Abstract
Most species of social insects have singly mated queens, but in some species each queen mates with numerous males to create a colony whose workers belong to multiple patrilines. This colony genetic structure creates a potential for intracolonial nepotism. One context with great potential for such nepotism arises in species, like honey bees, whose colonies reproduce by fissioning. During fissioning, workers might nepotistically choose between serving a young (sister) queen or the old (mother) queen, preferring the former if she is a full-sister but the latter if the young queen is only a half-sister. We examined three honeybee colonies that swarmed, and performed paternity analyses on the young (immature) queens and samples of workers who either stayed with the young queens in the nest or left with the mother queen in the swarm. For each colony, we checked whether patrilines represented by immature queens had higher proportions of staying workers than patrilines not represented by immature queens. We found no evidence of this. The absence of intracolonial nepotism during colony fissioning could be because the workers cannot discriminate between full-sister and half-sister queens when they are immature, or because the costs of behaving nepotistically outweigh the benefits.
Keywords: Apis mellifera, genetic relatedness, honeybee, kin recognition, intracolonial nepotism, queen rearing, swarming
1. Introduction
Although queens in most social insect species do not mate with multiple males (Strassmann 2001), polyandry is prominent in certain taxa including yellow jacket wasps (Vespula, Ross 1986), leaf-cutter ants (Atta, Fjerdingstad et al. 1998; Acromyrmex, Boomsma et al. 1999), army ants (Eciton, Denny et al. 2004; Dorylus, Kronauer et al. 2004), harvester ants (Pogonomyrmex, Rheindt et al. 2004; Wiernasz et al. 2004; Pol et al. 2008), desert ants (Cataglyphis, Timmermans et al. 2008) and honey bees (Apis, Estoup et al. 1994; Tarpy & Nielsen 2002). One consequence of this polyandry is that the females in a colony (queens and workers) are not all full-sisters. Instead, they constitute several patrilineal groups, with females in the same patriline related as full-sisters (r = 0.75) and those in different patrilines related as half-sisters (r = 0.25).
The genetic structure of multi-patriline colonies creates a potential for intracolonial nepotism in various contexts, including food-sharing and brood-rearing, though there is no convincing evidence that workers behave nepotistically in these two particular contexts (Breed et al. 1994; Tarpy et al. 2004; Châline et al. 2005). A third context with great potential for intracolonial nepotism arises in species, such as honey bees and army ants, whose colonies reproduce by fissioning (Wilson 1971). During this process of colony multiplication, the workers rear several young queens, all of whom are the workers' sisters. Eventually, once the original colony divides itself, one of these young (sister) queens will head one of the derivative colonies and typically the old (mother) queen heads the other derivative colony. Thus, the workers in a colony that is fissioning might choose between serving a young queen or the old queen. And in making this choice, a worker might act nepotistically, preferring to serve a young queen if she is likely to be a full-sister (r = 0.75) or preferring to serve the old queen (r = 0.50) if all the young queens are half-sisters (r = 0.25).
To date, two studies with honey bees have investigated whether workers nepotistically choose between a young (sister) queen and the old (mother) queen during colony fissioning, but neither study provides a definitive answer. Getz et al. (1982) established colonies, each of which was headed by a queen who was homozygous for a recessive body colour marker (cordovan) and was instrumentally inseminated with semen from one wild-type drone and one cordovan drone. Thus the patriline membership of each worker was indicated by her body colour. From each of the two colonies that fissioned (‘swarmed’), samples of workers were collected from the swarm and the nest, and the young queens were collected from the nest (in honey bees, the old queen leaves in the swarm). All the young queens were cordovan, and yet in both colonies the proportion of cordovan workers was higher in the group that left with the old queen than in the group that stayed with the young queen. These results contradict the prediction that workers should prefer to stay with the young queen if she is their full-sister. However, the use of the cordovan marker gene—which may be linked to genes conferring a propensity for leaving in a swarm (Breed et al. 1994)—and the use of colonies with only two patrilines—honeybee colonies typically contain ten or more patrilines (Tarpy & Nielsen 2002)—make it difficult to draw firm conclusions from this study regarding intracolonial nepotism during colony fissioning.
