Abstract
Economic models of animal behaviour assume that decision-makers are rational, meaning that they assess options according to intrinsic fitness value and not by comparison with available alternatives. This expectation is frequently violated, but the significance of irrational behaviour remains controversial. One possibility is that irrationality arises from cognitive constraints that necessitate short cuts like comparative evaluation. If so, the study of whether and when irrationality occurs can illuminate cognitive mechanisms. We applied this logic in a novel setting: the collective decisions of insect societies. We tested for irrationality in colonies of Temnothorax ants choosing between two nest sites that varied in multiple attributes, such that neither site was clearly superior. In similar situations, individual animals show irrational changes in preference when a third relatively unattractive option is introduced. In contrast, we found no such effect in colonies. We suggest that immunity to irrationality in this case may result from the ants’ decentralized decision mechanism. A colony's choice does not depend on site comparison by individuals, but instead self-organizes from the interactions of multiple ants, most of which are aware of only a single site. This strategy may filter out comparative effects, preventing systematic errors that would otherwise arise from the cognitive limitations of individuals.
Keywords: rationality, regularity, asymmetric dominance, collective decision-making, context-dependent preferences
1. Introduction
A basic assumption of behavioural ecology is that animals act to maximize their fitness. A similar expectation underpins the idea of rationality in neoclassical economics, where people are presumed to maximize utility, an implicit measure of net benefit or desirability (Arrow 1950; Sen 1993; Rieskamp et al. 2006). To do so, a rational decision-maker must consistently value each option on the basis of its contribution to utility. In a biological setting, rationality implies that decision-making animals should assign value according to an option's net fitness benefits. If these benefits are intrinsic to the option itself, its value should not change with the number and type of alternatives on hand. For example, an animal that prefers food type A to type B should not switch its preference to B if a third type C is also made available. Option A either confers higher fitness than B or it does not, and the presence of C is irrelevant. Similar reasoning applies when an individual does not always choose a particular option, but instead has some probability of picking A over B. This probability should not increase if the option set is expanded, a hallmark of rationality known as the principle of regularity (Rieskamp et al. 2006).
Regularity and other rationality principles have stirred controversy because they are repeatedly violated in both human and animal studies (Tversky 1969; Huber et al. 1982; Wedell 1991; Tversky & Simonson 1993; Shafir 1994; Shafir et al. 2002; Bateson et al. 2003). This typically happens when options vary in multiple attributes such that no option is superior in all of them. A widely seen pattern arises when a choice between two such options is supplemented by a third ‘decoy’ that is asymmetrically dominated (Huber et al. 1982). One option dominates another if it equals or exceeds it in all attributes and exceeds it in at least one attribute. An asymmetrically dominated decoy is dominated by one option but not by the other, and its presence can cause regularity violations by increasing the preference for the dominating option, relative to that option's popularity in a binary choice. Even when regularity is obeyed, the decoy can evoke violation of another rationality principle: the constant-ratio rule, which requires the relative preference between two options to remain unchanged by the addition of a third option (Luce 1959; Tversky 1972). Violations arise when some decision-makers switch their preference to the decoy, but do so disproportionately from the dominated option. Violations of either the constant-ratio rule or the regularity principle imply that decision-makers do not assign absolute values to options, as maximization requires, but instead rate each one by comparing it with the other options on hand.
Widespread irrationality appears to cast doubt on the idea that individuals maximize either utility or fitness. Some behavioural ecologists have responded by arguing that seemingly irrational behaviour is an artefact of experimental design (Schuck-Paim et al. 2004) or by pointing to particular circumstances in which comparative evaluation is in fact rational (Houston 1997). Others acknowledge irrational behaviour but attribute it to the environment in which a decision-maker is tested. Natural selection builds solutions to the problems encountered in the particular social and ecological setting in which an animal evolves, and these solutions may fail in novel contexts (Todd & Gigerenzer 2000; Laland & Brown 2002; Stephens et al. 2004).
Another line of reasoning notes that decision errors may arise even in the appropriate adaptive environment because selection is a process of fitness maximization under constraints. Some of these constraints are external: the world is complex and uncertain, making it impossible or prohibitively costly to acquire enough information for error-free decisions (Stephens & Krebs 1986; Houston 1997). Others are internal: even if complete information is available, organisms must process it with limited computational resources. This may select for simple heuristics or rules of thumb that economize on computation by excluding some information or processing it imperfectly (Todd & Gigerenzer 2000; Kahneman 2003; Hutchinson & Gigerenzer 2005; Waksberg et al. 2009). These rules are adequate in most circumstances, but may yield mistakes in certain challenging contexts. Moreover, recent theoretical work shows that even optimal brains of high complexity will be error-prone in the information-rich environments typical of real animals (Livnat & Pippenger 2008). Errors owing to computational constraints are especially interesting because they should depend systematically on an animal's particular cognitive mechanisms. Their analysis offers a window on these mechanisms and the selective forces that shaped them. Such a programme of study has been pioneered in experiments on individual animals, including honeybees, jays and hummingbirds (Shafir 1994; Waite 2001; Shafir et al. 2002; Bateson et al. 2003).
