Abstract
Despite the advent of modern molecular and computational methods, the phylogeny of the four major arthropod groups (Chelicerata, Myriapoda, Crustacea and Hexapoda, including the insects) remains enigmatic. One particular challenge is the position of myriapods as either the closest relatives to chelicerates (Paradoxopoda/Myriochelata hypothesis), or to crustaceans and hexapods (Mandibulata hypothesis). While neither hypothesis receives conclusive support from molecular analyses, most morphological studies favour the Mandibulata concept, with the mandible being the most prominent feature of this group. Although no morphological evidence was initially available to support the Paradoxopoda hypothesis, a putative synapomorphy of chelicerates and myriapods has recently been put forward based on studies of neurogenesis. However, this and other morphological characters remain of limited use for phylogenetic systematics owing to the lack of data from an appropriate outgroup. Here, we show that several embryonic characters are synapomorphies uniting the chelicerates and myriapods, as revealed by an outgroup comparison with the Onychophora or velvet worms. Our findings, thus provide, to our knowledge, first morphological/embryological support for the monophyly of the Paradoxopoda and suggest that the mandible might have evolved twice within the arthropods.
Keywords: arthropods, Onychophora, neurogenesis, neuroblast, synapomorphy, phylogeny
1. Introduction
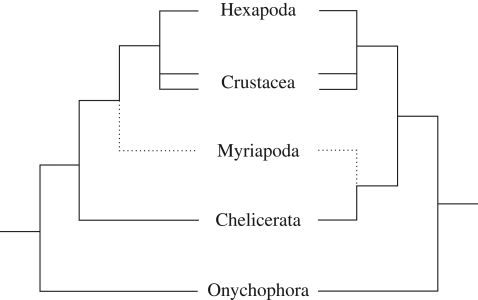
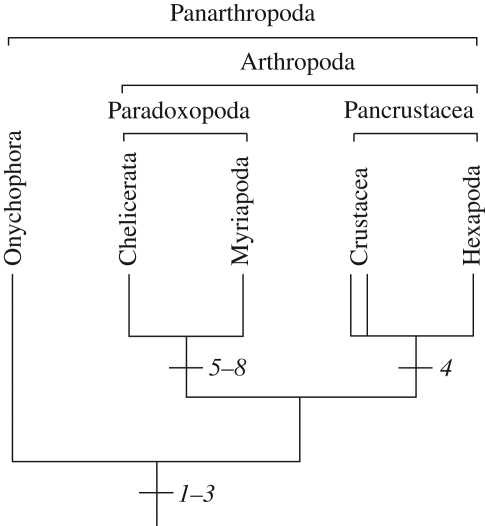
Historically, myriapods have been affiliated with virtually every major arthropod group, but only two competing hypotheses for their phylogenetic position currently remain in favour (figure 1). Most morphological, combined, and some molecular, studies unite myriapods with crustaceans and hexapods within the Mandibulata (Scholtz et al. 1998; Edgecombe et al. 2000; Giribet et al. 2001, 2005; Nielsen 2001; Kusche et al. 2002, 2003; Edgecombe 2004; Harzsch et al. 2005; Scholtz & Edgecombe 2005, 2006; Bourlat et al. 2008; Regier et al. 2008; Rota-Stabelli & Telford 2008). However, there is also strong molecular evidence for joining myriapods with chelicerates in a group dubbed Myriochelata or Paradoxopoda, the latter name reflecting the presumed absence of morphological evidence supporting this clade (Friedrich & Tautz 1995; Giribet et al. 1996; Cook et al. 2001; Hwang et al. 2001; Kusche & Burmester 2001; Mallatt et al. 2004; Negrisolo et al. 2004; Pisani et al. 2004; Hassanin 2006; Mallatt & Giribet 2006; Dunn et al. 2008; Gai et al. 2008; Podsiadlowski et al. 2008; Breugelmans et al. 2009). In fact, similarities have been recorded in chelicerate and myriapod development (Dove & Stollewerk 2003; Kadner & Stollewerk 2004; Stollewerk & Chipman 2006; McGregor et al. 2008). However, these similarities do not by themselves clarify the controversial position of Myriapoda because they might be symplesiomorphies (shared ancestral traits) rather than synapomorphies (shared derived characters). Hence, studies of an outgroup are necessary to polarize derived versus ancestral character states among the arthropods.
Figure 1.
Competing hypotheses on the relationship of panarthropods. The tree on the left represents the traditional Mandibulata hypothesis, with myriapods as sister group to crustaceans and hexapods. The tree on the right illustrates the Paradoxopoda/Myriochelata hypothesis uniting myriapods with chelicerates. The double lines for Crustacea indicate that this group might not be monophyletic.
Onychophora are the most promising candidates because they are the closest relatives of arthropods (Kusche et al. 2002; Mallatt & Giribet 2006; Dunn et al. 2008) and have changed little since the Early Cambrian, as evidenced by their resemblance to the fossil lobopodians (Budd 2001; Maas et al. 2007). We therefore analysed the development in representatives from two distantly related onychophoran groups that diverged prior to the break-up of Gondwana 175–140 Myr ago (Upchurch 2008) and display two different modes of embryogenesis. The embryo of Euperipatoides rowelli (Peripatopsidae), an ovoviviparous Australian species is surrounded by egg membranes and contains yolk, whereas that of Epiperipatus isthmicola (Peripatidae), a viviparous Costa Rican species, lacks embryonic envelopes and has placental structures instead. The study of development in both groups is likely to reveal the basic features for Onychophora.
2. Material and methods
(a). Obtaining specimens
Specimens of the onychophoran species E. rowelli Reid, 1996 and Ep. isthmicola (Bouvier, 1905) were collected from rotten logs in the Tallaganda State Forest (New South Wales, Australia) and the Reserva Biologica Hitoy-Cerere (Costa Rica) as described previously (Mayer & Tait in press). Cocoons of the spider Steatoda grossa (Koch, 1838) were obtained from the Department of Genetics (University of Melbourne, Australia). Specimens of the symphylan Hanseniella agilis Tiegs, 1939 were collected in the Dandenong Ranges (Melbourne) and maintained in the laboratory. The eggs were collected at regular intervals (Tiegs 1940). Prior to fixation, the spider and symphylan eggs were dechorionated by using household bleach according to standard protocols (e.g. Kadner & Stollewerk 2004).
(b). Scanning electron microscopy
Specimens were fixed in 4 per cent formaldehyde. After dehydration in an ethanol series, the specimens were dried in a CPD 030 Critical Point Dryer (BAL-TEC AG, Balzers, Liechtenstein), coated with gold in a SCD 040 Sputter Coater (BALZERS UNION, Balzers, Liechtenstein), and examined in a Quanta 200 Scanning Electron Microscope (FEI, Hillsboro, Oregon, USA).