The second study was done using colonies without the cordovan marker and with a natural number of patrilines (Kryger & Moritz 1997). This study looked at how the workers who stayed behind in the nest behaved when the remnant colony was strong enough to fission again, casting a second swarm (‘afterswarm’) that would be headed by one of the young queens. The authors predicted that workers are more likely to leave in the afterswarm than to stay in the nest if the afterswarm is headed by a full-sister queen rather than a half-sister queen. To test this prediction, they studied two colonies that produced both a prime swarm (containing the old, mother queen) and an afterswarm (containing a young, sister queen). Workers were sampled from both the prime swarm and the afterswarm. In both colonies, there was no difference in patriline composition between the prime swarm and the afterswarm, which suggests that in both colonies the workers in the patriline of the young queen heading the afterswarm had not increased their likelihood of leaving the nest between the prime swarm context and the afterswarm context. Unfortunately, the authors did not determine the patrilines of the queens in the afterswarm and in the nest, and they did not sample the workers who stayed behind in the nest, hence they were unable to make a full test of their hypothesis. Additionally, once the authors sampled the workers in the prime swarm, they returned the prime swarm to its hive (minus the roughly 200 workers collected from it) to encourage the production of an afterswarm. One wonders whether the worker-assortment patterns were the same for the prime swarm and the afterswarm because the authors returned the prime swarms to their nests, and the prime swarm bees left again in the afterswarms.
Because the evidence about intracolonial nepotism during colony fissioning in honey bees remains ambiguous, we examined three honeybee colonies that were headed by naturally mated queens and that were allowed to swarm naturally, to test the hypothesis that a worker bee is more likely to stay in the nest (with a young, sister queen) than to leave in the swarm (with the old, mother queen) if at least one of the young queens being reared in the nest is her full-sister. If this hypothesis is true, then patrilines that are represented by immature queens will have higher proportions of staying workers than will patrilines that are not represented by immature queens. The null hypothesis is that worker bees do not decide to stay or leave based on their genetic relatedness to the young queens being reared in the nest. If so, then the two groups of patrilines—those that are and are not represented by immature queens—should not differ in the proportion of workers who stay in the remnant colony.
2. Material and methods
(a). Study site and bees
We conducted our study at the Liddell Field Station of Cornell University in Ithaca, New York (42°26′ N, 76°30′ W). Three medium-sized honeybee colonies were used, all headed by naturally mated New World Carniolan queens (Apis mellifera carnica; Strachan Apiaries, Yuba City, CA, USA). On 13 May 2008, each colony was installed with its original queen in a three-frame observation hive (described by Seeley 1995). The three frames chosen for each colony were covered with adult bees (approx. 6000 workers), and were roughly half full of brood and half full of pollen and honey to simulate the conditions present in a natural colony that is preparing to swarm (Winston 1987). The observation hives were set up in a light-proof room to simulate the darkness inside natural honeybee nests, leaving only the hive entrance as a source of light. Two weeks after the colonies were established, the bees started to produce queen cells in preparation for swarming in late May to early June, the time of year when most swarms are issued in the Ithaca area (Fell et al. 1977).
Before placing the glass walls on each observation hive, we installed an electret condenser microphone (Radio Shack Model 33-3013, 70–16 000 Hz frequency response) at the centre of the bottom frame so we could hear worker piping, the mechanical–acoustic signal produced by a few dozen bees in colonies that are preparing to swarm (Rangel & Seeley 2008). We checked each observation hive daily, listening every 30 min for piping signals from10.00 to 16.00. Once a colony's piping rate was higher than three signals in 30 s, we monitored that colony closely until its swarm departed.
(b). Collection of samples
Once a swarm departed its nest, we waited until the swarm bees had settled on a tree branch and no more bees were exiting the hive to join the swarm, whereupon we collected workers from both the swarm and the remnant colony so that the workers' patriline memberships could be determined through genotyping. At the swarm, workers were collected randomly by gently carving out a side of the swarm from bottom to top so that the workers fell into a vial containing ethanol. At the remnant colony, we opened the glass walls of the observation hive and collected workers from both sides of the frames of comb at random, placing the bees in vials with ethanol. At least 120 workers were genotyped from both the swarm and the remnant colony (range = 120–131 workers per group across colonies). All immature queens were placed in individual vials with ethanol, and the developmental stage of each one was noted (i.e. larva, pupa or adult).
