Abstract
Balaenid whales perform long breath-hold foraging dives despite a high drag from their ram filtration of zooplankton. To maximize the volume of prey acquired in a dive with limited oxygen supplies, balaenids must either filter feed only occasionally when prey density is particularly high, or they must swim at slow speeds while filtering to reduce drag and oxygen consumption. Using digital tags with three-axis accelerometers, we studied bowhead whales feeding off West Greenland and present here, to our knowledge, the first detailed data on the kinematics and swimming behaviour of a balaenid whale filter feeding at depth. Bowhead whales employ a continuous fluking gait throughout the bottom phase of foraging dives, moving at very slow speeds (less than 1 m s−1), allowing them to filter feed continuously at depth. Despite the slow speeds, the large mouth aperture provides a water filtration rate of approximately 3 m3 s−1, amounting to some 2000 tonnes of water and prey filtered per dive. We conclude that a food niche of dense, slow-moving zooplankton prey has led balaenids to evolve locomotor and filtering systems adapted to work against a high drag at swimming speeds of less than 0.07 body length s−1 using a continuous fluking gait very different from that of nekton-feeding, aquatic predators.
Keywords: filter feeding, bowhead whale, kinematics
1. Introduction
Air-breathing aquatic animals display a number of adaptations to access two spatially separated, but vital resources: oxygen at the surface and food at depth. To get a sufficient net uptake of energy, aquatic carnivores must balance the metabolic costs of locomotion and prey acquisition against their oxygen reserves while foraging (Kramer 1988; Williams 1999). Most breath-holding marine predators capture food in discrete feeding events (Ropert-Coudert et al. 2006; Hassrick et al. 2007; Aguilar Soto et al. 2008), where they reduce oxygen consumption by gliding during parts of either ascent or descent (Williams et al. 2000) and employ a stroke-and-glide gait at depth to prolong foraging time (Crocker et al. 1997; Croll et al. 2001; Williams 2001; Wilson et al. 2002; Watanuki et al. 2003). Thus, locomotion is a major oxygen-consuming activity using up oxygen reserves while diving, and the stroke-and-glide strategy of most air-breathing marine animals allows them to perform longer breath-hold dives, maximizing access to food resources (Crocker et al. 1997; Williams et al. 2000; Watanuki et al. 2003).
In contrast to the discrete foraging events seen in most air-breathing marine predators, the large balaenids (right and bowhead whales) feed on aggregations of zooplankton through what has been termed continuous ram filtration, similar to the feeding behaviour of basking-, whale- and megamouth sharks (Pivorunas 1979; Diamond 1985; Sims 1999; Lambertsen et al. 2005).
The large head of bowhead whales comprises approximately one-third of its total body length and, with a mouth aperture of more than 4 m2, it forms an enormous filtering apparatus with the high curved maxillary and premaxillary bones supporting up to 4 m long baleen plates (Werth 2001, 2004; Lambertsen et al. 2005). When foraging at the surface, balaenid whales have been reported to swim with mean speeds of 1.1–2.5 m s−1 (Mayo et al. 2001; Baumgartner & Mate 2003; Werth 2004), which is comparable to their migration speeds (Heide-Jørgensen et al. 2006). Owing to lack of data, these speeds have been presumed to be maintained by whales feeding under water (Baumgartner & Mate 2003; Werth 2004). Swimming with an open mouth to force water past a dense curtain of baleen changes the hydrodynamic shape of the animal and increases the drag significantly (Sanderson & Wassersug 1990; Werth 2004). Despite this increased drag, balaenid whales perform long foraging dives lasting between 10 and 40 min (Werth 2004; Laidre et al. 2007). Given the expected large drag increment resulting from an open mouth, balaenids face a trade-off between the benefits of filtering large volumes of water per second and the energetic costs of swimming faster, thus reducing foraging time. This trade-off brings into question the assumption that whales diving to feed with their mouth open will swim as fast as those travelling with mouth closed or feeding at the surface: how can bowhead whales maintain high speeds while continuously working against a high drag during long breath-hold dives? One possible explanation is that balaenids do not employ continuous ram filtration when submerged, but only open their mouth in discrete events when the food density is particularly high. Drag would be reduced during the mouth-closed swimming, which, in combination with an energy-saving stroke-and-glide gait, could explain the long dive times at high mean speeds. An alternate hypothesis is that balaenids employ continuous ram filtration while at depth, but swim much slower than previously estimated from surface feeding whales, thereby reducing the drag forces and hence oxygen consumption during breath-hold dives.
Here, we test these two alternative hypotheses using multi-sensor archival digital tags (DTAGs) on filter feeding bowhead whales in West Greenland and provide, to our knowledge, the first detailed account of the behaviour and biomechanics of filter feeding in balaenid whales with implications for filtration rates and prey location. We show that feeding bowhead whales employ a continuous fluking gait and swim slowly at less than 0.07 of body length s−1, allowing them to ram filter feed continuously at depth during long breath-hold dives.
2. Material and methods
Bowhead whales south of Disko Island were tagged with DTAGs (Johnson & Tyack 2003) in the period from 2–16 May 2008. The whales were approached slowly with a dinghy, and the DTAGs were attached to the middle of their backs with four suction cups, using an 8 m hand-held carbon fibre pole. The DTAG released from the whale after a pre-programmed time period, and the tags were retrieved using VHF tracking (Johnson & Tyack 2003). Given the challenging field conditions of partial ice cover, variable weather and uncertain whale residence time in the area, we programmed tags to release after 3 h.
The DTAG contains a pressure sensor and three-axis magnetometers and accelerometers, each sampled at 50 Hz with 16-bit resolution (Johnson & Tyack 2003). For analysis, the sensor data were down-sampled to 5 Hz, and the accelerometer and magnetometer data were corrected for tag orientation on the whale by rotating each three-element vector to provide orientation data in whale frame coordinates (Johnson & Tyack 2003). All sensors were compensated for drift from the changing temperatures using a built-in temperature sensor (Johnson & Tyack 2003).
