Abstract
Lizards commonly climb in complex three-dimensional habitats, and gekkotans are particularly adept at doing this by using an intricate adhesive system involving setae on the ventral surface of their digits. However, it is not clear whether geckos always deploy their adhesive system, given that doing so may result in decreased (i.e. reduction in speed) locomotor performance. Here, we investigate circumstances under which the adhesive apparatus of clinging geckos becomes operative, and examine the potential trade-offs between speed and clinging. We quantify locomotor kinematics of a gecko with adhesive capabilities (Tarentola mauritanica) and one without (Eublepharis macularius). Whereas, somewhat unusually, E. macularius did not suffer a decrease in locomotor performance with an increase in incline, T. mauritanica exhibited a significant decrease in speed between the level and a 10° incline. We demonstrate that this results from the combined influence of slope and the deployment of the adhesive system. All individuals kept their digits hyperextended on the level, but three of the six individuals deployed their adhesive system on the 10° incline, and they exhibited the greatest decrease in velocity. The deployment of the adhesive system was dependent on incline, not surface texture (600 grit sandpaper and Plexiglas), despite slippage occurring on the level Plexiglas substrate. Our results highlight the type of sensory feedback (gravity) necessary for deployment of the adhesive system, and the trade-offs associated with adhesion.
Keywords: locomotion, Gekkota, Tarentola, Eublepharis, climbing, adhesion
1. Introduction
Among vertebrates, lizards are incredibly adept at moving through complex three-dimensional habitats (Higham et al. 2001; Higham & Jayne 2004; Mattingly & Jayne 2004). Whereas many lizards employ claws or prehensile feet to cling to the substrate, geckos are noteworthy among relatively large animals (body mass range 1–50 g) in being able to temporarily and reversibly bond with substrata (Dellit 1934; Hiller 1968; Maderson 1970; Autumn et al. 2000) that range from the molecularly smooth (Autumn et al. 2002) to the macroscopically rough (Russell & Johnson 2007). They do so by employing microscopic integumentary outgrowths (setae) on the ventral surfaces of the digits (Russell 2002) that are controlled by a hierarchy of anatomical components (Russell 1975, 2002). Adhesion occurs through the creation of high attachment forces generated via a combination of Van der Waals bonding (Autumn et al. 2000) and shear-based friction (Autumn et al. 2006a; Tian et al. 2006). The deployment of the adhesive system is integrated with locomotor kinematics, allowing for controllable attachment and release (Autumn et al. 2006b).
Recent investigations (Autumn et al. 2006b; Chen et al. 2006) demonstrated that pad-bearing geckos exhibit major energetic differences when moving on horizontal versus vertical surfaces. Potentially implicated in these changes is the deployment of the adhesive system and the concomitant clinging that is enabled. In vertical locomotion, Autumn et al. (2006b) reported that the digits of Hemidactylus unfurled onto the locomotor surface subsequent to heel strike and were hyperextended, effecting release of the adhesive system, prior to any heel movement. The time taken to attach and detach was rapid (5 ± 2 and 15 ± 4 ms, respectively) and attachment occupied 12.7 per cent of stance time (Autumn et al. 2006b). Digital action in the same species during horizontal running was, however, not reported (Chen et al. 2006). Differences in locomotor energetics on horizontal and vertical surfaces (Autumn et al. 2006b; Chen et al. 2006) are suggestive of a trade-off in locomotor performance in the transition from level running to climbing. This may be related to the deployment of the adhesive system in climbing and the additional level of control this necessitates.
To explore these hypotheses, we investigated circumstances under which the adhesive apparatus of clinging geckos becomes operative, and examined the potential trade-offs between speed and clinging. To do this, we quantified aspects of locomotor kinematics of a clinging gecko (Tarentola mauritanica) and a primitively padless gekkotan (Eublepharis macularius) moving on horizontal and inclined surfaces that provided either good or minimal traction. We did this to ascertain whether mechanical grip or the perception of changes in the vector of gravitational loading is primarily responsible for initiating the use of the adhesive system. Furthermore, we determined how incline running is accommodated by a clinging and a non-clinging gekkotan on surfaces that provide different degrees of traction.