(c). DNA extraction and microsatellite analysis
We used polymorphic DNA microsatellite markers to determine the patriline composition of the swarm and remnant-colony bees. Paternity was determined by analyzing seven microsatellite loci (Ap033, Ap068, A079, A113, Ap226, Ap256 and Ap289), which are highly variable and sufficient to assign a worker to a patriline in colonies with ten or more patrilines (Solignac et al. 2003; Schlüns et al. 2005). For each marker, the forward primer was labelled with one of four fluorescent phosphoramidites so that the polymerase chain reaction (PCR) products could be separated by size and fluorescence. We extracted DNA from the hind legs and thoraces of workers and from the whole bodies of immature queens with a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). PCR reactions were performed in a thermal cycler (Thermo Electron Corporation, Milford, MA) using a 10 µl mixture that contained 1 µl of DNA in solution, 5 µl of pre-mixed PCR reagents from a multiplexing kit (Qiagen, Valencia, CA), 1.2 µl of water, and 0.2 µl of each primer (for a total primer concentration of 2.0 M). The thermocycler was programmed at 95°C for 15 min, 94°C for 50 s, 57°C for 45 s, and 72°C for 90 s. The annealing temperature was dropped one degree per cycle for the first seven cycles, then the reactions were cycled 28 more times at 94°C for 50 s, 50°C for 45 s, and 72°C for 90 s. The PCR products were visualized with a 3730×l DNA analyzer (Applied BioSystems, Foster City, CA) at the Cornell University Core Laboratories Center using GeneMapper, v. 3.0 (Applied BioSystems, Foster City, CA).
For each colony, the queen's genotype for each locus was inferred by comparing the genotypes of immature queens and workers. Genotyped workers were assumed to belong to the same patriline if their profile of drone-derived alleles was the same.
(d). Statistical analysis
For each patriline represented in a colony, we calculated the proportion of workers who stayed in the nest (‘stayers’) by dividing the number of stayers in that patriline by the total number of workers (stayers and ‘leavers’) in the patriline. For each colony, we tested whether the proportion of stayers was higher in patrilines with immature queens than in those without immature queens. We used a one-sided t-test because our data met the assumption of normality for parametric tests. Also, for the one colony that contained adult virgin queens, we used a χ2-test to determine whether having an adult full-sister queen present in the nest increased a worker's tendency to stay. For this test, we compared the frequency of staying workers between patrilines with and without adult full-sister queens. Data are reported as mean proportions ± s.d. We set the level of significance of all tests at α = 0.05.
3. Results
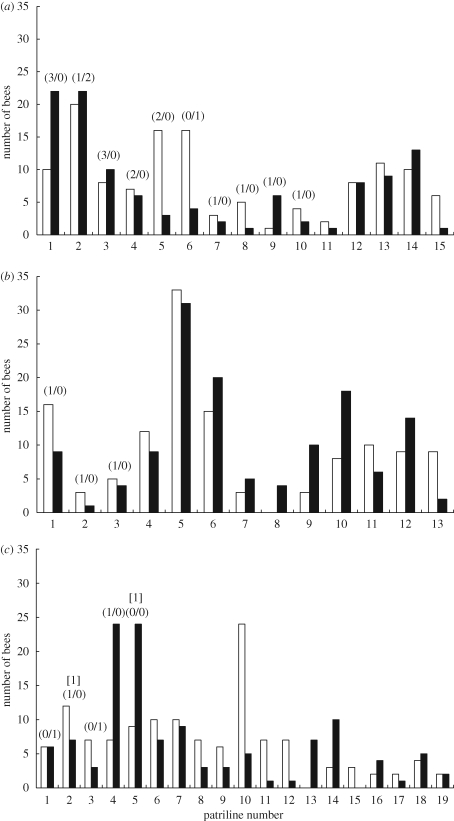
Colony 1 contained 15 patrilines, 10 of which were represented by immature queens (figure 1a). Colony 2 contained 13 patrilines, three of which were represented by immature queens (figure 1b). Colony 3 contained 19 patrilines, six of which were represented by immature queens (figure 1c). When we determined, for each patriline in each colony, the proportion of the sampled bees that stayed in the nest, we did not find higher proportions of stayers in patrilines that did, relative to those that did not, have immature queens developing in the nest (table 1).