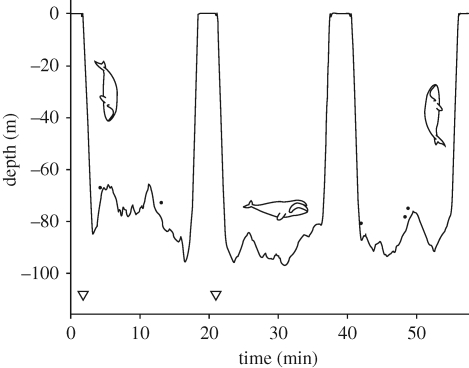
Following previous tagging studies of balaenids, two broad types of dives were identified: U-shaped and V-shaped dives (Baumgartner & Mate 2003; Laidre et al. 2007). Dives were divided into three phases: descent, bottom and ascent. Descents started when the whale left the surface and ended when the whale's pitch angle first became positive, indicating the first upward-pointing orientation (figure 1). Ascents started when the whale pitch last became negative and ended when the whale reached the surface (Sato et al. 2003; Miller et al. 2004; Watwood et al. 2006). The bottom phase of U-dives was the interval between the descent and ascent phases (figure 1). V-dives lacked a bottom phase and consisted of only ascent and descent phases. To compare speed estimates and fluke rates of U-dives with an equivalent part of V-dives, we defined a ‘bottom phase’ in V-dives. On average, the bottom time made up 79 per cent of the deepest samples of each U-dive. Based on this, we defined the bottom phase of V-dives as the 79 per cent deepest samples of each V-dive.
Figure 1.
Dive profile from a feeding bowhead whale tagged with a DTAG in Disko Bay, West Greenland. Small dots at the bottom of dives indicate the times at which rattle-like sounds were detected. The two triangles indicate the bottom depth measured with an echo-sounder from the tag boat.
The oscillations from fluke strokes of a swimming whale undulate through the whole body (Fish et al. 2003) and can be detected as cyclic variations in the pitch angle of the tag (Johnson & Tyack 2003). Using this signal, the fluking rate was computed as the inverse of the time between peaks in the pitch record averaged over 30 s bins and reported in Hertz (fluke strokes s−1).
Swimming speed is difficult to measure accurately for a submerged animal using a small tag without external localization methods such as acoustic tracking (Johnson & Tyack 2003). Swim speed is generally defined as the forward-directed movement along the longitudinal axis of the animal per unit of time. However, the body of a swimming whale undulates with each fluke stroke accelerating the tag perpendicular to the body axis, complicating speed measurements. In addition, animals moving in three dimensions are affected by lift, buoyancy and gravity forces influencing their forward speed. Therefore, we estimated swimming speed using two methods. In the first method, vertical speed, derived from the depth sensor, was multiplied by the arcsine of the pitch angle (Miller et al. 2004) and smoothed with a Kalman filter following Zimmer et al. (2005). This approach is a good proxy for speed provided (i) that the whale's specific acceleration is low (a requirement for the pitch estimate to be accurate), (ii) that the whale moves anteriorly in the direction of its body axis, and (iii) that the absolute pitch angle is far from zero. During the bottom phase of U-dives, bowhead whales regularly swim with pitch angles close to zero, rendering the speed estimate unreliable at these times. Therefore, following previous studies (Fletcher et al. 1996; Burgess et al. 1998; Goldbogen et al. 2006; Aguilar Soto et al. 2008), we used the low-frequency flow noise recorded by the tag as an alternative proxy for speed. For each tag placement, we computed the flow noise (noise power at 500 Hz band-pass filtered with a 2-pole Butterworth filter) during descents in 5 s bins along with the mean speed, in that bin derived from the mean vertical speed multiplied by the arcsine of the mean pitch angle over the same interval. We used descent for the speed–noise calibration because the tagged whales all fluked during descent and in the bottom phases, whereas many ascents had little fluking. Using regression analysis (sensu Goldbogen et al. 2006), we fitted a function k+α(20 log(noise power)) with a mean r2 value of 0.65 to the noise and speed data during descents for each whale. This flow noise/speed correlation was used to estimate the swimming speed in the bottom phases in U-dives. The speed estimate is probably an overestimate as any low frequency sounds associated with feeding, such as increased flow noise because of the changed body form when compared with the calibration epoch, will add to the noise level and thus the apparent speed.
The sound of baleen plates rattling during feeding has been described from surface-skimming Northern right whales (Watkins & Schevill 1976). We listened through the tag sound recordings using custom software and marked possible baleen rattles to identify the time and depth of these sounds. All analyses were performed using Matlab 6.5 (Mathworks).
3. Results
Seven bowhead whales were tagged, and a total of 13.9 h of dive data were obtained containing 52 dives, of which 33 and 19 were classified as V- and U-dives, respectively.