2. Material and methods
(a). Animals
Six Moorish geckos (T. mauritanica—average mass 8.0 ± 0.8 g and snout-vent length 57.8 ± 2.7 mm) and five juvenile leopard geckos (E. macularius—average mass 5.2 ± 0.4 g, snout-vent length 61.0 ± 0.7 mm) were obtained from a commercial supplier.
(b). Experimental protocol
We used a 1 m long, 10 cm wide wooden trackway, on the surface of which was mounted 600 grit aluminium oxide sandpaper or Plexiglas to provide a high-friction and smooth (presumed low-friction) surface, respectively. A single high-speed video camera (Photron APX-RS) was used to capture the movements of the lizards at 500 fps (1024 × 1024 pixels). The camera was oriented lateral to the trackway, and a mirror mounted at a 45° angle above the trackway provided a dorsal view of the lizard. The trackway was initially placed horizontally (0° of inclination), and was then raised to 10 and 30° of inclination for subsequent trials.
(c). Data analysis
The tip of the snout was digitized using DLTdataViewer3 (Hedrick 2008) in MatLab in order to obtain displacement. Velocity was calculated as the derivative of the displacement data. The extremely low noise in the velocity data obviated the need for smoothing. Comparisons between species and conditions were assessed using parametric t-tests and analyses of variance (the data were normally distributed).
3. Results and discussion
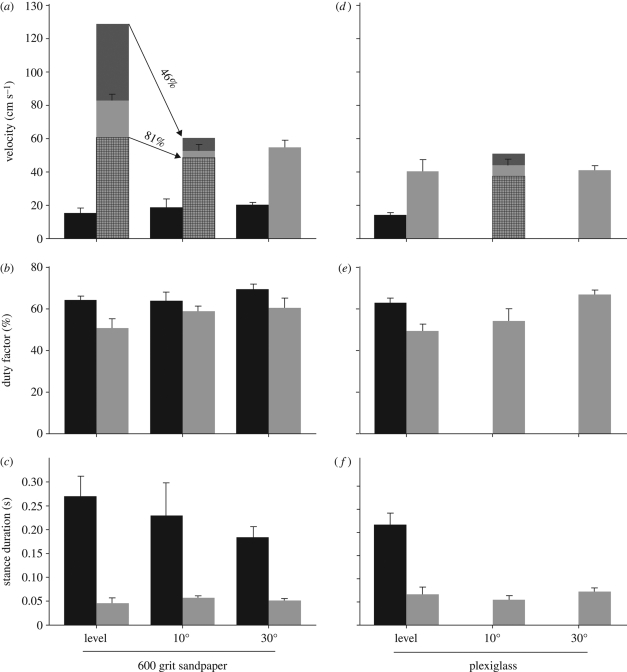
When running horizontally on the high-friction surface (600 grit aluminium oxide sandpaper), T. mauritanica averaged 80.0 ± 10.0 cm s−1 (figure 1a), a velocity that compares favourably with that of other pad-bearing geckos on horizontal surfaces (60–180 cm s−1 for Gekko gecko (Zaaf et al. 2001a,b)), but is considerably slower than the speeds of fast runners such as Callisaurus draconoides (354 ± 17.0 to 420 ± 10.0 cm s−1), Uma scoparia (390 ± 10.0 to 400 ± 20.0 cm s−1) and Aspidoscelis tigris (370 ± 5.0 cm s−1) (Irschick & Jayne 1998, 1999; Jayne & Ellis 1998). The speed of T. mauritanica, a pad-bearing gecko, was significantly faster, however, than that of the primitively padless and slow-moving Leopard gecko (E. macularius) traversing the same trackway (average velocity 16.0 ± 2.0 cm s−1; figure 1a). High-speed videography revealed that T. mauritanica achieved these velocities running with the subdigital pads held in a permanently hyperextended configuration. This posture noticeably foreshortens the portion of the foot able to transmit thrust to the substratum and is contributory to the smaller duty factor in this species (figure 1b) when compared with Eublepharis moving on level terrain (50.8 ± 4 versus 64.3 ± 2%). The duty factor for Hemidactylus garnotii, another pad-bearing gecko, running on level terrain was approximately 44 per cent (Chen et al. 2006). It is not known if the digits of Hemidactylus were held in a permanently hyperextended state (but it is likely that they were). Stance duration for Tarentola and Eublepharis (figure 1c) on level terrain is directly reflective of the difference in velocity achieved by these two taxa.