(c). Immunohistochemistry
Embryos were fixed overnight in 4 per cent paraformaldehyde in phosphate-buffered saline (PBS, 0.1 M, pH 7.4), rinsed in PBS and pre-incubated in 1 per cent bovine serum albumin and 0.3 per cent Triton X-100 in PBS for 3 h at room temperature. Incubations with antibodies were carried out overnight at 4°C. The following primary antibodies were used either separately or in combination diluted in PBS: (i) mouse monoclonal anti-acetylated α-tubulin IgG2b isotype (catalogue no. T6793, Sigma-Aldrich, St. Louis, MO, USA; diluted 1 : 500); (ii) rabbit polyclonal anti-phospho-histone H3 mitosis marker (α-PH3, catalogue no. 06-570, Upstate, Temecula, CA, USA; diluted 1 : 500); (iii) mouse monoclonal anti-actin, JLA20 (Developmental Studies Hybridoma Bank; diluted 1 : 10); and (iv) Cy5-conjugated goat anti-Horseradish Peroxidase (anti-HRP, catalogue no. 123-175-021, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA; diluted 1 : 100). Specimens were rinsed in several changes of PBS and incubated with secondary antibodies (Invitrogen, Carlsbad, CA, USA; each diluted 1 : 500 in PBS): (i) Alexa Fluor 488 goat anti-mouse IgG (catalogue no. A11001); or (ii) Alexa Fluor 488 goat anti-rabbit IgG (catalogue no. A11034) and/or (iii) Alexa Fluor 594 goat anti-rabbit IgG (catalogue no. A11037). Embryos were then incubated for 1 h in a solution containing phalloidin-rhodamine (catalogue no. R-415300, Molecular Probes (Invitrogen); 10 units per 100 µl PBS), after which the DNA-selective fluorescent dye Hoechst (Bisbenzimide, H33 258, catalogue no. 861405, Sigma-Aldrich; 1 µg ml−1 in PBS) was applied for 15 min. Embryos were rinsed in PBS and either mounted directly on glass slides in Vectashield Mounting Medium (catalogue no. H-1000, Vector Laboratories Inc., Burlingame, CA, USA), or dehydrated through an isopropanol series and mounted between two cover slips in a 2 : 1 mixture of benzyl benzoate and benzyl alcohol. Omission of the primary antibodies abolished staining.
(d). Confocal microscopy and image processing
Embryos were analysed with confocal laser-scanning microscopes LSM 510 META and LSM 5 PASCAL (Carl Zeiss MicroImaging GmbH, Jena, Germany). Optical sections were taken at intervals ranging from 0.18 to 11.1 µm, and the resulting image stacks were merged digitally into maximum projection micrographs with the Zeiss LSM Image Browser software (v. 4.0.0.241). Image intensity histograms were adjusted by using the Adobe (San Jose, CA, USA) Photoshop CS2. The adjustment was kept at a minimum to allow the micrographs of the same plate to have similar intensity. Final panels and diagrams were designed with an Adobe Illustrator CS2.
3. Results and discussion
(a). Comparison of early development in onychophorans and arthropods
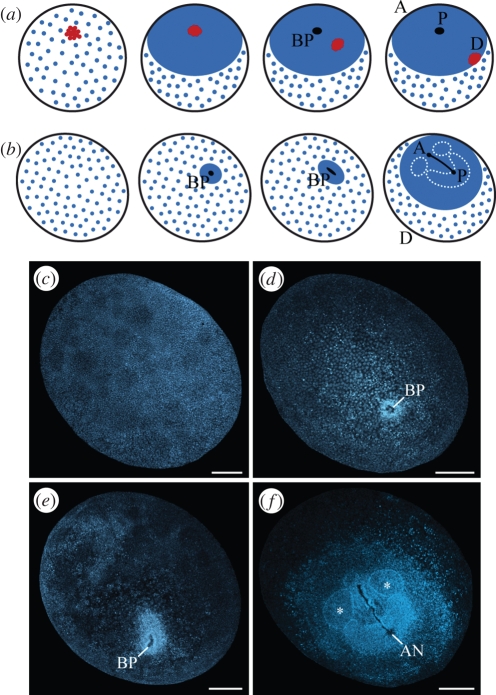
In embryos of chelicerates, the so-called cumulus (figure 2a), a conspicuous group of mesenchymal cells, initiates the breakdown of radial symmetry and leads to a dorsal split of the embryonic germ disc (Sekiguchi 1973; Chaw et al. 2007; McGregor et al. 2008). Transplantation or removal of the cumulus results in duplication or complete loss of the dorsal embryonic region (Holm 1952), while a gene expression study has revealed that the cumulus functions as an organizer that determines the dorsal region via decapentaplegic signalling (Akiyama-Oda & Oda 2003). A group of mesenchymal cells forms in an equivalent position in myriapod embryos and shows the same spatio-temporal pattern of movements as in chelicerates (Sakuma & Machida 2002, 2004; Janssen et al. 2004). However, a cumulus-like structure has never been reported in crustaceans or hexapods.
Figure 2.
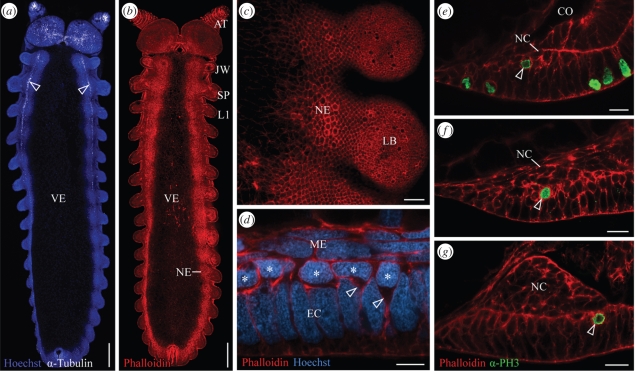
Early development in chelicerates and onychophorans. (a) Diagram of early development of spider embryos, in which a group of mesenchymal cells called the cumulus (red) arises early, migrates towards the edge of the germinal disc, and specifies the dorsal body region. Modified from McGregor et al. (2008) and Chaw et al. (2007). (b) Diagram of stages of onychophoran embryogenesis shown in (c–f), illustrating the absence of a cumulus-like structure. (c–f) Confocal images of E. rowelli embryos labelled with Hoechst. (c) Blastula. (d) Gastrula with blastopore arising in the middle of the germ disc. No cumulus-like morphological structure is seen. (e) Elongating embryo with blastopore being transformed into a slit. (f) Germ band stage embryo prior to lateral blastopore closure with anterior-most somites already established (asterisks). A, anterior; AN, anus; BP, blastopore; D, dorsal; P, posterior. Scale bars, 300 µm.