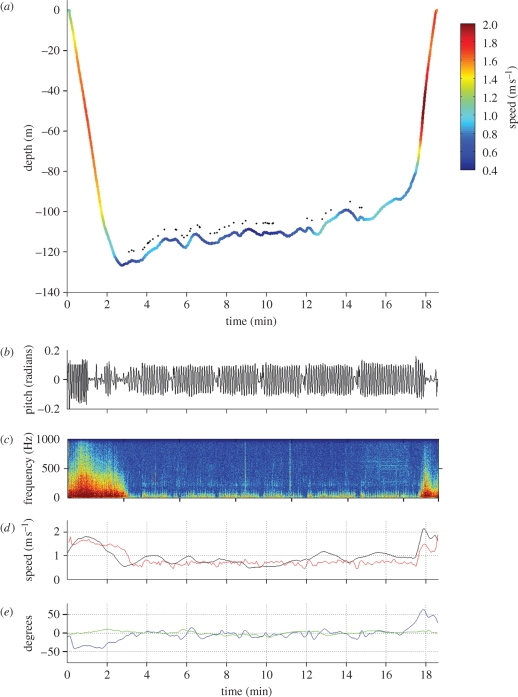
The maximum depth of V-dives ranged from 15 to 221 m, with a mean of 69 m (s.d. = 37). V-dives had an average duration of 9.0 min (s.d. = 5.1), with a range of 1.6–19 min (statistics for each animal are summarized in table 1). The maximum depths of U-dives varied across whales with an overall mean of 79 m (s.d. = 64) and range of 17–127 m. The duration of U-dives was 15.2 min (s.d. = 4.1), with a range of 7–21 min (table 1). When the whales left the surface descending on a typical U-dive, they pitched downwards and fluked continuously for the first 24–90 m (figure 2). One whale continued fluking throughout descents, but other whales adopted a stroke-and-glide gait, resulting in overall mean (over the dive phase) fluking rates of 0.08 Hz (s.d. = 0.03) during descent and 0.06 Hz (s.d. = 0.02) during ascent (table 1). The instantaneous fluking rate computed in the bouts of fluking during the initial part of the descent was 0.79 Hz (s.d. = 0.11), 10 times higher than the mean descent fluking rate (0.08 Hz, table 1). In comparison, whales fluked almost continuously during the bottom phases of U-dives, with a mean fluking rate of 0.12 Hz (s.d. = 0.08) (figure 2b and table 1). The mean fluking rate in the bottom phase was significantly higher than the overall mean descent and ascent fluking rate (non-parametric ANOVA p < 0.05, descent: H = 6.8, ascent: H = 9.1, d.f. = 1). However, the mean of the instantaneous fluking rate during the first part of the descent was significantly higher than the U-dive bottom-phase fluking rate (non-parametric ANOVA H = 55, p < 0.05, d.f. = 1). Despite the higher mean fluking rate during the bottom phase, the estimated swim speeds were 0.7 m s−1 (s.d. = 0.11) and 0.8 m s−1 (s.d. = 0.08) (Kalman-filtered and noise-based estimates, respectively), about one-half of the speeds of descent (1.4 m s−1) and ascent (1.2 m s−1) as estimated with the Kalman approach (table 1). The pitch angle was consistently close to zero (within ±10°) during the bottom phase, making the Kalman speed estimate suspect in this phase, but the general agreement with the noise-based speed estimate was good (table 1). The mean noise speed estimate of bottom phase in U-dives (0.8 m s−1) was significantly lower than the mean speed estimates of the bottom phase in V-dives (1.3 m s−1, s.d. = 0.39) (non-parametric ANOVA H = 25.21, p < 0.001, d.f. = 1). At the end of the bottom phase, the whales pitched towards the surface and switched to a stroke-and-glide gait. While all of the tagged whales fluked during at least part of the descents, some glided all the way to the surface presumably powered by positive buoyancy. The roll angle was ±10° during the bottom phase of all U-dives (figure 2).
Table 1.
Dive statistics of foraging and non-foraging bowhead whale dives. (Numbers in brackets indicate standard deviation.)
| Bm123a | Bm126a | Bm126b | Bm127a | Bm129a | Bm130a | Bm137a | mean | ||
|---|---|---|---|---|---|---|---|---|---|
| U-shaped foraging dives | n | 1 | 1 | 3 | 3 | 1 | 10 | 0 | |
| maximum depth (m) | 90 | 105 | 99 | 115 | 127 | 45 | — | ||
| mean maximum depth (m) | 90 | 105 | 97 (2.3) | 90 (36.1) | 127 | 31 (9.4) | — | ||
| range maximum depth (m) | 90 | 105 | 94–99 | 49–105 | 127 | 17–45 | — | ||
| mean dive time (min) | 20.6 | 11.2 | 13.4 | 18.4 | 12.5 | — | 15.2 | ||
| speed (m s−1), descent | 1.6 | 1.3 | 1.7 (0.26) | 1.5 (0.29) | 1.4 | 1.0 (0.17) | — | 1.43 | |
| speed (m s−1), ascent | 1.3 | 1.5 | 1.0 (0.32) | 1.5 (0.54) | 1.4 | 0.8 (0.07) | — | 1.24 | |
| speed (m s−1), bottom | 0.8 | 0.6 (0.33) | 0.6 (0.18) | 0.8 | 0.6 (0.08) | — | 0.67 | ||
| noise calibration speed (m s−1) | 0.8 (0.2) | 0.9 (0.1) | 0.9 (0.2) | 0.7 (0.1) | 0.8 (0.1) | — | 0.82 | ||
| fluking rate (Hz), descent | 0.05 (0.03) | 0.07 (0.03) | 0.13 (0.003) | 0.09 (0.01) | 0.07 (0.04) | — | 0.08 | ||
| fluking rate (Hz), ascent | 0.08 (0.13) | 0.03 | 0.05 (0.01) | 0.07 (0.02) | 0.09 | 0.05 (0.05) | — | 0.06 | |
| fluking rate (Hz), bottom | 0.13 | 0.07 | 0.11 (0.01) | 0.13 (0.01) | 0.13 | 0.15 (0.01) | — | 0.12 | |
| V-shaped search dives | n | 2 | 11 | 0 | 13 | 0 | 0 | 7 | |
| maximum depth (m) | 81 | 124 | — | 80 | — | — | 221 | ||
| mean maximum depth (m) | 76 (6.29) | 66 (42.5) | — | 33 (18.2) | — | — | 173 (43.8) | ||
| range maximum depth (m) | 72–81 | 15–124 | — | 16–80 | — | — | 93–221 | ||
| mean dive time (min) | 6.1 | 8.9 | — | 4.8 | — | — | 16.3 | 9.0 | |
| speed (m s−1), descent | 1.6 (0.02) | 0.8 (0.26) | — | 1.4 (0.27) | — | — | 1.3 (0.33) | 1.27 | |
| speed (m s−1), ascent | 1.5 (0.03) | 1.2 (0.38) | — | 1.1 (0.27) | — | — | 1.6 (0.42) | 1.34 | |
| fluking rate (Hz), descent | 0.07 (0.03) | 0.07 (0.02) | — | 0.12 (0.03) | — | — | 0.09 (0.02) | 0.09 | |
| fluking rate (Hz), ascent | 0.10 (0.02) | 0.05 (0.05) | — | 0.08 (0.04) | — | — | 0.04 (0.03) | 0.07 |
Figure 2.
