generation time of approximately 30 years and the average survival observed in several foraging societies (Kaplan et al. 2000), this means that the probability of a surviving maternal grandmother peaks early in marriage at around 0.7 and drops very low by the time the maximum caloric deficit is reached in the late 30s to mid-40s. Indeed, in the Ache population, only 32 per cent of women from age 36 to 45 had a surviving mother. In contrast, 59 per cent of the Ache women of that age lived in nuclear families with negative net caloric production and thus received consistent provisioning assistance from a male other than their husband. The co-residence patterns of hunter–gatherers further mitigate against grandmother provisioning because a residence pattern other than strict matrilocality lowers the probability of grandmother co-residence even further. For example, preliminary analyses from spot checks among the Ache during the pre-contact period shows that only approximately 36 per cent of adult women co-reside with their mothers even if they are alive. Multiplying the probability of mothers' survival (0.32) by the conditional probability of co-residing with her if she is alive (0.36) means that only approximately 10 per cent of middle-aged Ache women co-resided with their own mothers. Grandmothers cannot be the major food providers to their daughters at the ages when daughters will most need help.
Economic evidence also contradicts the grandmother provisioning hypothesis. Contrary to popular views, vertebrate prey makes up the most food energy and virtually all the protein–lipid in most modern foraging societies (Ember 1978; Cordain et al. 2000; Kaplan et al. 2000). In both our study populations, men are the main food providers (84% of total energy for Ache, 79% for Hiwi; Kaplan et al. 2000). In both societies the numerical importance of male helpers is augmented by intentionally male-biased adult sex ratios owing to infanticide and partial neglect of infant females (Hill & Hurtado 1996; Hill et al. 2007). Indeed, we suspect that female-biased infanticide/neglect may be a behavioural adaptation to produce a favourable cooperative breeding sex ratio in ecologies where male productivity is high. This speculation is supported by the positive relationship between overall male economic contribution and male-biased sex ratio observed in hunter–gatherer societies (Hewlett 1991).
Derived features of human life history may have coevolved with a cooperative breeding socio-economic system that transfers resources between age classes (Lee 2008). Hunter–gatherer children are born helpless, and undergo a long period of brain growth and slow body growth, which is associated with near-total resource dependency until full adult body size (Lancaster & Kaplan 2009) is obtained. The adolescent growth spurt must be subsidized by helpers because there is no sudden increase in productive ability of juveniles or their parents during teen years (Kaplan et al. 2000; Walker et al. 2006). Likewise, the high fertility rate in hunter–gatherers is only attainable by provisioning adult females and their offspring. Hunter–gatherer women typically achieve early adult fertility rates of approximately 0.3 offspring per year, whereas great ape fertility typically peaks around 0.2 offspring per year (Hewlett 1991; Kaplan et al. 2000). Moreover, female wild chimpanzees show interbirth intervals after surviving offspring that are nearly twice as long as hunter–gatherer women who are subsidized (approx. 70 months for chimpanzees in table 1 of Emery Thompson et al. 2007 versus 36 months for Ache in Hill & Hurtado 1996, p. 254). Finally, the cessation of fertility (menopause), when life expectancy is approximately 20 more years (Hill & Hurtado 1996, p. 427), is a hunter–gatherer life-history trait that only makes sense in a cooperative breeding context.
Post-reproductive human females continue to live for decades, engaging in indirect reproduction by helping their children and grandchildren to survive and reproduce (Hawkes et al. 1998; Sear & Mace 2008). But demographic gains in offspring fertility or grandoffspring survival do not appear large enough to account for the termination of reproductive function and the evolution of menopause (Hill & Hurtado 1991, 1996; Rogers 1994; Lee 2008). One interesting possibility is that reproductive senescence is favoured because of the investment patterns of helpers. Perhaps investors who will be related to newly produced offspring prefer to provision younger females of high intrinsic fertility rather than older females whose reproductive machinery has declined in efficiency owing to senescence. As a woman loses kin-based food subsidies, her reproductive output may drop low enough to favour termination of continued investment in her own reproductive function; Hill & Hurtado (1996, p. 32) show that a fertility drop to one-sixth of peak fertility would favour menopause in Ache women. One intriguing analogy comes from observations on the gamergates (female workers who develop ovarian function and reproduce upon death of the queen) of Hypergnathos saltator. In that species, gamergate fertility declines with ageing and is advertised through exoskeletal hydrocarbons, leading to a reduction in investment by helpers and cessation of reproduction by older gameragates, who then become post-reproductive helpers until the end of their lifespan (Hölldobler & Wilson 2008, pp. 336–355).