Figure 1.
The effects of incline and surface structure on gecko locomotion. (a,d) Mean velocity of E. macularius (black bars) and T. mauritanica (grey bars) running on a level, 10 and 30° surface covered with (a) 600 grit sandpaper and (d) Plexiglas. Note that for (a) and (d), dark grey bars represent the mean velocities for individuals of Tarentola that used their adhesive apparatus on the 10° incline, and cross-hatched bars represent the same for those that did not. Forty-six and 81 per cent refer to the velocities attained by those individuals of Tarentola that, respectively, employed and did not employ their adhesive apparatus on the 10° high-friction slope, expressed as a proportion of their velocities on the horizontal high-friction surface. (b,e) Mean duty factor for E. macularius (black bars) and T. mauritanica (grey bars) running on a level, 10 and 30° surface covered with (b) 600 grit sandpaper and (e) Plexiglas. (c,f) Mean stance duration for E. macularius (black bars) and T. mauritanica (grey bars) running on a level, 10 and 30° surface covered with (c) 600 grit sandpaper and (f) Plexiglas. All values are mean ± s.e. Note that E. macularius was unable to move on the inclined surfaces covered with Plexiglas.
When Tarentola and Eublepharis were run on the smooth surface (Plexiglas) positioned horizontally, both incurred severe slippage of the feet, especially so for the hind limbs and most evidently at push-off in the transition from stance to swing. Forward progress was severely hampered. We confirmed that Tarentola was able to cling to this Plexiglas sheet when oriented vertically. Thus, the lack of traction in running is accounted for by the failure to deploy the adhesive system and not by an inability to cling. Data for Tarentola and Eublepharis moving on the smooth horizontal surface indicate that velocity decreased by 51 and 7 per cent, respectively (figure 1d), but duty factor and stance duration did not change significantly (figure 1e,f). Reduced velocity is explained by slippage, not gait characteristics. Lack of traction was not a sufficient stimulus, however, to trigger deployment of the adhesive system in Tarentola.
When challenged to move on a slope of 10°, the average velocity of Eublepharis remained relatively unchanged on the high-friction surface (figure 1a), whereas the average velocity of Tarentola fell markedly to 63 per cent of that attained on the same surface in a horizontal orientation (figure 1a). Of significance in our observations was the fact that three of the six Tarentola deployed their adhesive system. Those that did so exhibited a much greater decrease in velocity (achieving only 46% of the speed attained on the level surface—figure 1a, dark grey bars) than those that did not (attaining 81% of the speed on the level surface—figure 1a, cross-hatched bars), highlighting the additional cost (i.e. decreased locomotor speed) associated with the activation of the adhesive system. The individuals that did not deploy the adhesive system exhibited a similar value for the decline in performance as that for the more rapid (compared with E. macularius) eublepharid, Coleonyx brevis (Farley 1997), on a similar incline (10 versus 20°). Those that did deploy the adhesive apparatus exhibited a much more drastic decline in performance.
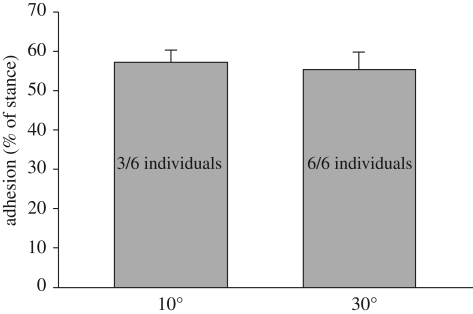
Eublepharis was not able to move on the smooth surface inclined at 10° (figure 1d–f). Tarentola was much less impacted, and those individuals that employed their adhesive apparatus performed better than those that did not (average velocity 51.5 versus 38.4 cm s−1) (figure 1d). This clearly demonstrates that deploying the adhesive system enhances locomotor ability on surfaces that furnish less traction (velocity achieved being 131% of that attained on the horizontally oriented smooth surface—figure 1d). The individuals that did not deploy the adhesive system achieved a similar velocity (91%) to that on the horizontal smooth surface (figure 1d). All individuals deployed their adhesive system on the 30° incline (figure 1d). Duty factor (figure 1b,e) shows an increase for Tarentola in relation to increasing slope. This is more pronounced for the smooth surface (figure 1e). Stance duration (figure 1c,f) remained essentially unchanged for Tarentola regardless of surface type or inclination, indicating a temporally consistent pattern for foot placement and release. This provides a stable interval into which the deployment of the adhesive apparatus can be inserted when operative. Further illustration of this is provided by the unchanging percentage of stance occupied by the adhesive phase of the cycle when the subdigital pads are deployed (figure 2).