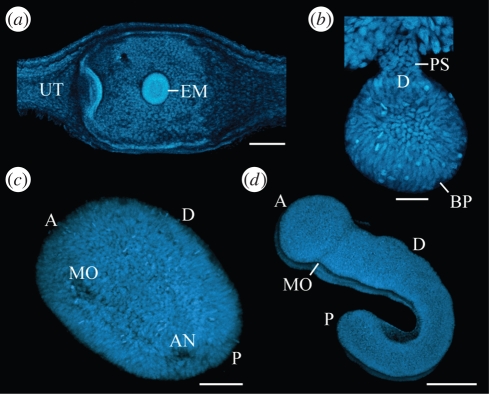
To establish whether a cumulus-like structure occurs in onychophoran development, or rather, is a derived feature of chelicerates and myriapods, we focused on early embryonic stages. In E. rowelli, we obtained embryos in the first six months of development at a blastula stage (figure 2b,c). After this stage, the embryo displays a condensed field of cells, within which the blastopore arises and transforms into a slit (figure 2d,e). The paired germ band develops at the posterior end of this slit, grows in the anterior direction and fuses in front of the future mouth, where the antennal somite is formed (figure 2b,f). The embryo elongates further and acquires a flexed posture. During this phase of development, the extra-embryonic ectoderm occupies most of the ventral and dorsal surface, but is later reduced in extent. In contrast to E. rowelli, the extra-embryonic ectoderm is hardly visible in the embryo of Ep. isthmicola, which is attached to the maternal uterus via a placental stalk, with the blastopore originating on the opposite side (figure 3a,b). While the embryo elongates, the mouth and anus separate and the embryonic segments arise in a rapid anterior to posterior progression (figure 3c,d). We did not observe a cumulus-like structure or a dorsal split of the germ disc in either of the two onychophoran species.
Figure 3.
Embryogenesis in the viviparous onychophoran Ep. isthmicola. A cumulus-like structure is not evident at any stage. Confocal micrographs of embryos stained with Hoechst. (a) Blastula stage embryo, which is implanted into the uterus wall of the mother. (b) Gastrula stage embryo attached to the uterus via a placental stalk. (c) Elongating germ band embryo with separate mouth and anus openings. (d) Elongating and segmenting embryo. A, anterior; AN, anus; BP, blastopore; D, dorsal; EM, embryo; MO, mouth; P, posterior; PS, placental stalk; UT, uterus. Scale bars, 200 µm (a,c,d) and 50 µm (b).
(b). Recruitment of single cells versus cell clusters during neurogenesis
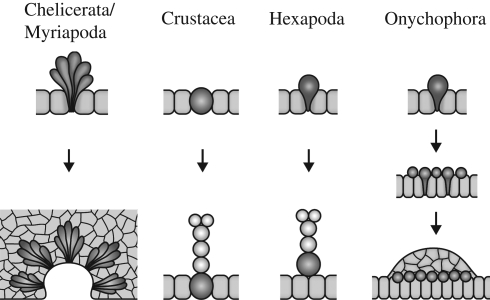
Additional similarities between chelicerates (Stollewerk et al. 2001; Mittmann 2002) and myriapods (Dove & Stollewerk 2003; Kadner & Stollewerk 2004) occur during neurogenesis. In embryos from both groups, the neuroectoderm generates numerous cell clusters with basally located cell bodies and apical cell processes that converge at the epithelial surface (figure 4). The clusters arise in a stereotypic pattern during development and finally dissociate from the ectodermal surface to give rise to neurons (Stollewerk et al. 2001; Dove & Stollewerk 2003; Kadner & Stollewerk 2004). Phalloidin-rhodamine staining of embryos of the spider S. grossa carried out in the current study reveals the same pattern of cell clusters previously reported in other chelicerates (see electronic supplementary material, figure S1a,b). In addition, we have observed similar cell clusters in the neuroectoderm of the symphylan H. agilis (see electronic supplementary material, figure S2a–c), showing that this is a widespread developmental phenomenon in the Myriapoda. In contrast to chelicerates and myriapods, cell clusters with convergent processes do not occur in the neuroectoderm of crustaceans and hexapods (Stollewerk & Simpson 2005; Ungerer & Scholtz 2008). Instead, individual cells called neuroblasts are segregated from the neuroectoderm in these animals (figure 4).
Figure 4.
Diagram of neurogenesis in arthropods and onychophorans. In chelicerates and myriapods, clusters of post-mitotic cells immigrate and the neuroectoderm invaginates in a segmental fashion (see text and figures in the electronic supplementary material S1a–c, S2a–d). In crustaceans, hexapods and onychophorans, single cells are segregated from the neuroectoderm to produce neurons by cell divisions. In (malacostracan) crustaceans (Dohle & Scholtz 1997; Ungerer & Scholtz 2008), these neural progenitor cells remain in the ectoderm.
To determine whether neuroectodermal cell clusters are an ancestral character state for the arthropods, we studied neurogenesis in Onychophora. Since cell lineage analyses cannot be performed in onychophorans, we have used a full complement of embryonic stages in two distantly related onychophoran species to obtain a detailed picture of neurogenesis. We found that despite considerable differences in development (yolky eggs versus a placenta), neurogenesis is remarkably similar in E. rowelli and Ep. isthmicola. In neither species are cell clusters evident in the neuroectoderm at any stage (figure 5a–g, see electronic supplementary material, figure S3a,b). Instead, single cells with a bottle-like shape migrate basally from the neuroectoderm, where they form a conspicuous layer of cells (figures 4, 5d, S5b, electronic supplementary material). Mitotic cell divisions are highly abundant in this cell layer, whereas cell divisions are virtually absent from the presumptive nervous tissue, i.e. the future nerve cords (figure 5e–g, see electronic supplementary material, figures S3c,d, S4a–i). Nevertheless, the nervous tissue rapidly increases in thickness and in the number of cells, which suggests that the neurons within each nerve cord originate from the conspicuous layer of dividing cells (figures 4, 5e–g). In addition, there is no apparent morphological structure apart from the ventral nerve cords, to which these dividing cells could give rise.
Figure 5.