Bowhead whale foraging dive. (a) Dive profile showing the slow estimated speed in the bottom phase of the dive compared with the descent and ascent phases (colour bar). The dots indicate the time of recorded rattle-like sounds. (b) Cyclic variation in the pitch angle because of fluke strokes, indicating constant fluking during the bottom phase of the dive. (c) Spectrogram of the sound recording showing the higher levels of low frequency sound during the fast descent and ascent phases (fast Fourier transform (FFT) size 1024). (d) Speed estimated from the change in depth corrected for the pitch angle, filtered with a Kalman filter (black) and speed estimated from the low-frequency flow noise (red). (e) Pitch angle (blue) and roll angle (green).
To evaluate the speed estimates during U-dives, we logged the geo-referenced position and time at the beginning and end of two U-dives. Knowing the pitch angles and speeds during the descent and ascent phases of these dives, we subtracted the distance covered during descent and ascent from the total distance covered during the dive to derive the distance covered during the bottom time. The mean speed during the bottom phase in the two U-dives derived in this way was below 0.8 m s−1 and hence close to the speed estimates calculated from flow noise and corrected vertical rate with a Kalman filter (0.8 and 0.7 m s−1, respectively; table 1).
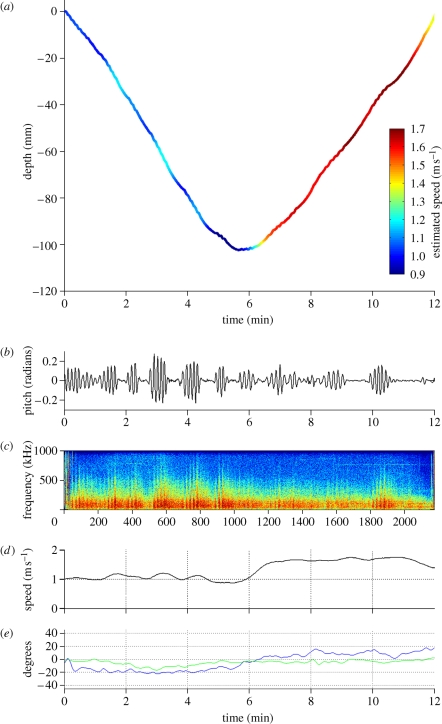
The speed estimates during descents and ascents of V-dives (mean 1.3 m s−1) were not significantly different from speeds in U-dive descents and ascents (non-parametric ANOVA, H = 5.1, d.f. = 3, p = 0.2; table 1). The descent and ascent phases of V-dives were similar to those described earlier for U-dives and thus were characterized by stroke-and-glide gait, with a higher overall mean fluking rate during descent (0.09 Hz, s.d. = 0.02) than during ascent (0.07 Hz, s.d. = 0.03) (figure 3 and table 1). Thus, the only dive phase in which whales fluked continuously was during the bottom phase of U-dives (figure 2).
Figure 3.
Bowhead whale non-foraging dive. (a) Dive profile showing the estimated Kalman-filtered speed in the colour bar. (b) The cyclic variation in pitch angle shows the bouts of fluke strokes, typical of a stroke-and-glide gait. (c) Spectrogram of sound recording showing the low-frequency sound recorded during descent and ascent of a non-foraging dive (FFT 1024). (d) Speed estimated from the change in depth corrected for pitch angle, filtered with a Kalman filter. (e) Pitch angle (blue) and roll angle (green).
Distinct rattle-like sounds were heard in six of the seven tag recordings. The remaining tag recording (Bm137a) contained only V-dives. The rattle sound pulses occurred with variable levels and pulse intervals and had a frequency range of 300–600 Hz. Except for recording Bm126a, in which rattle-like sounds were detected throughout the dive profile, rattles were only heard during the bottom phase of U-dives (figures 1,2 and 4). Tagged whales did not produce any detectable vocalizations while foraging, and the soundscape was dominated by cracking ice, and occasional calls from non-tagged bowheads.
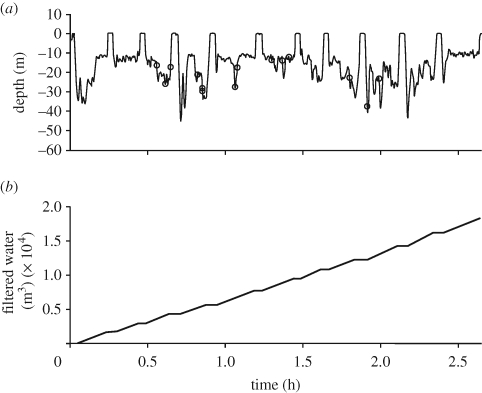
Figure 4.
Filtering capacity of a feeding bowhead whale. (a) Dive profile from a bowhead whale feeding at 10–50 m depth. Rings indicate time when rattle-like sounds were recorded. (b) Accumulated filtered water during a 2.7 h feeding event, assuming a mean effective filter area of 4.23 m2 and using the estimated swimming speeds in the bottom phases of the dives.
4. Discussion
Major questions when studying the behavioural ecology and field physiology of free-ranging animals pertain to when, how and by what energetic investments they acquire food (Costa & Sinervo 2004). Previous tagging studies on Northern right whales and bowhead whales have divided dives into two types—U- and V-dives—and suggested that balaenids employ ram filtration during the bottom phase of the U-dives (Nowacek et al. 2001; Baumgartner & Mate 2003; Laidre et al. 2007), whereas V-dives serve for travel and search for food (Laidre et al. 2007). Dive data presented here from bowhead whales in Greenland also follow a similar pattern of U- and V-dives, but the sensor array of the DTAG allowed us to test the assertion that U-dives are feeding dives.