Figure 1.
Comparison between the number of bees who stayed in the nest (black bars) and the number of bees who left in the swarm (white bars) for each patriline. The number of immature queens belonging to a patriline is noted above the bars in parentheses as: (number of larvae/number of pupae). Numbers inside brackets above the parentheses denote queens that had emerged as adults and were roaming the nest after the swarm departed.
Table 1.
Summary of the proportions of workers who stayed in the nest for both the patrilines that were represented by immature queens and the patrilines that were not represented by immature queens. Proportions are given as mean ± s.d.
| colony | number of immature queens | patrilines with immature queens |
patrilines without immature queens |
t | d.f. | p-value | ||
|---|---|---|---|---|---|---|---|---|
| number of patrilines | proportion of stayers | number of patrilines | proportion of stayers | |||||
| 1 | 18 | 10 | 0.46 ± 0.21 | 5 | 0.40 ± 0.17 | 0.61 | 13 | 0.28 |
| 2 | 3 | 3 | 0.35 ± 0.10 | 10 | 0.62 ± 0.23 | −1.63 | 11 | 0.87 |
| 3 | 6 | 5 | 0.57 ± 0.17 | 14 | 0.43 ± 0.25 | 1.16 | 17 | 0.13 |
We found two newly emerged virgin queens roaming inside the nest of colony 3 after the swarm had issued; they belonged to patrilines 2 and 5 (figure 1c). The worker bees in patriline 2 had fewer stayers than leavers (seven versus 12), while those in patriline 5 had more stayers than leavers (24 versus nine). In this colony, the workers who had an adult full-sister queen in the nest prior to swarming did not show a higher tendency to stay compared to workers who had an immature full-sister queen or no full-sister queen inside the nest (χ2 = 2.24, d.f. = 1, p = 0.1341).
4. Discussion
Our results show that honeybee workers are not more likely to stay in the nest rather than to leave in the swarm if at least one full-sister is being reared as a young queen prior to swarming. This finding indicates that workers do not show intracolonial nepotism during colony fissioning.
Our results are consistent with those of two previous studies that reported preliminary results suggesting an absence of intracolonial nepotism in honey bees during colony fissioning. Kryger & Moritz (1997) found no significant difference in patriline compositions between the prime swarm and the afterswarm in two colonies. Their results are similar to our results in that the workers in their two colonies showed no sign of deciding to leave in the swarm versus stay in the nest based on their genetic relatedness to the queen that they will serve. However, the study by Kryger and Moritz differs from ours in several aspects. Most importantly, after each colony produced the afterswarm, Kryger and Moritz did not sample any adult workers from the remnant colony to determine, for each patriline, the proportion of workers who stayed in the nest versus the proportion that left in the afterswarm. Thus, the proportion of adult workers who stayed in the nest was not compared between patrilines with and without full-sister queens in the nest. There is also the complication that the authors returned the prime swarm from each colony back to its hive to encourage the production of an afterswarm, and this manipulation by itself could have caused the similarity in patriline distributions between prime swarms and afterswarms.
The other study that attempted to look for nepotism during colony fissioning in honey bees was performed with two colonies headed by cordovan queens that were artificially inseminated with semen from one wild-type and one cordovan drone (Getz et al. 1982). After each of the two colonies swarmed, there was actually a higher proportion of cordovan workers in each swarm than in each remnant colony, even though all the virgin queens found in the two remnant colonies were cordovan. Evidently, workers did not show intracolonial nepotism. The results of this early study support the current view that cordovan workers may have a higher propensity to swarm relative to wild-type workers, and that colonies headed by queens artificially mated with a low number of drones are unnatural and their use may yield unrealistic results (Breed et al. 1994).
The present study is, to our knowledge, the first to test for intracolonial nepotism at the fissioning stage of the swarming process in undisturbed colonies headed by naturally mated queens. We are the first to identify the patrilines of adult workers in both the swarm and the remnant colony, and of all immature queens present in the nest. Also, we avoided using special genetic lines or returning swarms to colonies to encourage further swarming. Our negative results regarding intracolonial nepotism by workers during colony fissioning are similar to those from most studies of intracolonial nepotism by workers during queen rearing, which report no worker tendency to favour full-sister queens at the egg, larval or adult stage (see Breed et al. 1994 for review).