Figure 2.
Effects of incline on duration of adhesion. Mean adhesion time (% of stance) for individuals running on a 10 and 30° incline lined with 600 grit sandpaper. Surface structure did not influence these values. Values are mean ± s.e. Note that only three of the six individuals deployed their adhesive system on the 10° incline.
Our results reveal that the adhesive apparatus is activated on inclined surfaces, but not on smooth surfaces that are not inclined, even though Tarentola experienced significant amounts of slippage of the feet in such situations, resulting in greatly reduced sprinting velocity. At an incline of 10°, 50 per cent of the Tarentola engaged their adhesive apparatus on both the high-friction and smooth surfaces, indicating that such an angle lies close to the threshold for the perception of gravitational effects that differ from those experienced in horizontal running. This probably provides the feedback that initiates a differential neural response, resulting in the unfurling of the digits, and the associated generation of the perpendicular pre-load necessary for initiating adhesive contact of the setae (Autumn et al. 2000). Active (using the muscular and tendinous systems of the digits) (Russell 2002) and passive (employing gravitational loading along preferentially aligned digits) (Russell et al. 1997) loading is then potentially available to induce the parallel pre-load that fully exploits shear-based friction interactions (Autumn et al. 2006a; Tian et al. 2006) of the setae.
These observations are significant because on the high-friction surface Eublepharis suffered no decrease in velocity and showed no appreciable change in duty factor or stance duration when scaling a 10° incline. Thus, the switch of behaviour in Tarentola cannot be attributed to a decreased ability to achieve traction on such a combination of surface and incline, and indicates that the response is neurally programmed via feedback circuits. The shift in the pattern of digital usage in Tarentola was accompanied by a change in body posture, with the individuals keeping the body much closer to the substratum (hip height at the end of stance on the level: 1.4 cm; 10°: 1.2 cm; 30°: 0.9 cm) and the limbs adopting a more evident lateral sprawling posture. Change in body posture was much less evident for Eublepharis (see also Zaaf et al. 2001b) (hip height at the end of stance on the level: 1.2 cm; 10°: 1.3 cm; 30°: 1.2 cm). The marked decline in the velocity of Tarentola is associated with these postural changes, the added locomotor demand of controlling the attachment and detachment of the adhesive apparatus and the regulation of the fixed time of contact of the subdigital pads.
On the high-friction surface, Eublepharis maintained a similar velocity on the 30° incline as it did at 10°, indicating that it had not reached the maximum output of its locomotor apparatus (Farley 1997), but exhibited an increased duty factor and a decreased stance duration, indicative of shorter strides being taken. Eublepharis was unable to scale the smooth surface inclined at 10 or 30°.
Wassersug et al. (2005) noted that unlike most other squamates, geckos (including primitively padless forms such as Eublepharis) adopt a sky-diving posture in microgravity situations rather than thrashing wildly to seek contact with a surface. This indicates that geckos perceive gravitational loading (or the lack of it) differently from other squamates, a contention reinforced by the observations of Jusufi et al. (2008), and that their locomotor apparatus (both anatomical and neurological) reacts in a unique fashion. Such perceptive mechanisms appear to be involved in the observations reported here, whereby the adhesive apparatus is triggered to be deployed through proprioceptive feedback rather than through perceived physical tractive interaction between the feet and the substratum. These findings have implications not only for the patterns of deployment of the clinging apparatus of geckos as they scale surfaces of a variety of inclinations, but also for why the adhesive apparatus may become reduced in relation to secondary terrestriality (Johnson et al. 2005; Lamb & Bauer 2006). Carriage of the digits in a permanently hyperextended state while moving on horizontal surfaces has led to a distal displacement of the adhesive apparatus (Russell 1976), resulting in longer proximal portions of the digits being able to contribute to performance in horizontal sprinting (Bauer et al. 1996; Johnson & Russell 2009). In extreme cases, this has resulted in complete reduction of the external manifestation of the adhesive apparatus (Russell 1976).