An air-breathing diver should adopt an energy-efficient gait, matched to its instantaneous body form and behaviour throughout foraging dives so as to maximize net energy return. A filter-feeding balaenid passes through several body forms in a foraging dive from high buoyancy near the surface when the lungs are full of air to a lower buoyancy, more streamlined body form at greater depths (Nowacek et al. 2001). A third change in body form occurs when the whale opens its mouth to filter water, probably incurring a drastic increase in the drag coefficient (Werth 2001, 2004). Tagged bowhead whales showed clear changes in gait associated with these changes in body form. When leaving the surface, whales performed a burst of rapid fluking (0.79 Hz instantaneous), giving way to a stroke-and-glide gait with slower fluking (0.08 Hz average during descent). The bottom phase of U-dives had significantly higher mean fluking rates compared with the descents and ascents of both V- and U-dives; whales fluked almost continuously at the bottom of U-dives with only very short breaks in the fluking effort. However, the instantaneous fluking rate of 0.12 Hz during the bottom phase was substantially lower than during bursts of fluking in the descent and ascent, and only half of the 0.25 Hz fluking rate predicted from scaling across a wide range of air-breathing marine vertebrates (Sato et al. 2007).
Given the higher average fluking rate during the flat bottom phase of U-dives, one would expect an increase in speed compared with the lower mean fluking rates of ascents and descents if the drag coefficient was unchanged. However, the estimated speeds decrease to about half of the speeds during descent and ascent. Thus in the bottom part of the U-dives, the whales fluke at least 50 per cent more per unit of time than in descents while almost halving their speeds from 1.4 to 0.8 m s−1. The most parsimonious explanation for this reduction in speed is that their drag coefficient (Vogel 1994) has increased significantly through opening of their mouth for filter feeding (Werth 2004), an inference consistent with previous studies, proposing that whales feed during the bottom phase of U-dives. For this reason, we consider dives with flat bottom profiles, slow estimated speeds and continuous fluking to be foraging dives. Given the near-continuous fluking and slow estimated speeds during the bottom phase of foraging dives, we infer that the tagged whales filtered continuously throughout the bottom phase. That falsifies one of our initial hypotheses: that the relatively long foraging dives of bowheads result from intermittent filtering with the whale swimming with mouth closed in the intervening periods, allowing an oxygen-conserving stroke-and-glide gait. Instead, to cope with the high drag from the open mouth when ram filter feeding on plankton, bowhead whales use a slow continuous fluking gait that is radically different from the stroke-and-glide gait adopted by other nekton feeding air breathers at sea (Williams 2001; Sato et al. 2003, 2007; Woodward et al. 2006).
The buoyancy of a whale depends on the density of its tissue and the volume of air within its body (Nowacek et al. 2001; Miller et al. 2004). In this study, whales consistently fluked less but attained higher speeds during ascents (table 1), showing that they are positively buoyant even at depth. In fact, one individual ceased fluking altogether at 120 m depth on the ascent of a V-dive and drifted slowly towards the surface for some seconds, showing that their thick blubber layer may, in some cases, make these whales positively buoyant even when their lungs are compressed (Nowacek et al. 2001).
The dive times of large rorquals that employ lunge feeding are surprisingly short (5–10 min), and this has been explained by the high energetic costs of the lunges used to inflate their large buccal pouch with prey laden water (Acevedo-Gutiérrez et al. 2002). In comparison, foraging bowhead whales perform long foraging dives: the mean duration of 15.2 min (range 7–21 min, table 1) reported here is comparable to the mean dive times reported in another study of bowhead whales, 3–18 min (mean of U- and V-dives, Laidre et al. 2007), and of Northern right whales, 12.7 min (Baumgartner & Mate 2003). The dive duration varies with individual and, although the mean dive duration is just below 15 min, extreme dives of up to 48 min have been reported from some individuals (Laidre et al. 2007). Breath-hold dives of 12–15 min, in which the whale is continuously fluking for some 80 per cent of the time with an open mouth, would be very energetically costly if they happened at the normal swimming speeds of 1.5–2 m s−1 reported for cetaceans in general and adopted by balaenids feeding near the surface (Watkins & Schevill 1976; Mayo et al. 2001; Werth 2004). Instead, our speed estimates from the bottom phase of foraging dives support the alternate hypothesis of this paper, proposing that whales reduce the energetic cost of swimming with an open mouth by reducing their swimming speed and therefore their drag. As seen from figure 2 and table 1, both the Kalman-filtered speed estimates and speed estimates based on flow noise indicate that the speed drops significantly during the flat part of foraging dives, with mean speed estimates around 0.75 m s−1, which is some 60 per cent of the speed during ascent and descent phases.
We therefore conclude that feeding bowheads move forward at an average speed of less than 1 m s−1 at depth. More specifically, our data indicate a mean speed of some 0.75 m s−1, demonstrating that bowheads swim significantly slower when feeding at depth than reported in previous studies of whales observed at the surface (Watkins & Schevill 1976; Mayo et al. 2001; Werth 2004). It is also about half the stable average swim speeds between 1 and 2 m s−1 found across sizes ranging from 0.5 kg birds to 30 000 kg sperm whales (Sato et al. 2007). As drag increases with the square of speed at the Reynolds numbers in play here (Vogel 1994), a halving of speed should give a drag that is four times smaller for the same body shape. In our study, filter feeding bowhead whales swam at about one half of their descent and ascent speed while foraging, and yet needed an average fluking rate 1.5 times higher to maintain this low speed. This suggests that the drag coefficient increases by a factor of around 6 (1.5 × 4) when the whales swim with an open mouth, assuming a constant thrust per fluke stroke. For a given drag coefficient, the power, and hence oxygen consumption required to swim, increases with the cube of the swimming speed (Hind & Gurney 1997; Fish 2002). As the oxygen consumption sets the aerobic dive time (Kooyman et al. 1980), the slow swim speeds of feeding bowheads may represent an attempt to maximize dive time by reducing drag and hence oxygen consumption while swimming with the mouth open. Swimming at double the speed would, all other things being equal, increase oxygen consumption eightfold, while only doubling the volume of filtered water and prey.