In the current study, we apply the same logic in a novel setting: the consensus decisions of animal collectives. Group living is widespread, and many animals have evolved behavioural mechanisms to ensure consensus choice when splitting imposes net fitness costs on group members (Conradt & Roper 2005; King & Cowlishaw 2009). Particularly sophisticated decision-making is seen in eusocial insect colonies, where sterile workers advance their inclusive fitness largely through cooperative rearing of the queen's offspring. As a result, selection has acted at the level of the whole colony to shape highly integrated complex behaviours analogous to those of individual animals (Hölldobler & Wilson 2008; Gardner & Grafen 2009). These societies act as unitary decision-makers, able to jointly select a single travel direction, foraging location or nest site from many options (Seeley 1995; Camazine et al. 2001; Visscher 2007). Just as an individual's choice emerges from the complex interactions of a network of neurons, a colony's decision emerges from an analogous network of interacting insects (Passino et al. 2008; Marshall et al. in press). At both levels of organization, the occurrence of systematic errors may give insights into underlying decision-making mechanisms.
We sought evidence for irrational preferences in collective nest site selection by the ant Temnothorax curvispinosus. Like other members of the genus, these ants live in natural cavities such as hollow nuts or twigs and are adept at organizing emigrations when their current nest is damaged (Visscher 2007). Emigrating colonies can reach consensus on the better of two sites, distinguishing them on the basis of entrance size, cavity dimensions, interior light level and other attributes (Pratt & Pierce 2001; Franks et al. 2003; Pratt 2005). Detailed analysis of this species and similar behaviour in Temnothorax albipennis has shown how consensus depends on a minority of active ants that scout for potential homes and assess their quality. A successful scout summons nest-mates to a candidate site and these recruits summon still more, creating a positive feedback cascade that drives up the site's population. The strength of this cascade depends on site quality because each ant initiates recruitment at a rate that is higher for better sites (Mallon et al. 2001; Pratt 2005). The ants amplify this difference by a quorum rule under which they accelerate recruitment once the site's population reaches a threshold (Pratt et al. 2002). They achieve this by switching from slow recruitment via tandem runs, directed only at fellow scouts, to the faster method of social transport, directed at the colony's non-scouting majority (Möglich 1978; Mallon et al. 2001). Models and experimental analyses have shown how this quorum rule, combined with quality-dependent recruitment initiation, allows a colony to reach consensus on the best option, even when most individual ants do not visit most nests to compare them (Pratt et al. 2002, 2005). Site selection is thus a truly group-level property and the colony can be regarded as a unitary decision-maker.
With this system, we can readily challenge colonies with the same asymmetric dominance context known to evoke irrationality in individuals. Colonies evaluate multiple attributes, allowing straightforward design of appropriate competitors and decoys. Nest site selection also offers an advantage over analogous experiments with foraging decisions, in that no training phase is needed for test subjects to associate each option with its underlying attributes. This avoids the risk of training-induced changes in a state that may confound interpretation of apparently irrational behaviour (Schuck-Paim et al. 2004). We probed the susceptibility of colonies to systematic irrationality by comparing their site preferences in three conditions: (i) a binary choice between two target sites A and B that differed in two attributes such that A was better in one attribute and B was better in the other; (ii) a ternary choice between A, B and a decoy DA that was dominated by A but not by B; and (iii) a ternary choice between A, B and a decoy DB that was dominated by B but not by A. We predicted that colonies would show split decisions between A and B because neither is obviously superior. If colonies use a comparative heuristic for evaluating option quality, we further predicted that the addition of an asymmetrically dominated decoy would increase the preference for the dominant option, compared with the binary case.