Neurogenesis in the onychophoran E. rowelli. Confocal micrographs; anterior is up in (a–c), median is left in (c–g). (a) Embryo in ventral view showing an antero-posterior progression in neural development. At the anterior end, axonogenesis gives rise to two longitudinal tracts (=future nerve cord neuropils; arrowheads) whereas axons are not seen at the posterior end. Double-labelling with Hoechst (nuclei; blue) and an antibody raised against acetylated α-tubulin (neurotubules; white). (b) Embryo at the same developmental stage as in (a), labelled with phalloidin-rhodamine (f-actin; red). Note the absence of brightly stained dots in the neuroectoderm (NE). (c) Ventral view of the neuroectoderm from the same embryo as in (b), which does not show brightly stained dots representing convergent apical processes of immigrating cell clusters. (d) Cross-section of neuroectoderm with immigrating neuronal precursors, some of which still have a bottle-like shape (arrowheads). Note the basally displaced nuclei of neuronal precursors forming one layer (asterisks) and the columnar shape of large remaining ectodermal nuclei (EC). Double-labelling with phalloidin-rhodamine (red) and Hoechst (blue). Dorsal is up. (e–g) Further subsequent stages of neurogenesis. Optical cross-sections. Dorsal is up. After immigration, neuronal precursors divide mitotically (arrowheads) and the internal nervous tissue (NC) increases in thickness. Note the absence of cell divisions in the developing nervous tissue. Double-labelling with phalloidin-rhodamine (red) and α-PH3 antibody (prophase cells; green). AT, presumptive antenna; CO, coelomic cavity; EC, ectoderm; JW, presumptive jaw; L1, anlage of the first walking leg; LB, limb bud; ME, mesoderm; NC, presumptive nerve cord; NE, neuroectoderm; SP, presumptive slime papilla; VE, ventral extra-embryonic ectoderm. Scale bars, 300 µm (a,b), 50 µm (c), 10 µm (d) and 20 µm (e–g).
(c). Involvement of cell clusters in sensory structure formation
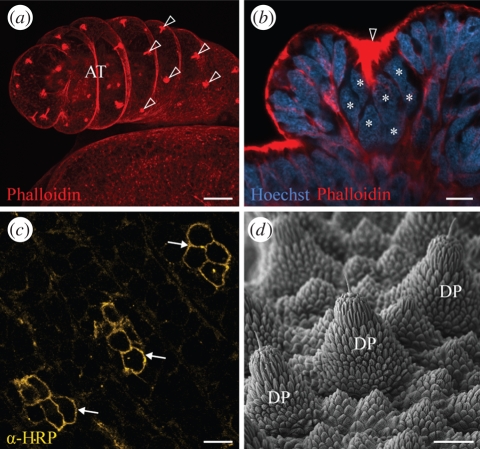
Despite the fact that cell clusters are absent from the onychophoran neuroectoderm, groups of cells morphologically resembling those in chelicerates (see electronic supplementary material, figure S1a,b; Stollewerk et al. 2001; Mittmann 2002) and myriapods (see electronic supplementary material, figure S2a–c; Kadner & Stollewerk 2004) do occur in the onychophoran embryos (figure 6a–c). The cell groups or clusters initially arise on the antennal ectoderm, but later in development cover the entire body surface, including the legs. Although the onychophoran cell clusters display the anti-HRP immunoreactivity characteristic of neurons (figure 6c), they do not generate neurons of the ventral cord, but rather, give rise to sensory structures on the body surface (figure 6d). It is of interest that in chelicerates, sensory organs also arise from cell clusters with convergent cell processes, whereas in hexapods these organs originate from single precursor cells (Stollewerk & Seyfarth 2008). Given that sensory structures originating from clusters of cells occur in embryos of chelicerates, myriapods and onychophorans, we suggest that this is an ancestral character of arthropods and, as such, should not be used to link the myriapods with chelicerates.
Figure 6.
Sensory structures and their formation in Onychophora. (a) Clusters of cells with convergent processes (arrowheads), which are brightly stained with phalloidin-rhodamine, occur on the antennae of E. rowelli embryos. These clusters give rise to the antennal sensillae. (b) As in chelicerates, the nuclei of each cell cluster are displaced basally (asterisks), whereas the apical cell processes converge at the ectodermal surface (arrowhead; Hoechst/Phalloidin-rhodamine double-labelling). (c) Anti-HRP immunoreactivity of cell clusters from body surface of Ep. isthmicola embryo (arrows). The clusters give rise to sensory dermal papillae. (d) Scanning electron micrograph of dermal papillae in an adult specimen of Ep. isthmicola. Note a sensory bristle on the tip of each papilla. AT, antenna; DP, dermal papilla. Scale bars, 50 µm (a,d) and 10 µm (b,c).
(d). Lack of segmental invaginations in onychophoran neurogenesis
A conspicuous phenomenon in the neurogenesis of myriapods is the invagination of the neuroectoderm in each hemisegment, once a large number of neurons has been generated. The invagination leads to the formation of epithelial vesicles (ventral organs), which enclose the remaining cell clusters in each hemisegment. This process has previously been documented in the Chilopoda and Diplopoda (Heymons 1901; Dohle 1964; Whitington et al. 1991; Dove & Stollewerk 2003; Kadner & Stollewerk 2004) and we have observed the same process in the symphylan H. agilis (see electronic supplementary material, figure S2c,d; see also Tiegs 1940). Reports of an apparently similar process of ventral organ formation can be found in the literature for a number of chelicerates, including pseudoscorpions and pycnogonids (e.g. Weygoldt 1964: figs 53 and 54; Winter 1980: fig. 1). We have observed a process of neuroectodermal invagination in the spider S. grossa (see electronic supplementary material, figure S1c), which mirrors closely that seen in the myriapods. Stollewerk (2004) reports an apparently different phenomenon in the spider Cupiennius salei, of epidermal cells ‘overgrowing’ the ventral neuromeres, although the same author also makes reference to ‘epithelial vesicles’ and ‘secondary invaginations’ in this species. Leaving aside the question of whether neuroectodermal invagination is a universal feature of spider development, it is clear that this process does not take place during the formation of the central nervous system in either crustaceans or hexapods (Stollewerk & Simpson 2005; Ungerer & Scholtz 2008).
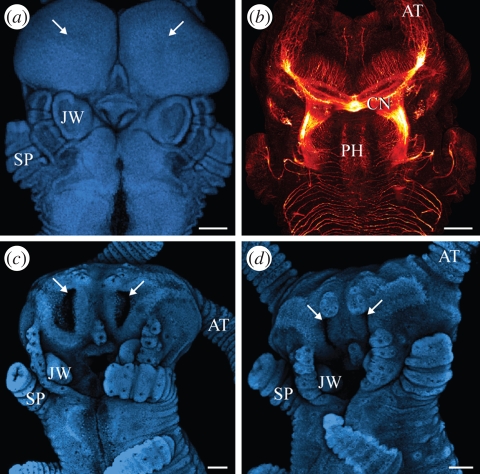
Our data exclude the possibility that ectodermal invaginations, as seen in chelicerate and myriapod embryos, play a role in onychophoran neurogenesis, because such invaginations occur only in the antennal segment. These invaginations, which give rise to the hypocerebral organs/glands (Eriksson et al. 2005; Mayer et al. 2005), only appear when the brain is at a relatively advanced developmental stage (figure 7a–d) and so cannot play a role in neurogenesis. The absence of segmental invaginations from the onychophoran neuroectoderm thus contrasts with the occurrence of the ‘ventral organs’ or ‘epithelial vesicles’ in chelicerates and myriapods, which are incorporated into developing ganglia (Dohle 1964; Weygoldt 1964; Anderson 1973; Whitington et al. 1991; Kadner & Stollewerk 2004; Stollewerk 2004; Whitington 2007).