It may seem ironic that one of the largest carnivores on the planet can capture enough prey by moving forward at less than 0.07 body length s−1. However, whereas many nekton-eating predators need to swim at high speeds to locate and subdue agile but calorific prey, the copepod prey of bowhead whales moves slowly (approx. 4 mm s−1, Wong 1988) and is found in extensive patches. Nonetheless, these small prey must be acquired in large numbers, calling for a large filter aperture that, in turn, creates a large drag coefficient and requires a lower foraging speed to maximize net energy gain. This implies that the minimum cost of transport for feeding bowheads occurs at speeds about one half of those found for similar sized fusiform marine endotherms (Sato et al. 2007). We conclude that balaenid ram filter feeding is a highly specialized behaviour, where not only the morphology of the filter apparatus of the predator is optimized for the capture of its slow small prey in dense patches (Werth 2004; Lambertsen et al. 2005), but also the locomotor system and the physiology that fuels it are adapted to work against a high drag at slow speeds using a continuous fluking gait very different from other air-breathing predators at sea.
The filter apparatus of an adult bowhead whale has an estimated mean effective cross-sectional area of 4.23 m2 (Werth 2004). If we assume that the whales keep a constant gape and a mean swimming speed of 0.75 m s−1 (table 1) while feeding, the filtration rate is around 3.2 m3 s−1. So despite the slow swimming speed, the large mouth aperture can filter a remarkable volume of water over time. Figure 4 shows the estimated filtered water volume using the estimated speeds during ram filtration of one of the tagged whales. Over a period of 2.7 h, an estimated water volume of 18 000 m3 passed through the filter of the whale. That raises the questions of how much food the whales collect over time and how often they must empty their filtering apparatus.
Overall, the whales in this study spent 29 per cent of the total tagged time feeding (i.e. at the bottom of a foraging dive moving at a slow speed with continuous fluking), resulting in an estimated daily filtering rate of some 80 000 m3 of water per whale, assuming that the short tagging periods are representative of time allocation over a diurnal cycle. Based on a copepod concentration of 0.001 kg m−3, Laidre et al. (2007) concluded that a bowhead whale should filter more than 800 000 m3 of water per day just to meet its estimated field metabolic rate (FMR). If a whale spends only 7 h d−1 (29% of the time) with its mouth open, it would have to filter 31.7 m3 of water s−1 when feeding at these copepod densities. With a mean effective filter area of 4.23 m2, it would translate into a mean swimming speed of 7.5 m s−1. This is about an order of magnitude above our estimate and highly unlikely given the 100-fold higher drag force at such a speed. While the 29 per cent foraging time may be an underestimate, it is evident that either the FMR is widely overestimated or that the prey density where the whales feed has been grossly underestimated, as suggested by Laidre et al. (2007). Assuming that the FMR estimate of Laidre et al. (2007) is correct, whales moving at 0.75 m s−1 would require copepod patches with a mean density of 0.01 kg m−3, 10 times higher than Laidre's figure but in line with the prey densities reported in the vicinity of foraging Northern right whales (Mayo & Goldman 1992; Beardsley et al. 1996).
During the bottom phase of foraging dives, we observed brief pauses in fluking with durations around 2 s (i.e. the duration of about half a fluke stroke when feeding) at fairly regular intervals with a mean interval of 2.4 min (s.d.=0.65) (figure 2). Similar brief pauses have also been observed in ram filtering Northern right whales (Nowacek et al. 2001). Although ram filter feeding whales are believed to be able to continuously filter for hours (Baumgartner & Mate 2003; Laidre et al. 2007), they will probably need to clean the collected prey from the baleen plates periodically to ingest prey and maintain water flow through the baleen (Werth 2001). Cleaning could be achieved by shaking the head, using the muscular tongue to scrape off prey, back flushing trapped prey, or a combination of all three methods (Werth 2001). If we interpret the regular pauses in the fluking correctly to be cleaning of the baleen, our data suggest that this happens every 2.5 min, corresponding to some 480 m3 of filtered water. The regularity of the gesture suggests that prey is acquired at a fairly constant rate consistent with the idea that the whales are feeding in an extensive patch with sufficiently high prey density to support continuous filtration.
Other ram filter feeding animals carefully balance food uptake with energy consumption and oxygen assimilation over the gills (Sims & Quayle 1998; Sims 1999). For example, basking sharks decrease locomotion in low food concentrations to save energy when food concentrations are low (Sims & Quayle 1998; Sims 1999). Herring switch from ram filtering to particulate feeding when food densities drop and the increased food uptake of filter feeding no longer compensates the increased energetic cost of ram filter feeding (Gibson & Ezzi 2006). Similarly, it seems energetically important that bowhead whales only open their mouths in areas of high food density and keep the mouth shut when energetic costs of ram filter feeding are too high relative to the food intake (Mayo & Marx 1990). The non-foraging dives observed here generally reach the same depth as feeding dives and may reflect that the whales search for food patches in some of those dives, but that they do not encounter prey densities worth targeting. We have shown that the bowhead whales feed in all parts of the water column, not only close to the surface or bottom where there are physical boundaries to guide the whales to the food patches or constrain the prey (figures 1 and 4). This fact, along with the need for much higher copepod densities than found on average, suggests that bowhead whales employ sensory cues to locate high density food patches and guide them as to when to open their mouth. How and with what means they locate these food patches offers an intriguing challenge for future studies on these large, slow filter feeders of the Arctic.
Acknowledgements
The research was conducted under a permit granted by the Greenland Government to GINR.
We thank A. Brandt, A. Isaksen, Arctic Station and F. Ugarte for invaluable help in the field. F. Ugarte, A. Werth, M. Baumgartner, D. Costa, M. P. Heide-Jørgensen, K. L. Laidre and two reviewers are thanked for helpful discussions and critique. M.S. was funded by the Oticon Foundation and KIIIN, Greenlandic Home Rule, P.T.M. by Steno and frame grants from the National Danish Science Foundation and M.J. and P.L.T. by NOPP and SERDP grants.