2. Material and methods
(a). Nest designs
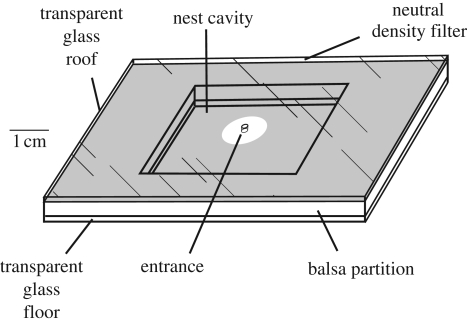
Nests were made from a balsa wood slat (2.4 mm thick) sandwiched between two glass microscope slides (50 × 75 mm). A rectangular nest cavity (25 × 33 mm) was cut through the middle of the slat and a round entrance hole drilled through the centre of the roof slide (figure 1). For binary choices, colonies chose between two target nests A and B designed such that neither was clearly superior to the other. Nest A had a dimmer interior than nest B, but a larger entrance size. Colonies strongly prefer small entrances and darker interiors (Pratt & Pierce 2001; Pratt 2005); hence the choice imposed a significant trade-off. The entrance diameter of nest A was 4.8 mm, while that of nest B was 1.6 mm. Interior light level was adjusted by inserting thin, plastic neutral-density filters (Rosco Cinegel) between the roof slide and the balsa slat. For nest type A, we used a 3-stop filter, thus reducing interior illumination by approximately a factor of eight from the ambient level of 300 lux. For nest type B we used a 1-stop filter, reducing ambient levels by a factor of two.
Figure 1.
Design of nests used in preference tests.
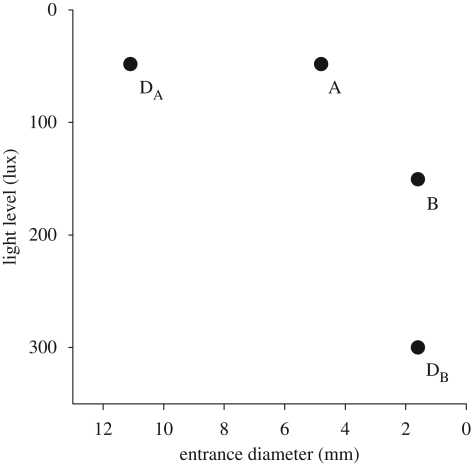
For ternary choices, colonies were given options A and B, as well as a third decoy nest, either DA or DB. Decoy DA was dominated by A, but not by B; it had the same interior illumination as A (40 lux), but a larger, and thus less preferred, entrance size (9.5 mm diameter). It was not dominated by B because DA had a darker, and thus more preferred, interior light level. Decoy DB, on the other hand, was dominated by B, but not by A; it had the same entrance size as B (1.6 mm), but a brighter, and thus less preferred, light level (300 lux), achieved by fitting it with a transparent plastic sheet (Grafix Dura-lar) instead of a neutral-density filter. It was not dominated by A because DB had a smaller, and thus more preferred, entrance size. Figure 2 summarizes the dominance relationships between targets and decoys.
Figure 2.
Values of target and decoy nests in two attributes: interior light level and entrance diameter. Target A is superior to target B in light level (i.e. darker), but inferior in entrance diameter (i.e. larger). Decoy DA is dominated by A but not by B. Decoy DB is dominated by B but not by A.
For all nest designs, the neutral-density filter or clear film had a 9.5 mm diameter hole its centre. Consistent hole size ensured that illumination was independent of entrance size. All filters were lightly coated with an anti-static spray to prevent an electrostatic charge build-up, which ants find repellent.
(b). Subjects
Twenty-six colonies of T. curvispinosus were collected between 28 April and 14 June 2005 in Princeton, NJ, USA. All colonies were queenright, with worker populations ranging from 12 to 90 and brood populations from 3 to 150. Each colony was housed in a nest like those described above, but with no light filter and with a small entrance (1.6 mm diameter). Each nest was kept in a Petri dish (15 mm diameter), the walls of which were coated with Fluon to prevent the ants from escaping. Each dish contained a water-filled plastic tube capped with cotton, and an ad libitum supply of fruitflies and an agar-based diet. Colonies were kept in a laboratory maintained at a 13 : 11 light : dark schedule.
(c). Preference tests
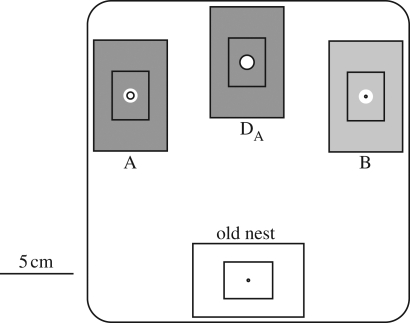
Nest site preferences were assayed by inducing colonies to emigrate in the presence of candidate sites and observing which one they moved into. For each test, a colony in its home nest was placed near the centre of one wall of a 20 × 20 cm tray with 2-cm-tall sides. Two unoccupied candidate nests were placed near the opposite corners of the tray; for ternary choices, a third option was placed between them such that the distance to each new nest from the centre of the old nest was identical (figure 3). Tray walls were coated with Fluon, and escapes were further limited by covering each tray with a clear plastic lid. Emigrations were induced by removing the roof slide of the old nest, motivating the colony to find a new home. Colonies were checked hourly and the emigration was deemed complete once no ants or brood items remained in the old nest (typically within 7 h of the start of the experiment). At this point, a photograph was taken of the newly occupied nest (or nests, if the colony split between them). Colonies sometimes emigrate in stages, moving entirely or partially to one site before relocating to another site. To catch such secondary moves, we left the colonies and nests in their trays until 24 h after the start of the emigration, when all occupied nests were again photographed. Each colony was then returned to its home Petri dish.