Figure 7.
Ectodermal invaginations in Onychophora. Confocal micrographs. Anterior end of embryos in ventral view, anterior is up. (a,b) Head of Ep. isthmicola embryo prior to invagination of hypocerebral glands in the antennal segment. Double-labelling with Hoechst (blue) and an antibody raised against acetylated α-tubulin (red). (a) Future position of invaginations is indicated by arrows. (b) Note that the brain is at an advanced developmental stage in the same embryo. (c,d) Heads of E. rowelli embryos at subsequent developmental stages showing paired invaginations of hypocerebral glands in the antennal segment (arrows) and their subsequent closure. Hoechst labelling. AT, position of antenna; CN, central brain neuropil; JW, jaw anlage; PH, pharynx; SP, slime papilla. Scale bar, 100 µm.
(e). Bipotential fate of the onychophoran neuroectoderm
The fate of the neuroectoderm is another important issue when considering relationships between the arthropods. We have observed that the onychophoran neuroectoderm contributes cells to both nervous tissue and epidermis. During their basal migration within the neuroectoderm, neuronal progenitors are intermingled with other cells with large columnar nuclei (figure 5d), which become even more conspicuous later in development (figure S5a,b, electronic supplementary material).These specialized cells retain their positions within the epidermis and give rise to the so-called ventral organs (Mayer & Whitington in press). Even though the onychophoran ventral organs have been homologized with the homonymous structures in chelicerates and myriapods (Tiegs 1940; Pflugfelder 1948; Anderson 1973), their homology is unsubstantiated as there are no correspondences in their composition, role and embryonic fate (Whitington 2007; Mayer & Whitington in press). Furthermore, there is no embryological evidence that the ventral organs contribute cells to the central nervous system in Onychophora (Sedgwick 1887; von Kennel 1888; Mayer & Whitington in press; but see Eriksson et al. 2003). They, rather, have a different fate and persist as epidermal structures in adults (von Kennel 1888; Mayer 2007). Since the onychophoran neuroectoderm gives rise to both neurons of the nerve cords and epidermal cells (ventral organs), the onychophorans share with crustaceans and hexapods this bipotential fate of the neuroectoderm. This contrasts with the exclusively neural fate of the central area of the neuroectoderm in chelicerates and myriapods (Dove & Stollewerk 2003; Stollewerk 2004).
4. Conclusions and perspectives
To determine the ancestral versus derived states among different embryological characters, we have used Onychophora as an outgroup. Our data show that a cumulus-like structure does not occur in onychophoran development, which suggests that the cumulus is not an ancestral arthropod character, as proposed previously (McGregor et al. 2008), but rather a unique feature uniting the chelicerates and myriapods (figure 8). In order to further substantiate this hypothesis, expression studies of decapentaplegic (dpp), a gene specifying the dorsal body region, might be helpful. If dpp is expressed along the dorsal body axis in Onychophora, as for example in hexapods (Carroll et al. 2005), this pattern would support the monophyly of the Paradoxopoda, whereas an expression in a cumulus-like group of cells would suggest that this feature is a ground pattern character of Panarthropoda.
Figure 8.
Summary of major findings from study of onychophoran development and polarized characters supporting the monophyly of Paradoxopoda. 1–3, ground pattern of Panarthropoda: 1, segregation and immigration of single cells during neurogenesis; 2, mitotic activity of neuronal precursors; 3, dual nature of neuroectoderm giving rise to both neurons and epidermis; 4, autapomorphy of Pancrustacea: neuronal stem cells, which divide asymmetrically (see Harzsch 2001); 5–8, autapomorphies of Paradoxopoda: 5, immigrating clusters of post-mitotic cells during neurogenesis; 6, segmental invaginations of neuroectoderm; 7, exclusive generation of neurons in central neuroectoderm of each hemisegment; 8, cumulus determining dorsal body region (function of the cumulus-like structure remains to be clarified in myriapods).
Our data revealed that the mode of neurogenesis in onychophorans is more similar to that found in hexapods and crustaceans than that in chelicerates and myriapods as the onychophoran neuroectoderm shows neither post-mitotic cell clusters nor segmental invaginations. In Onychophora, instead, single precursors are recruited for neuronal fate and migrate internally as bottle-like cells, which is similar to the mode found in hexapods (figure 4). These immigrated cells are mitotically active, and in this respect resemble the neuronal stem cells (neuroblasts) of both crustaceans and hexapods (Harzsch 2001; Stollewerk & Simpson 2005; Ungerer & Scholtz 2008), even though they do not show asymmetric cell divisions. Our findings thus suggest that immigration of single cells, followed by their mitotic activity, is an ancestral feature of arthropod neurogenesis, while asymmetric cell divisions are a synapomorphy of crustaceans and hexapods (figure 8). The absence of the following three characters in onychophorans, crustaceans and hexapods suggests that they are derived features uniting the chelicerates and myriapods as sister groups: (i) clusters of immigrating post-mitotic cells; (ii) paired segmental invaginations; and (iii) exclusively neural fate of the central area of the neuroectoderm (figure 8). These findings, in addition to the occurrence of a cumulus in chelicerates and myriapods, argue against the proposed sister-group relationship of onychophorans and chelicerates (Strausfeld et al. 2006).
Our study, based on various lines of evidence and an outgroup comparison, provides, to our knowledge, the first morphological/embryological evidence for joining myriapods with chelicerates as sister taxa and thus challenges the traditional Mandibulata concept. However, since only few morphological data are currently available for either hypothesis (for Mandibulata, see reviews Edgecombe 2004; Scholtz & Edgecombe 2006), it is premature to perform a phylogenetic analysis including these characters. We predict, however, that a targeted search for similarities between chelicerates and myriapods might reveal additional synapomorphies in future studies, which could well be used for cladistic analyses.