References
- Acevedo-Gutiérrez A., Croll D. A., Tershy B. R.2002High feeding costs limit dive time in the largest whales. J. Exp. Biol. 205, 1747–1753 [DOI] [PubMed] [Google Scholar]
- Aguilar Soto N., Johnson M., Madsen P. T., Díaz F., Domínguez I., Brito A., Tyack P.2008Cheetahs of the deep sea: deep foraging sprints in short-finned pilot whales off Tenerife (Canary Islands). J. Anim. Ecol. 77, 936–947 (doi:10.1111/j.1365-2656.2008.01393.x) [DOI] [PubMed] [Google Scholar]
- Baumgartner M. F., Mate B. R.2003Summertime foraging ecology of North Atlantic right whales. Mar. Ecol. Prog. Ser. 264, 123–135 (doi:10.3354/meps264123) [Google Scholar]
- Beardsley R., Epstein A. W., Chen C., Wishner K. F., Macaulay M. C., Kenney R. D.1996Spatial variability in zooplankton abundance near feeding right whales in the Great South Channel. Deep Sea Res. Part II. Topical Stud. Oceanogr. 43, 1601–1625 (doi:10.1016/S0967-0645(96)00050-1) [Google Scholar]
- Burgess W. C., Tyack P. L., Le Boeuf B. J., Costa D. P.1998A programmable acoustic recording tag and first results from free-ranging northern elephant seals. Deep Sea Res. II 45, 1327–1351 (doi:10.1016/S0967-0645(98)00032-0) [Google Scholar]
- Costa D. P., Sinervo B.2004Field physiology: physiological insights from animals in nature. Annu. Rev. Physiol. 66, 23.1–23.30 [DOI] [PubMed] [Google Scholar]
- Crocker D. E., Le Boeuf B. J., Costa D. P.1997Drift diving in female northern elephant seals: implications for food processing. Can. J. Zool. 75, 27–39 (doi:10.1139/z97-004) [Google Scholar]
- Croll D. A., Acevedo-Gutiérrez A., Tershy B., Urbán-Ramírez J.2001The diving behavior of blue and fin whales: is dive duration shorter than expected based on oxygen stores? Comp. Biochem. Physiol. A 129, 797–809 (doi:10.1016/S1095-6433(01)00348-8) [DOI] [PubMed] [Google Scholar]
- Diamond J. A.1985Filter-feeding on a grand scale. Nature 316, 679–680 (doi:10.1038/316679a0) [Google Scholar]
- Fish F. E.2002Speed. In Encyclopaedia of marine mammals (eds Perrin W. F., Würsig B., Thewissen J. G. M.), pp. 1161–1163 San Diego, CA: Academic Press [Google Scholar]
- Fish F. E., Peacock J. E., Rohr J. J.2003Stabilization mechanism in swimming odontocete cetaceans by phased movements. Mar. Mamm. Sci. 19, 515–528 (doi:10.1111/j.1748-7692.2003.tb01318.x) [Google Scholar]
- Fletcher S., Le Boeuf B. J., Costa D. P., Tyack P. L., Blackwell S. B.1996Onboard acoustic recording from diving northern elephant seals. J. Acoust. Soc. Am. 100, 2531–2539 (doi:10.1121/1.417361) [DOI] [PubMed] [Google Scholar]
- Gibson R. N., Ezzi I. A.2006The relative profitability of particulate- and filter-feeding in the herring, Clupea harengus L. J. Fish Biol. 40, 577–590 (doi:10.1111/j.1095-8649.1992.tb02607.x) [Google Scholar]
- Goldbogen J. A., Calambokidis J., Shadwick R. E., Oleson E. M., McDonald M. A., Hildebrand J. A.2006Kinematics of foraging dives and lunge-feeding in fin whales. J. Exp. Biol. 209, 1231–1244 (doi:10.1242/jeb.02135) [DOI] [PubMed] [Google Scholar]
- Hassrick J. L., Crocker D. E., Zeno R. L., Blackwell S. B., Costa D. P., Le Boeuf B. J.2007Swimming speed and foraging strategies of northern elephant seals. Deep Sea Res. II 54, 369–383 (doi:10.1016/j.dsr2.2006.12.001) [Google Scholar]
- Heide-Jørgensen M. P., Laidre K. L., Jensen M. V., Dueck L., Postma L. D.2006Dissolving stock discreteness with satellite tracking: bowhead whales in Baffin Bay. Mar. Mamm. Sci. 22, 34–45 [Google Scholar]
- Hind A. T., Gurney W. S. C.1997The metabolic cost of swimming in marine homeotherms. J. Exp. Biol. 200, 531–542 [DOI] [PubMed] [Google Scholar]
- Johnson M. P., Tyack P. L.2003A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean Eng. 28, 2–12 (doi:10.1109/JOE.2002.808212) [Google Scholar]
- Kooyman G. L., Wahrenbrock E. A., Castellini M. A., Davis R. W., Sinnett E. E.1980Aerobic and anaerobic metabolism during voluntary diving in Weddel seals: evidence of preferred pathways from blood chemistry and behavior. J. Comp. Physiol. 138, 335–346 [Google Scholar]
- Kramer D. L.1988The behavioural ecology of air breathing by aquatic animals. Can. J. Zool. 66, 89–94 (doi:10.1139/z88-012) [Google Scholar]
- Laidre K. L., Heide-Jørgensen M. P., Nielsen T. G.2007Role of the bowhead whale as a predator in West Greenland. Mar. Ecol. Prog. Ser. 346, 285–297 (doi:10.3354/meps06995) [Google Scholar]
- Lambertsen R. H., Rasmussen K. J., Lancaster W. C., Hintz R. J.2005Functional morphology of the mouth of the bowhead whale and its implications for conservation. J. Mamm. 86, 342–352 (doi:10.1644/BER-123.1) [Google Scholar]
- Mayo C. A., Goldman L.1992Right whale foraging and the plankton resources in Cape Cod and Massachusetts Bays. In The right whale in the western North Atlantic: a science and management workshop (ed. Hain J.), pp. 43–44 Northeast Fish Sci Cent Ref Doc. 92-05 [Google Scholar]