Figure 3.
Layout of nests for ternary preference tests. This diagram shows decoy DA, but DB was tested in the same configuration. Binary tests were similar except that no decoy was present.
Each colony carried out three emigrations: a binary choice between A and B, a ternary choice between A, B and DA, and a ternary choice between A, B and DB. Because the experience of one choice might influence later preferences, colonies received the treatments in different orders, such that all six possible orderings of the three treatments were equally represented. In addition, at least 5 days intervened between treatments to allow possible learning effects to fade (Langridge et al. 2008). To control for any directional preferences, half of the colonies always had option A to the left and option B to the right, and the other half had the opposite arrangement.
Choice tests were carried out in six sessions, each consisting of 12 simultaneous emigrations. The 12 trays were arrayed on a bench top in two rows of six. To prevent colonies from being influenced by the behaviour of neighbouring colonies, the sides of each tray were shielded with white index cards. During emigrations, the room's only illumination was provided by two 1.2-m-long 32 W fluorescent lamps arrayed end to end above the bench. The lamps were dimmed by covering their lower surfaces with a sheet of 2-stop neutral-density filter. Their height above the bench was then adjusted to achieve illumination of roughly 300 lux at the bench top, as determined by a light meter (Lutron LX101). Illumination was measured independently at the bench locations of all 12 emigration trays and was found to vary among them (range: 289–339 lux; mean: 319 lux). To minimize the influence of this variation, each colony was always tested at the same bench position, so that it experienced a consistent illumination for each nest type.
Tray floors were wiped with ethanol between emigrations to remove any chemical cues or signals that may have been deposited by the ants. Glass and plastic components of the nests were re-used between emigrations, after washing in soap and water. Balsa slats were used only once.
(d). Testing for violation of regularity
Colonies violate regularity if their preference for either target is higher in the presence of a decoy than in its absence. We predicted that an asymmetrically dominated decoy would cause a regularity violation by increasing the preference for the dominant target. To test this, we determined whether the proportion choosing A from the set A, B and DA was higher than the proportion choosing A from the set A and B. Likewise, we determined whether the proportion choosing B from the set A, B and DB was higher than the proportion choosing B from the set A and B. Treatments were compared with McNemar's test for a difference between paired proportions.
(e). Testing for violation of the constant-ratio rule
Even if colonies have regular preferences, they may still violate the constant-ratio rule and, thus, the expectation of rationality. This rule is violated if the relative preference for A versus B is altered by the addition of either decoy. This could happen without violating regularity if some colonies switch their preference to the decoy, but do so disproportionately from one target rather than the other. We predicted that an asymmetrically dominated decoy would violate the constant-ratio rule by attracting more switches from the non-dominant target. To test this, we determined whether the relative preference for nest A over B was higher in the presence of DA than in the binary choice. Likewise, we determined whether the relative preference for nest A over B was lower in the presence of DB than in the binary choice. Relative preference was measured as follows:
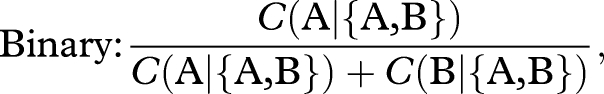 |
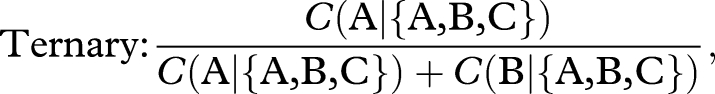 |
where C(x|{x,y,z}) is the number of colonies choosing option x from the set x,y,z. For the binary choice, the denominator is simply the total number of colonies because A and B were the only choices available. For the ternary choices, the denominator excludes any colonies that chose the decoy. Treatments were compared using the simple asymptotic method for comparing two proportions (Newcombe 1998), as implemented in the function prop.test of the statistical software package R (v. 2.8.0).
(f). Split decisions
Colonies usually showed an unambiguous preference for a single site, but they sometimes split between sites. In these cases, the numbers of workers and brood items in each occupied nest were counted from photographs. For the analyses described above, split colonies were scored as having chosen the nest containing the majority of the colony's workers. To account for split decisions more directly, we also carried out alternative tests based on the average fraction of each colony in a given nest type, rather than the number of colonies choosing each type. For example, to detect departures from regularity, we compared the mean fraction occupying nest A in the binary choice with the mean fraction occupying A in the ternary choice with decoy DA. Treatments were compared with Wilcoxon's signed-rank test.