The proposed monophyly of the Paradoxopoda gives a new view of the evolution of the arthropod ‘head’, as it suggests that the mandible has evolved independently in myriapods and pancrustaceans (crustaceans+hexapods, also called Tetraconata). Similar mandibular organization in these groups (Edgecombe et al. 2003; Edgecombe 2004) may be the result of functional adaptation (Hwang et al. 2001). The alternative view, that the mandible is a plesiomorphy of arthropods (Cook et al. 2001), implies a removal of the mandible from the chewing chamber of the mouth and its reversal to a biramous walking limb in chelicerates. Placement of the myriapods with the chelicerates also suggests that the first antenna has either been transformed into the chelicerae of chelicerates (Budd 2002; Chen et al. 2004) or has, like the mandible, evolved independently in myriapods and pancrustaceans. Since the segment of onychophorans corresponding to the first antennal segment of arthropods bears jaws, which are functional correlates of chelicerae (Scholtz & Edgecombe 2006) rather than sensory antennae, we favour the hypothesis of an independent origin of antennae in myriapods and pancrustaceans.
Acknowledgements
The staff of the Instituto Nacional de Biodiversidad (INBio, Heredia, Costa Rica), the National System of Conservation Areas (SINAC, MINAE, Costa Rica) and State Forests NSW (New South Wales, Australia) are gratefully acknowledged for providing permits. G.M. is thankful to Alvaro Herrera, Paul Sunnucks and Noel Tait for their assistance with collection of specimens and to Ayscha Hill-Williams for providing spider cocoons. We thank Gerhard Scholtz for useful discussions and Greg Edgecombe, Ina Mayer and Eli Mrkusich for helpful comments on a previous version of the manuscript. This work was supported by grants from the German Research Foundation (DFG, Ma 4147/1-1) and the Department of Anatomy and Cell Biology (University of Melbourne) to G.M.
References
- Akiyama-Oda Y., Oda H.2003Early patterning of the spider embryo: a cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disc epithelial cells. Development 130, 1735–1747 (doi:10.1242/dev.00390) [DOI] [PubMed] [Google Scholar]
- Anderson D. T.1973Embryology and phylogeny in annelids and arthropods Oxford, UK: Pergamon Press [Google Scholar]
- Bourlat S. J., Nielsen C., Economou A. D., Telford M. J.2008Testing the new animal phylogeny: a phylum level molecular analysis of the animal kingdom. Mol. Phyl. Evol. 49, 23–31 (doi:10.1016/j.ympev.2008.07.008) [DOI] [PubMed] [Google Scholar]
- Breugelmans B., Simonet G., Van Hoef V., Van Soest S., Vanden Broeck J.2009Identification, distribution and molecular evolution of the pacifastin gene family in Metazoa. BMC Evol. Biol. 9, 97 (doi:10.1186/1471-2148-9-97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd G. E.2001Why are arthropods segmented? Evol. Dev. 3, 332–342 (doi:10.1046/j.1525-142X.2001.01041.x) [DOI] [PubMed] [Google Scholar]
- Budd G. E.2002A palaeontological solution to the arthropod head problem. Nature 417, 271–275 (doi:10.1038/417271a) [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Grenier J. K., Weatherbee S. D.2005From DNA to diversity. Molecular genetics and the evolution of animal design, 2nd edn.Malden, MA: Blackwell Publishing [Google Scholar]
- Chaw R. C., Vance E., Black S. D.2007Gastrulation in the spider Zygiella x-notata involves three distinct phases of cell internalization. Dev. Dyn. 236, 3484–3495 (doi:10.1002/dvdy.21371) [DOI] [PubMed] [Google Scholar]
- Chen J., Waloszek D., Maas A.2004A new ‘great appendage’ arthropod from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia 37, 3–20 [Google Scholar]
- Cook C. E., Smith M. L., Telford M. J., Bastianello A., Akam M.2001Hox genes and the phylogeny of the arthropods. Curr. Biol. 11, 759–763 (doi:10.1016/S0960-9822(01)00222-6) [DOI] [PubMed] [Google Scholar]
- Dohle W.1964Die Embryonalentwicklung von Glomeris marginata (Villers) im Vergleich zur Entwicklung anderer Diplopoden. Zool. Jb Anat. 81, 241–310 [Google Scholar]
- Dohle W., Scholtz G.1997How far does cell lineage influence cell fate specification in crustacean embryos? Cell Dev. Biol. 8, 379–390 (doi:10.1006/scdb.1997.0162) [DOI] [PubMed] [Google Scholar]
- Dove H., Stollewerk A.2003Comparative analysis of neurogenesis in the myriapod Glomeris marginata (Diplopoda) suggests more similarities to chelicerates than to insects. Development 130, 2161–2171 (doi:10.1242/dev.00442) [DOI] [PubMed] [Google Scholar]
- Dunn C. W., et al. 2008Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 (doi:10.1038/nature06614) [DOI] [PubMed] [Google Scholar]
- Edgecombe G. D.2004Morphological data, extant Myriapoda, and the myriapod stem-group. Contrib. Zool. 73 See http://dpc.uba.uva.nl/ctz/vol73/nr03/art02. [Google Scholar]
- Edgecombe G. D., Wilson G. D. F., Colgan D. J., Gray M. R., Cassis G.2000Arthropod cladistics: combined analysis of histone H3 and U2 snRNA sequences and morphology. Cladistics 16, 155–203 (doi:10.1111/j.1096-0031.2000.tb00352.x) [DOI] [PubMed] [Google Scholar]
- Edgecombe G. D., Richter S., Wilson G. D. F.2003The mandibular gnathal edges: homologous structures throughout Mandibulata? Afr. Invertebr. 44, 115–135 [Google Scholar]
- Eriksson B. J., Tait N. N., Budd G. E.2003Head development in the onychophoran Euperipatoides kanangrensis. With particular reference to the central nervous system. J. Morphol. 255, 1–23 (doi:10.1002/jmor.10034) [DOI] [PubMed] [Google Scholar]
- Eriksson B. J., Tait N. N., Norman J. M., Budd G. E.2005An ultrastructural investigation of the hypocerebral organ of the adult Euperipatoides kanangrensis (Onychophora, Peripatopsidae). Arthropod Struct. Dev. 34, 407–418 (doi:10.1016/j.asd.2005.03.002) [Google Scholar]