The question remains whether workers are unable to discriminate among full-sister and half-sister queens, or whether they have not been selected to make this discrimination because the costs of discrimination outweigh the gains, or both.
In theory, honeybee workers are predicted to use self-referent phenotype matching based on genetically based odour cues to discriminate between full-sister and half-sister immature queens at the time of swarming (Visscher 1986). It has been shown that, at least under certain experimental conditions, workers can discriminate full-sister from half-sister workers (Getz 1991), and a few laboratory studies have shown that cuticular hydrocarbons that provide odour cues may indicate a queen's patriline membership and may be used by workers to discriminate full-sister from half-sister queens (Moritz & Crewe 1988; Getz & Page 1991; Page et al. 1991; Arnold et al. 1995). However, in colonies kept in natural settings there seems to be a weak discrimination between full-sister and half-sister queens, perhaps because of a low allelic diversity of genetic odour cues used in recognition (Ratnieks 1991). It is also possible that the weak discrimination between full-sister and half-sister queens reflects selection for the muting or scrambling of kin recognition cues by the queens to prevent half-sister workers (the vast majority) from withholding resources (Reeve 1998).
Workers may not discriminate among full-sister and half-sister queens before swarming for other reasons. Ratnieks & Reeve (1991) proposed that high colony-level costs of kin discrimination (i.e. reduction in the colony's total production of queens) may outweigh the benefit that a worker gains from her selfish interest to help support a full-sister queen. Another possibility is that extreme polyandry results in so many patrilines in a colony that a worker's probability of encountering and detecting a full-sister queen is low. This last assertion is especially probable when one considers the highly congested environment inside a colony that is preparing to swarm.
Our finding of a lack of intracolonial nepotism during colony fissioning in honey bees is consistent with the negative results reported in most studies on intracolonial nepotism in species of social insects whose colonies are composed of multiple patrilines or matrilines. Although little is known about whether individuals behave nepotistically during colony fissioning in other polyandrous species (e.g. army ants), several studies of polygynous ant and wasp species have failed to detect nepotism by workers toward the brood of particular queens (see Carlin et al. 1993; DeHeer & Ross 1997; Holzer et al. 2006 for ants; and Queller et al. 1990; Solis et al. 1998; Strassmann et al. 2000, for wasps). What are especially needed now are studies of intracolonial nepotism during colony fissioning in other species of social insects with polyandrous queens, most notably the army ants.
Acknowledgements
We are indebted to Madeline Girard for helping set up the observation hives, collect worker samples and extract DNA. We thank Elizabeth Hunter and Steve Bogdanowicz for help with DNA extraction and genotyping. We are grateful to the editors and three anonymous reviewers for insightful comments on the manuscript. Funding was provided to J.R. by a U.S. National Science Foundation Graduate Research Fellowship (award no. DGE 0707428), a State University of New York Graduate Underrepresented Minority Fellowship, and the Eastern Apicultural Society Annual Research Grant for 2008. The study was also funded by a grant to T.D.S. and H.R.M. from the Cooperative State Research, Education and Extension Service National Research Initiative (Grant no. 2007-35 302-18174.).