Recognition that sensory feedback is crucial to the deployment of the clinging apparatus provides new impetus for understanding the neural control of gekkotan adhesion. A switching point at approximately 10° of slope seems to trigger this locomotor transition. At any angle other than 0°, gravitational loading will be perceived differently by the vestibular apparatus and the digits will be physically loaded along their long axes if aligned with the pull of gravity. Up to an inclination of 90°, this gravitationally mediated stimulus will be effective. There is much yet to be learned about how geckos control neuromuscular output in relation to changing sensory feedback conditions.
Acknowledgements
This work was conducted in accordance with the University of Calgary Animal Use Protocol BI 2008-24 (Evolutionary Morphology of the Gekkotan Adhesive System) issued to A.R. and with the Clemson University Institutional Animal Care and Use Committee Protocol AUP2008-059 issued to T.H.
Financial support came from an NSERC Discovery grant to A.P.R. and start-up funds from Clemson University to T.E.H.
References
- Autumn K., Liang Y. A., Hsieh S. T., Zesch W., Chan W. P., Kenny T. W., Fearing R., Full R. J.2000Adhesive force of a single gecko foot-hair. Nature (Lond.) 405, 681–685 (doi:10.1038/35015073) [DOI] [PubMed] [Google Scholar]
- Autumn K., et al. 2002Evidence for Van der Waals adhesion in gecko setae. Proc. Natl Acad. Sci. USA 99, 12 252–12 256 (doi:10.1073/pnas.192252799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autumn K., Dittmore A., Santos D., Spenko M., Cutkosky M.2006aFrictional adhesion: a new angle on gecko attachment. J. Exp. Biol. 209, 3569–3579 (doi:10.1242/jeb12486) [DOI] [PubMed] [Google Scholar]
- Autumn K., Hsieh S. T., Dudek D. M., Chen J., Chitaphan C., Full R. J.2006bDynamics of geckos running vertically. J. Exp. Biol. 209, 260–272 (doi:10.1242/jeb01980) [DOI] [PubMed] [Google Scholar]
- Bauer A. M., Russell A. P., Powell G. L.1996The evolution of locomotor morphology in Rhoptropus (Squamata : Gekkonidae): functional and phylogenetic considerations. Afr. J. Herpetol. 45, 8–30 [Google Scholar]
- Chen J. J., Peattie A. M., Autumn K., Full R. J.2006Differential leg function in a sprawled-posture quadrupedal trotter. J. Exp. Biol. 209, 249–259 (doi:10.1242/jeb.01979) [DOI] [PubMed] [Google Scholar]
- Dellit F.1934Zur Anatomie und Physiologie der Geckozehe. Jena Z. Naturwiss. 68, 613–656 [Google Scholar]
- Farley C. T.1997Maximum speed and mechanical power output in lizards. J. Exp. Biol. 200, 2189–2195 [DOI] [PubMed] [Google Scholar]
- Hedrick T. L.2008Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinsp. Biomim. 3, 034001 (doi:10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- Higham T. E., Jayne B. C.2004Locomotion of lizards on inclines and perches: hindlimb kinematics of an arboreal specialist and a terrestrial generalist. J. Exp. Biol. 207, 233–248 (doi:10.1242/jeb.00763) [DOI] [PubMed] [Google Scholar]
- Higham T. E., Davenport M. S., Jayne B. C.2001Maneuvering in an arboreal habitat: the effects of turning angle on the locomotion of three sympatric ecomorphs of Anolis lizards. J. Exp. Biol. 204, 4141–4155 [DOI] [PubMed] [Google Scholar]
- Hiller U.1968Untersuchungen zum feinbau und zur function der haftborsten von reptilien. Morph. Tiere 62, 307–362 (doi:10.1007/BF00401561) [Google Scholar]
- Irschick D. J., Jayne B. C.1998Effects of incline on speed, acceleration, body posture and hindlimb kinematics in two species of lizard, Callisaurus draconoides and Uma scoparia. J. Exp. Biol. 201, 273–287 [DOI] [PubMed] [Google Scholar]