Whatever the ultimate evolutionary causes, current data suggest that the observed hunter–gatherer life history is not possible without alloparental food subsidies. This pattern may partially explain why humans have recently evolved extensive non-kin cooperation, ‘other-regarding preferences’ demonstrated in economic experiments, and a suite of prosocial behaviours and emotions unlike the other great apes (Hrdy 2009; Hill et al. in press). We have shown in two well-studied foraging societies that food subsidies are provided mainly by adult males. It remains to be determined whether this pattern is typical during much of our evolutionary history.
References
- Allen-Arave W., Gurven M., Hill K.2008Reciprocal altruism, rather than kin selection, maintains nepotistic food transfers on an Ache reservation. Evol. Hum. Behav. 29, 305–318 (doi:10.1016/j.evolhumbehav.2008.03.002) [Google Scholar]
- Bailey R. C.1991The behavioral ecology of Efe pygmy men in the Ituri Forest, Zaire. Anthropological Papers no. 86. Ann Arbor, MI: Museum of Anthropology, University of Michigan [Google Scholar]
- Bowles S.2009Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324, 1293–1298 (doi:10.1126/science.1168112) [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H.2006Cooperative breeding in mammals. In Cooperation in primates and humans (eds Kappeler P. M., van Schaik C. P.), pp. 173–190 Berlin, Germany: Springer Verlag [Google Scholar]
- Cordain L., Miller J. B., Eaton S. B., Mann N., Holt S. H., Speth J. D.2000Plant–animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 71, 682–692 [DOI] [PubMed] [Google Scholar]
- Ellison P., Gray P.2009Endocrinology of social relationships Cambridge, MA: Harvard University Press [Google Scholar]
- Ember C.1978Myths about hunter-gatherers. Ethnology 17, 439–448 (doi:10.2307/3773193) [Google Scholar]
- Emery Thompson M., et al. 2007Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr. Biol. 17, 2150–2156 (doi:10.1016/j.cub.2007.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S.1995An evolutionary theory of the family. Proc. Natl Acad. Sci. USA 92, 8092–8099 (doi:10.1073/pnas.92.18.8092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M.2004To give and to give not: the behavioral ecology of human food transfers. Behav. Brain Sci. 27, 543–583 [Google Scholar]
- Gurven M., Hill K.2009Hunting as subsistence and mating effort? A re-evaluation of ‘Man the Hunter’, the sexual division of labor and the evolution of the nuclear family. Curr. Anthropol. 5, 51–74 (doi:10.1017/S0140525X04000123) [DOI] [PubMed] [Google Scholar]
- Gurven M., Walker R.2006Energetic demand of multiple dependents and the evolution of slow human growth. Proc. R. Soc. B 273, 835–841 (doi:10.1098/rspb.2005.3380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M., Hill K., Hurtado A. M., Kaplan H., Lyles B.2000aFood transfers among Hiwi foragers of Venezuela. J. Hum. Ecol. 28, 171–218 (doi:10.1023/A:1007067919982) [Google Scholar]
- Gurven M., Allen-Arave W., Hill K., Hurtado A. M.2000b‘It's a wonderful life’: signaling generosity among the Ache of Paraguay. Evol. Hum. Behav. 21, 263–282 (doi:10.1016/S1090-5138(00)00032-5) [DOI] [PubMed] [Google Scholar]
- Gurven M., Hill K., Kaplan H.2002From forest to reservation: transitions in food sharing behavior among the Ache of Paraguay. J. Anthropol. Res. 58, 91–118 [Google Scholar]
- Hames R., Draper P.2004Women's work, childcare, and helpers-at-the-nest in a hunter-gatherer society. Hum. Nat. 15, 319–341 (doi:10.1007/s12110-004-1012-x) [DOI] [PubMed] [Google Scholar]
- Hawkes K., O'Connell J. F., Blurton Jones N. G.1989Hardworking Hadza grandmothers. In Comparative socioecology: the behavioural ecology of humans and other mammals (eds Standen V., Foley R. A.), pp. 341–366 London, UK: Basil Blackwell [Google Scholar]
- Hawkes K., O'Connell J. F., Blurton Jones N. G., Charnov E. L., Alvarez H.1998Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA 95, 1336–1339 (doi:10.1073/pnas.95.3.1336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K., O'Connell J. F., Blurton Jones N. G.2001Hunting and nuclear families. Curr. Anthropol. 42, 681–709 (doi:10.1086/322559) [Google Scholar]