References
- Arnold G., Quenet B., Cornuet J. M., Masson C., DeShepper B., Estoup A., Gasqui P.1995Kin recognition in honey bees. Nature 379, 498 (doi:10.1038/379498a0) [Google Scholar]
- Boomsma J. J., Fjerdingstad E. J., Frydenberg J.1999Multiple paternity, relatedness, and genetic diversity in Acromyrmex leaf-cutter ants. Proc. R. Soc. Lond. B 266, 249–254 (doi:10.1098/rspb.1999.0629) [Google Scholar]
- Breed M. D., Welch C. K., Cruz R.1994Kin discrimination within honey bee (Apis mellifera) colonies: an analysis of the evidence. Behav. Proc. 33, 25–40 (doi:10.1016/0376-6357(94)90058-2) [DOI] [PubMed] [Google Scholar]
- Carlin N. F., Reeve H. K., Cover S. P.1993Kin discrimination and division of labour among matrilines in the polygynous carpenter ant, Camponotus planatus. In Queen number and sociality in insects (ed. Keller L.), pp. 362–401 Oxford, UK: Oxford University Press [Google Scholar]
- Châline N., Martin S. J., Ratnieks F. L. W.2005Absence of nepotism toward imprisoned young queens during swarming in the honey bee. Behav. Ecol. 16, 403–409 (doi:10.1093/beheco/ari003) [Google Scholar]
- DeHeer C. J., Ross K. G.1997Lack of detectable nepotism in multiple-queen colonies of the fire ant Solenopsis invicta (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 40, 27–33 (doi:10.1007/s002650050312) [Google Scholar]
- Denny A. J., Franks N. R., Powell S., Edwards K. J.2004Exceptionally high levels of multiple mating in an army ant. Naturwissenschaften 91, 396–399 (doi:10.1007/s00114-004-0546-4) [DOI] [PubMed] [Google Scholar]
- Estoup A., Solignac M., Cornuet M.1994Precise assessment of the number of patrilines and of genetic relatedness in honeybee colonies. Proc. R. Soc. Lond. B 258, 1–7 (doi:10.1098/rspb.1994.0133) [Google Scholar]
- Fell R. D., Ambrose J. T., Burgett M., De Jong D., Morse R. A., Seeley T. D.1977The seasonal cycle of swarming in honeybees. J. Apic. Res. 16, 170–173 [Google Scholar]
- Fjerdingstad E. J., Boomsma J. J., Thorén P.1998Multiple paternity in the leafcutter ant Atta colombica—a microsatellite DNA study. Heredity 80, 118–126 (doi:10.1038/sj.hdy.6882470) [Google Scholar]
- Getz W. M.1991The honey bee as a model kin recognition system. In Kin recognition (ed. Hepper P. G.), pp. 358–412 Cambridge, UK: Cambridge University Press [Google Scholar]
- Getz W. M., Page R. E.1991Chemosensory kin communication systems and kin recognition in honey bees. Ethology 87, 298–315 [Google Scholar]
- Getz W. M., Brückner D., Parisian T. R.1982Kin structure and the swarming behavior of the honey bee Apis mellifera. Behav. Ecol. Sociobiol 10, 265–270 (doi:10.1007/BF00302815) [Google Scholar]
- Holzer B., Kümmerli R., Keller L., Chapuisat M.2006Sham nepotism as a result of intrinsic differences in brood viability in ants. Proc. R. Soc. B 273, 2049–2052 (doi:10.1098/rspb.2006.3553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronauer D. J. C., Schöning C., Pedersen J. S., Boomsma J. J., Gadau J.2004Extreme queen-mating frequency and colony fission in African army ants. Mol. Ecol. 13, 2381–2388 (doi:10.1111/j.1365-294X.2004.02262.x) [DOI] [PubMed] [Google Scholar]
- Kryger P., Moritz R. F. A.1997Lack of kin recognition in swarming honeybees (Apis mellifera). Behav. Ecol. Sociobiol. 40, 271–276 (doi:10.1007/s002650050342) [Google Scholar]
- Moritz R. F. A., Crewe R. M.1988Chemical signals of queens in kin recognition of honeybees, Apis mellifera L. J. Comp. Physiol. A 164, 83–89 (doi:10.1007/BF00612721) [Google Scholar]
- Page R. E., Jr, Metcalf R. A., Mecalf R. L., Erickson E. H., Lampman R. L.1991Extractable hydrocarbons and kin recognition in the honey bee (Apis mellifera L.). J. Chem. Ecol. 17, 745–756 (doi:10.1007/BF00994197) [DOI] [PubMed] [Google Scholar]
- Pol R. G., Lopez de Casenave J., Feldhaar H., Milesi F. A., Gadau J.2008Polyandry in two South American harvester ants. Insectes Soc. 55, 91–97 (doi:10.1007/s00040-007-0975-0) [Google Scholar]