3. Results
(a). Colonies do not violate regularity
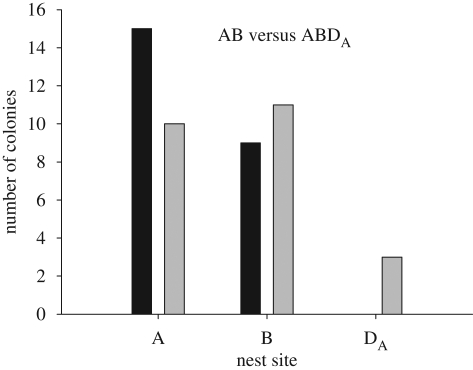
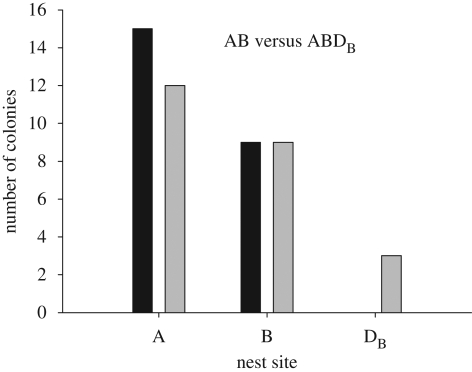
In the binary choice between A and B, colonies showed no strong preference for either site, with 15 choosing A and 9 choosing B (binomial test: p = 0.42). This confirmed that the trade-off between entrance size and dimness posed the desired decision-making challenge to the colonies. However, comparison of the binary and ternary results showed no effect of asymmetrically dominated decoys on the regularity of the colonies' preferences. That is, the proportion of colonies choosing nest A did not increase in the presence of decoy DA (figure 4), nor did the proportion choosing B increase in the presence of decoy DB (figure 5). Indeed, there were no significant changes in the proportion choosing A or B in the presence of either decoy. The same results held in the alternative analysis that took into account the 36 per cent of colonies that split between sites; neither decoy significantly influenced the mean fraction of each colony occupying sites A and B (electronic supplementary material).
Figure 4.
Outcome of binary choices between A and B (black bars) and ternary choices between A, B and DA (grey bars). Bar heights show the number of colonies choosing each option at the end of the emigration. The addition of DA had no effect on the proportion of colonies choosing site A (McNemar test: χ12 = 1.45, n = 24, p = 0.23) or site B (McNemar test: χ12 = 0.13, n = 24, p = 0.72).
Figure 5.
Outcome of binary choices between A and B (black bars) and ternary choices between A, B and DB (grey bars). Bar heights show the number of colonies choosing each option at the end of the emigration. The addition of DB had no effect on the proportion of colonies choosing site A (McNemar test: χ12 = 0.57, n = 24, p = 0.45) or site B (McNemar test: χ12 = 0.0, n = 24, p = 1.0).
The observations above were made at the time the old nest was abandoned, within 7 h of the start of emigration. When colonies were re-examined the following day, some had changed nest sites. Most changes simply resolved a split decision in favour of the nest that held the colony's majority, reducing the percentage of split colonies from 36 to 19 per cent. In two cases, however, the colony changed its choice, one shifting from a split in favour of B to unanimity for A (ternary choice ABDB) and the other shifting from a split in favour of A to unanimity for B (binary choice). The modified choices still showed no significant effect of either decoy on the proportion choosing A or B (electronic supplementary material).
(b). Colonies do not violate the constant-ratio rule
Even when regularity is satisfied, rationality may still be violated if the relative popularity of A and B is changed by the presence of a decoy. However, we found no significant change in the relative number of colonies choosing A and B in the presence of either DA (proportion test: χ12 = 0.49, n = 45 emigrations, p = 0.48) or DB (proportion test: χ12 = 0.004, n = 45 emigrations, p = 0.95). This remained true when preferences were scored as colony fractions rather than discrete choices (decoy DA, Wilcoxon test: V = 118.5, n = 24 colonies, p = 0.70; decoy DB, Wilcoxon test: V = 96, n = 22 colonies, p = 0.68). It was also true when choices were re-examined the day after the emigration (electronic supplementary material).