- Irschick D. J., Jayne B. C.1999Comparative three-dimensional kinematics of the hind limbs for high-speed bipedal and quadrupedal locomotion of lizards. J. Exp. Biol. 202, 1047–1065 [DOI] [PubMed] [Google Scholar]
- Jayne B. C., Ellis R. V.1998How inclines affect the escape behaviour of a dune dwelling lizard, Uma scoparia. Anim. Behav. 55, 1115–1130 (doi:10.1006/anbe.1997.0655) [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Russell A. P.2009Configuration of the setal fields of Rhoptropus (Gekkota:Gekkonidae): functional, evolutionary, ecological and phylogenetic implications of observed pattern. J. Anat. 214b, 937–955 (doi:10.1111/j1469-7580.2009.01075.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Russell A. P., Bauer A. M.2005Locomotor morphometry of the Pachydactylus radiation of lizards (Gekkota:Gekkonidae): a phylogenetically and ecologically informed analysis. Can. J. Zool. 83, 1511–1524 (doi:10.1139/z05-112) [Google Scholar]
- Jusufi A., Goldman D. I., Revzen S., Full R. J.2008Active tails enhance arboreal acrobatics in geckos. Proc. Natl Acad. Sci. USA 105, 4125–4129 (doi:10.1073/pnas.0711944105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T., Bauer A. M.2006Footprints in the sand: independent reduction of subdigital lamellae in the Namib-Kalahari burrowing geckos. Proc. R. Soc. B 273, 855–864 (doi:10.1098/rspb.2005.3390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderson P. F. A.1970Lizard hands and lizard glands: models for evolutionary study. Forma et Functio 3, 179–204 [Google Scholar]
- Mattingly W. B., Jayne B. C.2004Resource use in arboreal habitats: structure affects locomotion of four ecomorphs of Anolis lizards. Ecology 85, 1111–1124 (doi:10.1890/03-0293) [Google Scholar]
- Russell A. P.1975A contribution to the functional analysis of the foot of the Tokay, Gekko gecko (Reptilia : Gekkonidae). J. Zool. Lond. 176, 437–476 [Google Scholar]
- Russell A. P.1976Some comments concerning interrelationships amongst gekkonine geckos. In Morphology and biology of reptiles (eds d'A. Bellairs A., Cox C. B.), Linnean Society Symposium Series 3, pp. 217–244 London, UK: Academic Press [Google Scholar]
- Russell A. P.2002Integrative functional morphology of the gekkotan adhesive system (Reptilia:Gekkota). Integr. Comp. Biol. 42, 1154–1163 (doi:10.1093/icb/42.6.1154) [DOI] [PubMed] [Google Scholar]
- Russell A. P., Johnson M. K.2007Real world challenges to, and capabilities of, the gekkotan adhesive system: contrasting the rough and the smooth. Can. J. Zool. 85, 1228–1238 (doi:10.1139/207-103) [Google Scholar]
- Russell A. P., Bauer A. M., Laroyia R.1997Morphological correlates of the secondarily symmetrical pes of gekkotan lizards. J. Zool. Lond. 241, 767–790 (doi:10.1111/j.1469-7998.1997.tb05747.x) [Google Scholar]
- Tian Y., Pesika N., Zeng H., Rosenberg K., Zhao B., McGuiggan P., Autumn K.2006Adhesion and friction in gecko toe attachment and detachment. Proc. Natl Acad. Sci. USA 103, 19 320–19 325 (doi:10.1073/pnas.0608841103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassersug R. J., Roberts L., Gimian J., Hughes E., Saunders R., Devison D., Woodbury J., O'Reilly J. C.2005The behavioral responses of amphibians and reptiles to microgravity on parabolic flights. Zoology 108, 107–120 (doi:10.1016/jzool.2005.03.001) [DOI] [PubMed] [Google Scholar]
- Zaaf A., Van Damme R., Herrel A., Aerts P.2001aLimb joint kinematics during vertical climbing and level running in a specialist climber: Gekko gecko Linnaeus 1758 (Lacertilia:Gekkonidae). Belg. J. Zool. 131, 173–182 [Google Scholar]
- Zaaf A., Van Damme R., Herrel A., Aerts P.2001bSpatio-temporal gait characteristics of level and vertical locomotion in a ground-dwelling and a climbing gecko. J. Exp. Biol. 204, 1233–1246 [DOI] [PubMed] [Google Scholar]