- Headland T.1986. Why foragers do not become farmers: a historical study of a changing ecosystem and its effect on a Negrito hunter-gatherer group in the Philippines. PhD dissertation, University of Hawaii Ann Arbor, MI: University Microfilms International [Google Scholar]
- Hermann E., Call J., Hernandez-Lloreda M. V., Hare B., Tomasello M.2007Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366 (doi:10.1126/science.1146282) [DOI] [PubMed] [Google Scholar]
- Hewlett B.1991Demography and childcare in preindustrial societies. J. Anthropol. Res. 47, 1–37 [DOI] [PubMed] [Google Scholar]
- Hewlett B., Lamb M.2005Hunter-gatherer childhoods: evolutionary, cultural, and developmental perspectives. New York, NY: Aldine Transaction [Google Scholar]
- Hill K.2002Altruistic cooperation during foraging by the Ache, and the evolved human predisposition to cooperate. Hum. Nat. 13, 105–128 (doi:10.1007/s12110-002-1016-3) [DOI] [PubMed] [Google Scholar]
- Hill K., Hurtado A. M.1991The evolution of reproductive senescence and menopause in human females. Hum. Nat. 2, 315–350 (doi:10.1007/BF02692196) [DOI] [PubMed] [Google Scholar]
- Hill K., Hurtado A. M.1996Ache life history: the ecology and demography of a foraging people. New York, NY: Aldine [Google Scholar]
- Hill K., Kintigh K.2009Can anthropologists distinguish good from poor hunters: implications for hunting hypotheses, sharing conventions, and cultural transmission. Curr. Anthropol. 50, 369–377 (doi:10.1086/597981) [Google Scholar]
- Hill K., Kaplan H., Hawkes K., Hurtado A. M.1985Men's time allocation to subsistence work among the Ache of eastern Paraguay. Hum. Ecol. 13, 29–47 (doi:10.1007/BF01531087) [Google Scholar]
- Hill K., Hurtado A. M., Walker R.2007High adult mortality among Hiwi hunter-gatherers: implications for human evolution. J. Hum. Evol. 52, 443–454 (doi:10.1016/j.jhevol.2006.11.003) [DOI] [PubMed] [Google Scholar]
- Hill K., Barton M., Hurtado A. M.In press The origins of human uniqueness: the evolution of characters underlying behavioral modernity. Evol. Anthropol [Google Scholar]
- Hölldobler B., Wilson E. O.2008The super-organism: the beauty, elegance, and strangeness of insect societies. New York, NY: W. W. Norton [Google Scholar]
- Hrdy S. B.1999Mother nature: a history of mothers, infants, and natural selection New York, NY: Pantheon; [DOI] [PubMed] [Google Scholar]
- Hrdy S. B.2005Comes the child before the man: how cooperative breeding and prolonged postweaning dependence shaped human potentials. In Hunter-gatherer childhoods: evolutionary, developmental, and cultural perspectives (eds Hewlett B., Lamb M.), pp. 65–91 New York, NY: Aldine [Google Scholar]
- Hrdy S. B.2009Mother and others: the evolutionary origins of mutual understanding Cambridge, MA: Belknap, Harvard University Press [Google Scholar]
- Hurtado A. M., Hill K.1987Early dry season subsistence ecology of the Cuiva foragers of Venezuela. Hum. Ecol. 15, 163–187 (doi:10.1007/BF00888379) [Google Scholar]
- Hurtado A. M., Hill K.1990Seasonality in a foraging society: variation in diet, work effort, fertility, and the sexual division of labor among the Hiwi of Venezuela. J. Anthropol. Res. 46, 293–346 [Google Scholar]
- Hurtado A. M., Hill K.1992Paternal effect on offspring survivorship among Ache and Hiwi hunter-gatherers: implications for modeling pair-bond stability. In The father–child relationship (ed. Hewlett B.), pp. 31–56 Chicago, IL: Aldine [Google Scholar]
- Hurtado A., Hawkes K., Hill K., Kaplan H.1985Female subsistence strategies among Ache hunter-gatherers of eastern Paraguay. Hum. Ecol. 13, 1–28 (doi:10.1007/BF01531086) [Google Scholar]
- Hurtado A., Hill K., Kaplan H., Hurtado I.1992Tradeoffs between female food acquisition and child care among Hiwi and Ache foragers. Hum. Nat. 3, 185–216 (doi:10.1007/BF02692239) [DOI] [PubMed] [Google Scholar]
- Kaplan H.1994Evolutionary and wealth flows theories of fertility: empirical tests and new models. Popul. Dev. Rev. 20, 753–791 (doi:10.2307/2137661) [Google Scholar]