4. Discussion
The results of this study show no evidence for irrationality by ant colonies making collective choices between nest sites. Specifically, preference between two target sites was not affected by a third site dominated by one target but not by the other. In previous studies of individual decision-making by humans and other animals, such asymmetrically dominated options have increased the absolute or relative preference for the dominant target over the non-dominant one. These preference changes are irrational because they are not consistent with maximization of fitness or utility. Although the meaning and cause of these violations remain controversial, many explanations emphasize constraints on decision performance imposed by limited information and cognitive resources (Todd & Gigerenzer 2000; Kahneman 2003; Hutchinson & Gigerenzer 2005; Livnat & Pippenger 2008). Thus, a decision-maker might rely on mechanisms that yield fitness-reducing preference reversals because it lacks adequate resources for consistent identification of the best choice. Because these mechanisms work well in most circumstances, their use is only revealed in certain contexts that test their limits. Asymmetrically dominated decoys are one such context for a variety of individual decision-makers, but they evoked no such departure for the ant colonies in this experiment. Why might this be so?
One explanation that can probably be rejected is that we did not truly provide the context known to evoke irrationality in other cases. Irrationality is generally seen when options vary in multiple attributes such that no option is clearly superior. In simpler cases, when all information points to the same choice, it is easier for a computationally constrained decision-maker to choose the maximizing option. For example, earlier work on collective nest site preferences showed that Temnothorax colonies conform strongly to transitivity, another hallmark of rationality (Franks et al. 2003; Pratt 2005; Healey & Pratt 2008). That is, colonies that preferred A to B and B to C also preferred A to C. However, transitive ranking of options was tested only for sites that did not require trade-offs between different attributes. Under these conditions, strategies that simplify decision-making by ignoring some information can achieve consistent preference rankings across contexts (e.g. most attributes can be ignored if all yield the same ranking). When no single option is best in all attributes, these decision strategies can lead to preference reversals and intransitivity (Tversky 1969, 1972; Huber et al. 1982; Rieskamp et al. 2006; Livnat & Pippenger 2008). We aimed to create a similarly challenging context by designing target options A and B such that neither was clearly superior to the other. The behaviour of colonies in binary choices confirmed that this was so: at most, they showed a weak partiality for A, similar in magnitude to preferences seen in other experiments where decision-makers behaved irrationally (Shafir et al. 2002; Bateson et al. 2003).
If our experimental design was adequate, the explanation for the colonies' robust rationality may lie in their decision-making strategy. The highly distributed algorithm used by colonies may impose absolute rather than comparative evaluation of each option, thus leading to rational choice (Robinson et al. 2009). This algorithm does not require individual scouts to visit more than one of the options available to the colony. Instead, a scout can contribute to the collective decision by visiting and assessing only a single site and basing its recruitment decisions on the quality of that site alone. Thus, from the point of view of an ant assessing site A or B, there is no difference between the binary and ternary treatments; the ant receives the same information and will behave the same way. As a result, the information that the colony receives from these scouts is not influenced by the number or quality of other options. This independent valuation is exactly what is needed for rationality.
A possible objection to this idea is that many scouts do in fact visit multiple options. Mallon et al. (2001) found that as few as 32 per cent but as many as 86 per cent of scouts visited both available candidate sites at some point during the emigration. Robinson et al. (2009), looking at a larger sample of emigrations, found that 27 ± 11% of active ants visited both sites before the start of transport. Thus, while most ants do not have the opportunity to compare sites, a significant minority do. If these better-informed ants use comparative heuristics to evaluate the sites that they see, then we might expect their presence to place the colony's overall choice at risk of irrational errors. There are two possible explanations for why this did not occur. First, the colony's collective choice may be more heavily influenced by those ants that visit only one site—a large majority in most cases where their numbers have been counted. Although some evidence suggests that ants visiting more than one site may have a particularly strong influence (Robinson et al. 2009), their relative importance has not been investigated conclusively. Second, even ants that have the opportunity may not make direct comparisons between sites (Robinson et al. 2009). Current evidence is ambiguous about whether well-informed ants make direct comparisons (Mallon et al. 2001; Robinson et al. 2009). This study can shed no further light, as we did not monitor how many individuals visited more than one site. Further experimental work will be required to understand how both the opportunity and the capacity for individual comparison affect collective decision-making.
This study set out to analyse the mechanistic basis of collective decision-making by seeking evidence for systematic errors. Although we did not see the irrational behaviour predicted by previous research on solitary animals, this finding itself supports a novel hypothesis about the effect of collective mechanisms on decision performance. That is, the highly parallel nature of collective choice, in which single individuals lack either the opportunity or the ability to compare directly the full range of available options, may offer a bulwark against certain kinds of irrational, fitness-reducing error. Rigorous evaluation of this hypothesis will require that larger numbers of colonies be presented with a greater range of options and decoy designs, and that other rationality principles, such as transitivity, be tested. In addition, experiments should address decoy effects on decision speed, as well as accuracy. It will be especially important to determine unequivocally whether individuals can make direct comparisons between nest sites and whether they are subject to predictable irrationality. If so, it will be valuable to test the prediction that whole colonies are rational in the same conditions that evoke irrationality in individuals.