- Friedrich M., Tautz D.1995Ribosomal DNA phylogeny of the major extant arthropod classes and the evolution of myriapods. Nature 376, 165–167 (doi:10.1038/376165a0) [DOI] [PubMed] [Google Scholar]
- Gai Y., Song D., Sun H., Yang Q., Zhou K.2008The complete mitochondrial genome of Symphylella sp. (Myriapoda : Symphyla): extensive gene order rearrangement and evidence in favor of Progoneata. Mol. Phyl. Evol. 49, 574–585 (doi:10.1016/j.ympev.2008.08.010) [DOI] [PubMed] [Google Scholar]
- Giribet G., Carranza S., Baguñà J., Riutort M., Ribera C.1996First molecular evidence for the existence of a Tardigrada+Arthropoda clade. Mol. Biol. Evol. 13, 76–84 [DOI] [PubMed] [Google Scholar]
- Giribet G., Edgecombe G. D., Wheeler W. C.2001Arthropod phylogeny based on eight molecular loci and morphology. Nature 413, 157–161 (doi:10.1038/35093097) [DOI] [PubMed] [Google Scholar]
- Giribet G., Richter S., Edgecombe G. D., Wheeler W. C.2005The position of crustaceans within the Arthropoda: evidence from nine molecular loci and morphology. In Crustacea and Arthropod relationships (eds Koenemann S., Jenner R.), pp. 307–352 Boca Raton, FL: CRC Press [Google Scholar]
- Harzsch S.2001Neurogenesis in the crustacean ventral nerve cord: homology of neuronal stem cells in Malacostraca and Brachiopoda? Evol. Dev. 3, 154–169 (doi:10.1046/j.1525-142x.2001.003003154.x) [DOI] [PubMed] [Google Scholar]
- Harzsch S., Müller C. H. G., Wolf H.2005From variable to constant cell numbers: cellular characteristics of the arthropod nervous system argue against a sister-group relationship of Chelicerata and ‘Myriapoda’ but favour the Mandibulata concept. Dev. Genes Evol. 215, 53–68 (doi:10.1007/s00427-004-0451-z) [DOI] [PubMed] [Google Scholar]
- Hassanin A.2006Phylogeny of Arthropoda inferred from mitochondrial sequences: strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution. Mol. Phyl. Evol. 38, 100–116 (doi:10.1016/j.ympev.2005.09.012) [DOI] [PubMed] [Google Scholar]
- Heymons R.1901Die Entwicklungsgeschichte der Scolopender. Zoologica 33, 1–244 [Google Scholar]
- Holm A.1952Experimental studies on development and developmental physiology of the spider embryo (in German). Zool. Bidr. Uppsala 29, 293–424 [Google Scholar]
- Hwang U. W., Friedrich M., Tautz D., Park C. J., Kim W.2001Mitochondrial protein phylogeny joins myriapods with chelicerates. Nature 413, 154–157 (doi:10.1038/35093090) [DOI] [PubMed] [Google Scholar]
- Janssen R., Prpic N.-M., Damen W. G. M.2004Gene expression suggests decoupled dorsal and ventral segmentation in the millipede Glomeris marginata (Myriapoda : Diplopoda). Dev. Biol. 268, 89–104 (doi:10.1016/j.ydbio.2003.12.021) [DOI] [PubMed] [Google Scholar]
- Kadner D., Stollewerk A.2004Neurogenesis in the chilopod Lithobius forficatus suggests more similarities to chelicerates than to insects. Dev. Genes Evol. 214, 367–379 (doi:10.1007/s00427-004-0419-z) [DOI] [PubMed] [Google Scholar]
- Kusche K., Burmester T.2001Diplopod hemocyanin sequence and the phylogenetic position of the Myriapoda. Mol. Biol. Evol. 18, 1566–1573 [DOI] [PubMed] [Google Scholar]
- Kusche K., Ruhberg H., Burmester T.2002A hemocyanin from the Onychophora and the emergence of respiratory proteins. Proc. Natl Acad. Sci. USA 99, 10 545–10 548 (doi:10.1073/pnas.152241199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusche K., Hembach A., Hanger-Holler S., Gebauer W., Burmester T.2003Complete subunit sequences, structure and evolution of the 6 X 6-mer hemocyanin from the common house centipede, Scutigera coleoptrata. Eur. J. Biochem. 270, 2860–2868 (doi:10.1046/j.1432-1033.2003.03664.x) [DOI] [PubMed] [Google Scholar]
- Maas A., Mayer G., Kristensen R. M., Waloszek D.2007A Cambrian micro-lobopodian and the evolution of arthropod locomotion and reproduction. Chin. Sci. Bull. 52, 3385–3392 (doi:10.1007/s11434-007-0515-3) [Google Scholar]
- Mallatt J., Giribet G.2006Further use of nearly complete 28S and 18S rRNA genes to classify Ecdysozoa: 37 more arthropods and a kinorhynch. Mol. Biol. Evol. 40, 772–794 [DOI] [PubMed] [Google Scholar]
- Mallatt J. M., Garey J. R., Shultz J. W.2004Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Mol. Phyl. Evol. 31, 178–191 (doi:10.1016/j.ympev.2003.07.013) [DOI] [PubMed] [Google Scholar]
- Mayer G.2007Metaperipatus inae sp. nov. (Onychophora : Peripatopsidae) from Chile with a novel ovarian type and dermal insemination. Zootaxa 1440, 21–37 [Google Scholar]
- Mayer G., Tait N. N.In press Position and development of oocytes in velvet worms shed light on the evolution of the ovary in Onychophora and Arthropoda. Zool. J. Linn. Soc. [Google Scholar]
- Mayer G., Whitington P. M.In press Neural development in Onychophora (velvet worms) suggests a non-segmental ancestry of the arthropod nervous system. Dev. Biol. [DOI] [PubMed] [Google Scholar]
- Mayer G., Bartolomaeus T., Ruhberg H.2005Ultrastructure of mesoderm in embryos of Opisthopatus roseus (Onychophora, Peripatopsidae): revision of the ‘Long Germ Band’ hypothesis for Opisthopatus. J. Morphol. 263, 60–70 (doi:10.1002/jmor.10289) [DOI] [PubMed] [Google Scholar]
- McGregor A. P., Hilbrant M., Pechmann M., Schwager E. E., Prpic N.-M., Damen W. G. M.2008Cupiennius salei and Achaearanea tepidariorum: spider models for investigating evolution and development. BioEssays 30, 487–498 (doi:10.1002/bies.20744) [DOI] [PubMed] [Google Scholar]
- Mittmann B.2002Early neurogenesis in the horseshoe crab Limulus polyphemus and its implication for arthropod relationships. Biol. Bull. 203, 221–222 (doi:10.2307/1543407) [DOI] [PubMed] [Google Scholar]
- Negrisolo E., Minelli A., Valle G.2004The mitochondrial genome of the house centipede Scutigera and the monophyly versus paraphyly of myriapods. Mol. Biol. Evol. 21, 770–780 (doi:10.1093/molbev/msh078) [DOI] [PubMed] [Google Scholar]