- Queller D. C., Hughes C. R., Strassmann J. E.1990Wasps fail to make distinctions. Nature 344, 388 (doi:10.1038/344388a0) [Google Scholar]
- Rangel J., Seeley T. D.2008The signals initiating the mass exodus of a honey bee swarm from its nest. Anim. Behav. 76, 1943–1952 (doi:10.1016/j.anbehav.2008.09.004) [Google Scholar]
- Ratnieks F. L. W.1991The evolution of genetic odor-cue diversity in social Hymenoptera. Am. Nat. 137, 202–226 (doi:10.1086/285154) [Google Scholar]
- Ratnieks F. L. W., Reeve H. K.1991The evolution of queen-rearing nepotism in social Hymenoptera: effects of discrimination costs in swarming species. J. Evol. Biol. 4, 93–115 (doi:10.1046/j.1420-9101.1991.4010093.x) [Google Scholar]
- Reeve H. K.1998Game theory, reproductive skew, and nepotism. In Game theory and animal behavior (eds Dugatkin L. A., Reeve H. K.), pp. 118–145 Oxford, UK: Oxford University Press [Google Scholar]
- Rheindt F. E., Gadau J., Strehl C.-P., Hölldobler B.2004Extremely high mating frequency in the Florida harvester ant (Pogonomyrmex badius). Behav. Ecol. Sociobiol. 56, 472–481 (doi:10.1007/s00265-004-0808-3) [Google Scholar]
- Ross K. G.1986Kin selection and the problem of sperm utilization in social insects. Nature 323, 798–800 (doi:10.1038/323798a0) [Google Scholar]
- Schlüns H., Moritz R. F. A., Neumann P., Kryger P., Koeniger G.2005Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim. Behav. 70, 125–131 (doi:10.1016/j.anbehav.2004.11.005) [Google Scholar]
- Seeley T. D.1995The wisdom of the hive Cambridge, MA: Harvard University Press [Google Scholar]
- Solignac M., Vautrin D., Loiseau A., Mougel F., Baudry E., Estoup A., Garnery L., Haberl M., Cornuet M.2003Five hundred and fifty microsatellite markers for the study of the honeybee (Apis mellifera L.) genome. Mol. Ecol. Notes 3, 307–311 (doi:10.1046/j.1471-8286.2003.00436.x) [Google Scholar]
- Solis C. R., Hughes C. R., Klingler C. J., Strassmann J. E., Queller D. C.1998Lack of kin discrimination during wasp colony fission. Behav. Ecol. 9, 172–176 (doi:10.1093/beheco/9.2.172) [Google Scholar]
- Strassmann J.2001The rarity of multiple mating by females in the social Hymenoptera. Insectes Soc. 48, 1–13 (doi:10.1007/PL00001737) [Google Scholar]
- Strassmann J. E., Seppa P., Queller D. C.2000Absence of within-colony kin discrimination: foundresses of the social wasp, Polistes carolina, do not prefer their own larvae. Naturwissenschaften 87, 266–269 (doi:10.1007/s001140050718) [DOI] [PubMed] [Google Scholar]
- Tarpy D. R., Nielsen D. I.2002Sampling error, effective paternity, and estimating the genetic structure of honey bee colonies (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 95, 513–528 (doi:10.1603/0013-8746(2002)095[0513:SEEPAE]2.0CO;2) [Google Scholar]
- Tarpy D. R., Gilley D. C., Seeley T. D.2004Levels of selection in a social insect: a review of conflict and cooperation during honey bee (Apis mellifera) queen replacement. Behav. Ecol. Sociobiol. 55, 513–523 (doi:10.1007/s00265-003-0738-5) [Google Scholar]
- Timmermans I., Hefetz A., Fournier D., Aron S.2008Population genetic structure, worker reproduction and thelytokous parthenogenesis in the desert ant Cataglyphis sabulosa. Heredity 101, 490–498 (doi:10.1038/hdy.2008.72) [DOI] [PubMed] [Google Scholar]
- Visscher P. K.1986Kinship discrimination in queen rearing in honey bees (Apis mellifera). Behav. Ecol. Sociobiol. 18, 453–460 (doi:10.1007/BF00300521) [Google Scholar]
- Wiernasz D. C., Perroni C. L., Cole B. J.2004Polyandry and fitness in the western harvester ant Pogonomyrmex occidentalis. Mol. Ecol. 13, 1601–1606 (doi:10.1111/j.1365-294X.2004.02153.x) [DOI] [PubMed] [Google Scholar]
- Wilson E. O.1971The insect societies Cambridge, MA: Harvard University Press [Google Scholar]
- Winston M. L.1987The biology of the honey bee Cambridge, MA: Harvard University Press [Google Scholar]