- Kaplan H., Hill K.1985aHunting and reproductive success among male Ache foragers: preliminary results. Curr. Anthropol. 26, 131–133 (doi:10.1086/203235) [Google Scholar]
- Kaplan H., Hill K.1985bFood sharing among Ache foragers: tests of explanatory hypotheses. Curr. Anthropol. 26, 223–245 (doi:10.1086/203251) [Google Scholar]
- Kaplan H., Gurven M.2005The natural history of human food sharing and cooperation: a review and a new multi-individual approach to the negotiation of norms. In Moral sentiments and material interests: the foundations of cooperation in economic life (eds Gintis H., Bowles S., Boyd R., Fehr E.), pp. 75–113 Cambridge, MA: MIT Press [Google Scholar]
- Kaplan H., Hill K., Hawkes K., Hurtado A. M.1984Food sharing among the Ache hunter-gatherers of eastern Paraguay. Curr. Anthropol. 25, 113–115 (doi:10.1086/203089) [Google Scholar]
- Kaplan H., Hill K., Lancaster J., Hurtado A. M.2000A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [Google Scholar]
- Koenig W., Dickinson J.2004Ecology and evolution of cooperative breeding in birds. Cambridge, UK: Cambridge University Press [Google Scholar]
- Lancaster J., Kaplan H.2009The endocrinology of the adaptive human complex. In Endocrinology of social relationships (eds Ellison P., Gray P.), pp. 95–120 Cambridge, MA: Harvard University Press [Google Scholar]
- Lee R.2008Sociality, selection, and survival: simulated evolution of mortality with intergenerational transfers and food sharing. Proc. Natl Acad. Sci. USA 105, 7124–7128 (doi:10.1073/pnas.0710234105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace R., Sear R.2005Are humans cooperative breeders? In Grandmotherhood: the evolutionary significance of the second half of female life (eds Voland E., Chasiotis A., Schiefenhövel W.), pp. 143–159 New Brunswick, NJ: Rutgers University Press [Google Scholar]
- McMillan G.2001. Ache residential grouping and social foraging. PhD dissertation, University of New Mexico [Google Scholar]
- Rogers A. R.1994Evolution of time preference by natural selection. Am. Econ. Rev. 84, 460–481 [Google Scholar]
- Sear R., Mace R.2008Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18 (doi:10.1016/j.evolhumbehav.2007.10.001) [Google Scholar]
- Sugiyama L.2004aIllness, injury, and disability among Shiwiar forager-horticulturalists: implications of health-risk buffering for the evolution of human life history. Am. J. Phys. Anthropol. 123, 371–389 (doi:10.1002/ajpa.10325) [DOI] [PubMed] [Google Scholar]
- Sugiyama L. S.2004bPatterns of Shiwiar health insults indicate that provisioning during health crises reduces juvenile mortality. In Socioeconomic aspects of human behavioral ecology: research in economic anthropology, vol. 23 (ed. Alvard M.), pp. 377–400 New York, NY: Elsevier [Google Scholar]
- Sugiyama L. S., Chacon R.2000Effects of illness and injury on foraging among the Yora and Shiwiar: pathology risk as adaptive problem. In Human behavior and adaptation: an anthropological perspective (eds Cronk L., Chagnon N. A., Irons W.), pp. 371–396 New York, NY: Aldine [Google Scholar]
- Walker R. S., Hill K.2003Modeling growth and senescence in physical performance among the Ache of Eastern Paraguay. Am. J. Hum. Biol. 15, 196–208 (doi:10.1002/ajhb.10135) [DOI] [PubMed] [Google Scholar]
- Walker R., Hill K., Kaplan H., McMillan G.2002Age-dependency in skill, strength and hunting ability among the Ache of eastern Paraguay. J. Hum. Evol. 42, 639–657 (doi:10.1006/jhev.2001.0541) [DOI] [PubMed] [Google Scholar]
- Walker R., et al. 2006Growth rates and life histories in twenty-two small-scale societies. Am. J. Hum. Biol. 18, 295–311 (doi:10.1002/ajhb.20510) [DOI] [PubMed] [Google Scholar]
- Wiessner P.2002Hunting, healing, and hxaro exchange: a long-term perspective on !Kung (Ju/’hoansi) large-game hunting. Evol. Hum. Behav. 23, 407–436 (doi:10.1016/S1090-5138(02)00096-X) [Google Scholar]
- Wiessner P.2009Parent–offspring conflict in marriage: implications for social evolution and material culture among the Ju/’hoansi Bushmen. In Pattern and process in cultural evolution (ed. Shennan S.). Berkeley, MA: University of California Press [Google Scholar]