- Mayo C. A., Marx M. K.1990Surface foraging behaviour of the North Atlantic right whale, Eubalaena glacialis, and associated zooplankton characteristics. Can. J. Zool. 68, 2214–2220 (doi:10.1139/z90-308) [Google Scholar]
- Mayo C. A., Letcher B. H., Scott S.2001Zooplankton filtering efficiency of the baleen of a North Atlantic right whale, Eubalaena glacialis. J. Cetacean Res. Manag. Special Issue 2, 225–229 [Google Scholar]
- Miller P. J. O., Johnson M. P., Tyack P. L., Terray E. A.2004Swimming gaits, passive drag and buoyancy of diving sperm whales Physeter macrocephalus. J. Exp. Biol. 207, 1953–1967 (doi:10.1242/jeb.00993) [DOI] [PubMed] [Google Scholar]
- Nowacek D. P., Johnson M., Tyack P., Shorter K. A., McLellan W. A., Pabst D. A.2001Buoyant balaenids: the ups and downs of buoyancy in right whales. Proc. R. Soc. Lond. B 268, 1811–1816 (doi:10.1098/rspb.2001.1730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivorunas A.1979The feeding mechanisms of baleen whales. Am. Sci. 67, 432–440 [Google Scholar]
- Ropert-Coudert Y., Kato A., Wilson R. P., Cannell B.2006Foraging strategies and prey encounter rate in free-ranging Little Penguins. Mar. Biol. 149, 139–148 (doi:10.1007/s00227-005-0188-x) [Google Scholar]
- Sanderson S. L., Wassersug R.1990Suspension-feeding vertebrates. Sci. Am. 262, 96–101 [Google Scholar]
- Sato K., Mitani Y., Cameron M. F., Siniff D. B., Naito Y.2003Factors affecting stroking patterns and body angle in diving Weddell seals under natural conditions. J. Exp. Biol. 206, 1461–1470 (doi:10.1242/jeb.00265) [DOI] [PubMed] [Google Scholar]
- Sato K., et al. 2007Stroke frequency, but not swimming speed, is related to body size in free-ranging seabirds, pinnipeds and cetaceans. Proc. R. Soc. B 274, 471–477 (doi:10.1098/rspb.2006.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims D. W.1999Threshold foraging behaviour of basking sharks on zooplankton: life on an energetic knife-edge? Proc. R. Soc. Lond. B 266, 1437–1443 (doi:10.1098/rspb.1999.0798) [Google Scholar]
- Sims D. W., Quayle V. A.1998Selective foraging behaviour of basking sharks on zooplanton in a small-scale front. Nature 393, 460–464 (doi:10.1038/30959) [Google Scholar]
- Vogel S.1994Life in moving fluids. The physical biology of flow Princeton, NJ: Princeton University Press [Google Scholar]
- Watanuki Y., Niizuma Y., Gabrielsen G. W., Sato K., Niato Y.2003Stroke and glide of wing-propelled divers: deep diving seabirds adjust surge frequency to buoyancy change with depth. Proc. R. Soc. Lond. B 270, 483–488 (doi:10.1098/rspb.2002.2252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins W. A., Schevill W. E.1976Right whale feeding and baleen rattle. J. Mamm. 57, 58–66 (doi:10.2307/1379512) [Google Scholar]
- Watwood S. L., Miller P. J. O., Johnson M., Madsen P. T., Tyack P.2006Deep-diving foraging behaviour of sperm whales (Physeter macrocephalus). J. Anim. Ecol. 75, 814–825 (doi:10.1111/j.1365-2656.2006.01101.x) [DOI] [PubMed] [Google Scholar]
- Werth A. J.2001How do mysticetes remove prey trapped in baleen? Bull. Mus. Comp. Zool. 156, 189–203 [Google Scholar]
- Werth A. J.2004Models of hydrodynamic flow in the bowhead whale filter feeding apparatus. J. Exp. Biol. 207, 3569–3580 (doi:10.1242/jeb.01202) [DOI] [PubMed] [Google Scholar]
- Williams T. M.1999The evolution of cost efficient swimming in marine mammals: limits to energetic optimization. Phil. Trans. R. Soc. Lond. B 354, 193 (doi:10.1098/rstb.1999.0371) [Google Scholar]
- Williams T. L.2001Intermittent swimming by marine mammals: a strategy for increasing energetic efficiency during diving. Am. Zool. 41, 166–176 (doi:10.1668/0003-1569(2001)041[0166:ISBMAS]2.0.CO;2) [Google Scholar]
- Williams T. M., Davis R. W., Fuiman L. A., Francis J., Le Boeuf B. J., Horning M., Calambokidis J., Croll D. A.2000Sink or swim: strategies for cost-efficient diving by marine mammals. Science 288, 133–136 (doi:10.1126/science.288.5463.133) [DOI] [PubMed] [Google Scholar]
- Wilson R. P., Ropert-Coudert Y., Kato A.2002Rush and grab strategies in foraging marine endotherms: the case for haste in penguins. Anim. Behav. 63, 85–95 (doi:10.1006/anbe.2001.1883) [Google Scholar]
- Wong C. K.1988The swimming behavior of the copepod Metridia pacifica. J. Plankt. Res. 10, 1285–1290 (doi:10.1093/plankt/10.6.1285) [Google Scholar]
- Woodward B. L., Winn J. P., Fish F. E.2006Morphological specializations of baleen whales according to ecological niche. J. Morphol. 267, 1284–1294 (doi:10.1002/jmor.10474) [DOI] [PubMed] [Google Scholar]
- Zimmer W. M. X., Johnson M., Madsen P. T., Tyack P.2005Echolocation clicks of free-ranging Cuvier's beaked whales (Ziphius cavirostris). J. Acoust. Soc. Am. 117, 3919–3927 (doi:10.1121/1.1910225) [DOI] [PubMed] [Google Scholar]