Problem solving by insect societies relies on highly decentralized information processing. This partly reflects cognitive and information-processing constraints: individual insects cannot handle these problems alone, and colonies lack the hierarchical structures that might foster centralized decision-making. On the other hand, it has long been recognized that the decentralized design of insect societies offers great benefits in robustness and resilience. The results of this study support another advantage: the filtering out of systematic errors that would otherwise arise from the cognitive limitations of individual animals.
Acknowledgements
This work was supported by the Pew Charitable Trusts (award 2000-002 558). We thank Adi Livnat for stimulating discussions that inspired this project.
References
- Arrow K. J.1950A difficulty in the concept of social welfare. J. Polit. Econ. 58, 328–346 (doi:10.1086/256963) [Google Scholar]
- Bateson M., Healy S. D., Hurly T. A.2003Context-dependent foraging decisions in rufous hummingbirds. Proc. R. Soc. Lond. B 270, 1271–1276 (doi:10.1098/rspb.2003.2365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camazine S., Deneubourg J. L., Franks N. R., Sneyd J., Theraulaz G., Bonabeau E.2001Self-organization in biological systems Princeton, NJ: Princeton University Press [Google Scholar]
- Conradt L., Roper T.2005Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456 (doi:10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- Franks N. R., Mallon E. B., Bray H. E., Hamilton M. J., Mischler T. C.2003Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 65, 215–223 (doi:10.1006/anbe.2002.2032) [Google Scholar]
- Gardner A., Grafen A.2009Capturing the superorganism: a formal theory of group adaptation. J. Evol. Biol. 22, 659–671 (doi:10.1111/j.1420-9101.2008.01681.x) [DOI] [PubMed] [Google Scholar]
- Healey C. I. M., Pratt S. C.2008The effect of prior experience on nest site evaluation by the ant Temnothorax curvispinosus. Anim. Behav. 76, 893–899 (doi:10.1016/j.anbehav.2008.02.016) [Google Scholar]
- Hölldobler B., Wilson E. O.2008The superorganism New York, NY: Norton [Google Scholar]
- Houston A. I.1997Natural selection and context-dependent values. Proc. R. Soc. Lond. B 264, 1539–1541 (doi:10.1098/rspb.1997.0213) [Google Scholar]
- Huber J., Payne J. W., Puto C.1982Adding asymmetrically dominated alternatives: violations of regularity and the similarity hypothesis. J. Consum. Res. 9, 90–98 (doi:10.1086/208899) [Google Scholar]
- Hutchinson J. M. C., Gigerenzer G.2005Simple heuristics and rules of thumb: where psychologists and behavioural biologists might meet. Behav. Process. 69, 97–124 (doi:10.1016/j.beproc.2005.02.019) [DOI] [PubMed] [Google Scholar]
- Kahneman D.2003Maps of bounded rationality: psychology for behavioral economics. Am. Econ. Rev. 93, 1449–1475 (doi:10.1257/000282803322655392) [Google Scholar]
- King A., Cowlishaw G.2009Leaders, followers and group decision-making. Commun. Integr. Biol. 2, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland K. N., Brown G. R.2002Sense and nonsense: evolutionary perspectives on human behaviour Oxford, UK: Oxford University Press [Google Scholar]
- Langridge E. A., Sendova-Franks A. B., Franks N. R.2008How experienced individuals contribute to an improvement in collective performance in ants. Behav. Ecol. Sociobiol. 62, 447–456 (doi:10.1007/s00265-007-0472-5) [Google Scholar]
- Livnat A., Pippenger N.2008Systematic mistakes are likely in bounded optimal decision-making systems. J. Theor. Biol. 250, 410–423 (doi:10.1016/j.jtbi.2007.09.044) [DOI] [PubMed] [Google Scholar]
- Luce R. D.1959Individual choice behavior: a theoretical analysis New York, NY: Wiley [Google Scholar]
- Mallon E. B., Pratt S. C., Franks N. R.2001Individual and collective decision-making during nest site selection by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 50, 352–359 (doi:10.1007/S002650100377) [Google Scholar]
- Marshall J. A. R., Bogacz R., Dornhaus A., Planqué R., Kovacs T., Franks N. R.In press On optimal decision-making in brains and social insect colonies. J. R. Soc. Interface [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möglich M.1978Social organization of nest emigration in Leptothorax (Hym., Form.). Insectes Soc. 25, 205–225 (doi:10.1007/BF02224742) [Google Scholar]