- Nielsen C.2001Animal evolution: interrelationships of the living phyla, 2nd edn.Oxford, UK: Oxford University Press [Google Scholar]
- Pflugfelder O.1948Entwicklung von Paraperipatus amboinensis n. sp. Zool. Jb Anat. 69, 443–492 [Google Scholar]
- Pisani D., Poling L. L., Lyons-Weiler M., Hedges S. B.2004The colonization of land by animals: molecular phylogeny and divergence times among arthropods. BMC Biology 2 (doi:10.1186/1741-7007-2-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlowski L., Braband A., Mayer G.2008The complete mitochondrial genome of the onychophoran Epiperipatus biolleyi reveals a unique transfer RNA set and provides further support for the Ecdysozoa hypothesis. Mol. Biol. Evol. 25, 42–51 (doi:10.1093/molbev/msm223) [DOI] [PubMed] [Google Scholar]
- Regier J. C., et al. 2008Resolving arthropod phylogeny: exploring phylogenetic signal within 41 kb of protein-coding nuclear gene sequence. Syst. Biol. 57, 920–938 (doi:10.1080/10635150802570791) [DOI] [PubMed] [Google Scholar]
- Rota-Stabelli O., Telford M. J.2008A multi criterion approach for the selection of optimal outgroups in phylogeny: recovering some support for Mandibulata over Myriochelata using mitogenomics. Mol. Phyl. Evol. 48, 103–111 (doi:10.1016/j.ympev.2008.03.033) [DOI] [PubMed] [Google Scholar]
- Sakuma M., Machida R.2002Germ band formation of a centipede Scolopocryptops rubiginosus L. Koch (Chilopda : Scolopendromorpha). Proc. Arthropod. Embryol. Soc. Jpn 37, 19–23 [Google Scholar]
- Sakuma M., Machida R.2004Germ band formation of a centipede Scolopendra subspinipes L. Koch (Chilopoda : Scolopendromorpha). Proc. Arthropod. Embryol. Soc. Jpn 39, 41–43 [Google Scholar]
- Scholtz G., Edgecombe G. D.2005Heads, Hox and the phylogenetic position of trilobites. In Crustacea and arthropod phylogeny (eds Koenemann S., Jenner R., Vonk R.), pp. 139–165 Boca Raton, FL: CRC Press [Google Scholar]
- Scholtz G., Edgecombe G. D.2006The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216, 395–415 (doi:10.1007/s00427-006-0085-4) [DOI] [PubMed] [Google Scholar]
- Scholtz G., Mittmann B., Gerberding M.1998The pattern of Distal-less expression in the mouthparts of crustaceans, myriapods and insects: new evidence for a gnathobasic mandible and the common origin of Manibulata. Int. J. Dev. Biol. 42, 801–810 [PubMed] [Google Scholar]
- Sedgwick A.1887The development of the Cape species of Peripatus. Part III. On the changes from stage A to stage F. Q. J. Microsc. Sci. 27, 467–550 [Google Scholar]
- Sekiguchi K.1973A normal plate of the development of the Japanese horse-shoe crab, Tachypleus tridentatus. Sci. Rep. Tokyo Kyoiku Daigaku Sect. B 15, 153–162 [Google Scholar]
- Stollewerk A.2004Secondary neurons are arrested in an immature state by formation of epithelial vesicles during neurogenesis of the spider Cupiennius salei. Front. Zool. 1, 3 (doi:10.1186/1742-9994-1-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollewerk A., Chipman A. D.2006Neurogenesis in myriapods and chelicerates and its importance for understanding arthropod relationships. Integr. Comp. Biol. 46, 195–206 (doi:10.1093/icb/icj020) [DOI] [PubMed] [Google Scholar]
- Stollewerk A., Seyfarth A.2008Evolutionary changes in sensory precursor formation in arthropods: embryonic development of leg sensilla in the spider Cupiennius salei. Dev. Biol. 313, 659–673 (doi:10.1016/j.ydbio.2007.11.003) [DOI] [PubMed] [Google Scholar]
- Stollewerk A., Simpson P.2005Evolution of early development of the nervous system: a comparison between arthropods. BioEssays 27, 874–883 (doi:10.1002/bies.20276) [DOI] [PubMed] [Google Scholar]
- Stollewerk A., Weller M., Tautz D.2001Neurogenesis in the spider Cupiennius salei. Development 128, 2673–2688 [DOI] [PubMed] [Google Scholar]
- Strausfeld N. J., Strausfeld C. M., Loesel R., Rowell D., Stowe S.2006Arthropod phylogeny: onychophoran brain organization suggests an archaic relationship with a chelicerate stem lineage. Proc. R. Soc. B 273, 1857–1866 (doi:10.1098/rspb.2006.3536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs O. W.1940The embryology and affinities of the Symphyla, based on a study of Hanseniella agilis. Q. J. Microsc. Sci. 82, 1–225 [Google Scholar]
- Ungerer P., Scholtz G.2008Filling the gap between identified neuroblasts and neurons in crustaceans adds new support for Tetraconata. Proc. R. Soc. B 275, 369–376 (doi:10.1098/rspb.2007.1391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch P.2008Gondwanan break-up: legacies of a lost world? Trends Ecol. Evol. 23, 229–236 (doi:10.1016/j.tree.2007.11.006) [DOI] [PubMed] [Google Scholar]
- von Kennel J.1888Entwicklungsgeschichte von Peripatus edwardsii Blanch. und Peripatus torquatus n.sp. II. Theil. Arb. Zool. -Zootom. Inst. Würzburg 8, 1–93 [Google Scholar]
- Weygoldt P.1964Vergleichend-embryologische Untersuchungen an Pseudoscorpionen (Chelonethi). Z. Morph. Ökol. Tiere 54, 1–106 (doi:10.1007/BF00404408) [Google Scholar]
- Whitington P. M.2007The evolution of arthropod nervous systems: insights from neural development in the Onychophora and Myriapoda. In Theories, development, invertebrates (eds Striedter G. F., Rubenstein J. L. R.), pp. 317–336 Oxford, UK: Academic Press [Google Scholar]
- Whitington P. M., Meier T., King P.1991Segmentation, neurogenesis and formation of early axonal pathways in the centipede, Ethmostigmus rubripes (Brandt). Roux Arch. Dev. Biol. 199, 349–363 (doi:10.1007/BF01705928) [DOI] [PubMed] [Google Scholar]
- Winter G.1980Beiträge zur Morphologie und Embryologie des vorderen Körperabschnitts (Cephalosoma) der Pantopoda Gerstaecker, 1863. I. Entstehung und Struktur des Zentralnervensystems. Z. Zool. Syst. Evolutionsforsch. 18, 27–61 [Google Scholar]