- Newcombe R. G.1998Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat. Med. 17, 873–890 (doi:10.1002/(SICI)1097-0258(19980430)17:8<873::AID-SIM779>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- Passino K., Seeley T. D., Visscher P.2008Swarm cognition in honey bees. Behav. Ecol. Sociobiol. 62, 401–414 (doi:10.1007/s00265-007-0468-1) [Google Scholar]
- Pratt S. C.2005Behavioral mechanisms of collective nest-site choice by the ant Temnothorax curvispinosus. Insectes Soc. 52, 383–392 (doi:10.1007/s00040-005-0823-z) [Google Scholar]
- Pratt S. C., Pierce N. E.2001The cavity-dwelling ant Leptothorax curvispinosus uses nest geometry to discriminate between potential homes. Anim. Behav. 62, 281–287 (doi:10.1006/anbe.2001.1777) [Google Scholar]
- Pratt S. C., Mallon E. B., Sumpter D. J. T., Franks N. R.2002Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 52, 117–127 (doi:10.1007/s00265-002-0487-x) [Google Scholar]
- Pratt S. C., Sumpter D. J. T., Mallon E. B., Franks N. R.2005An agent-based model of collective nest choice by the ant Temnothorax albipennis. Anim. Behav. 70, 1023–1036 (doi:10.1016/j.anbehav.2005.01.022) [Google Scholar]
- Rieskamp J., Busemeyer J. R., Mellers B.2006Extending the bounds of rationality: evidence and theories of preferential choice. J. Econ. Lit. 44, 631–661 (doi:10.1257/jel.44.3.631) [Google Scholar]
- Robinson E. J. H., Smith F. D., Sullivan K. M. E., Franks N. R.2009Do ants make direct comparisons? Proc. R. Soc. B 276, 2635–2641 (doi:10.1098/rspb.2009.0350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck-Paim C., Pompilio L., Kacelnik A.2004State-dependent decisions cause apparent violations of rationality in animal choice. PLoS Biol. 2, e402 (doi:10.1371/journal.pbio.0020402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley T. D.1995The wisdom of the hive Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- Sen A. K.1993Internal consistency of choice. Econometrica 61, 495–521 (doi:10.2307/2951715) [Google Scholar]
- Shafir S.1994Intransitivity of preferences in honey bees: support for comparative evaluation of foraging options. Anim. Behav. 48, 55–67 (doi:10.1006/anbe.1994.1211) [Google Scholar]
- Shafir S., Waite T. A., Smith B. H.2002Context-dependent violations of rational choice in honeybees (Apis mellifera) and gray jays (Perisoreus canadensis). Behav. Ecol. Sociobiol. 51, 180–187 (doi:10.1007/s00265-001-0420-8) [Google Scholar]
- Stephens D. W., Krebs J. R.1986Foraging theory. Monographs in Behavior and Ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- Stephens D., Kerr B., Fernández-Juricic E.2004Impulsiveness without discounting: the ecological rationality hypothesis. Proc. R. Soc. Lond. B 271, 2459–2465 (doi:10.1098/rspb.2004.2871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. M., Gigerenzer G.2000Precis of simple heuristics that make us smart. Behav. Brain Sci. 23, 727–780 (doi:10.1017/S0140525X00003447) [DOI] [PubMed] [Google Scholar]
- Tversky A.1969Intransitivity of preferences. Psychol. Rev. 76, 31–48 (doi:10.1037/h0026750) [Google Scholar]
- Tversky A.1972Elimination by aspects: a theory of choice. Psychol. Rev. 79, 281–299 (doi:10.1037/h0032955) [Google Scholar]
- Tversky A., Simonson I.1993Context-dependent preferences. Manag. Sci. 39, 1179–1189 (doi:10.1287/mnsc.39.10.1179) [Google Scholar]
- Visscher P. K.2007Group decision making in nest-site selection among social insects. Annu. Rev. Entomol. 52, 255–275 (doi:10.1146/annurev.ento.51.110104.151025) [DOI] [PubMed] [Google Scholar]
- Waite T. A.2001Intransitive preferences in hoarding gray jays (Perisoreus canadensis). Behav. Ecol. Sociobiol. 50, 116–121 (doi:10.1007/s002650100346) [Google Scholar]
- Waksberg A., Smith A., Burd M.2009Can irrational behaviour maximise fitness? Behav. Ecol. Sociobiol. 63, 461–471 (doi:10.1007/s00265-008-0681-6) [Google Scholar]
- Wedell D.1991Distinguishing among models of contextually induced preference reversals. J. Exp. Psychol. Learn. Memory Cogn. 17, 767–778 (doi:10.1037/0278-7393.17.4.767) [Google Scholar]