Abstract
Culture has long been assumed to be uniquely human but recent studies, in particular on great apes, have suggested that cultures also occur in non-human primates. The most apparent cultural behaviours in great apes involve tools in the subsistence context where they are clearly functional to obtain valued food. On the other hand, tool-use to modify acoustic communication has been reported only once and its function has not been investigated. Thus, the question whether this is an adaptive behaviour remains open, even though evidence indicates that it is socially transmitted (i.e. cultural). Here we report on wild orang-utans using tools to modulate the maximum frequency of one of their sounds, the kiss squeak, emitted in distress. In this variant, orang-utans strip leaves off a twig and hold them to their mouth while producing a kiss squeak. Using leaves as a tool lowers the frequency of the call compared to a kiss squeak without leaves or with only a hand to the mouth. If the lowering of the maximum frequency functions in orang-utans as it does in other animals, two predictions follow: (i) kiss squeak frequency is related to body size and (ii) the use of leaves will occur in situations of most acute danger. Supporting these predictions, kiss squeaks without tools decreased with body size and kiss squeaks with leaves were only emitted by highly distressed individuals. Moreover, we found indications that the calls were under volitional control. This finding is significant for at least two reasons. First, although few animal species are known to deceptively lower the maximum frequency of their calls to exaggerate their perceived size to the listener (e.g. vocal tract elongation in male deer) it has never been reported that animals may use tools to achieve this, or that they are primates. Second, it shows that the orang-utan culture extends into the communicative domain, thus challenging the traditional assumption that primate calling behaviour is overall purely emotional.
Keywords: orang-utan, tool use, sound, deception, innovation, culture
1. Introduction
Studies of animal cultures have often focused on tool-use innovations for subsistence purposes (e.g. Whiten et al. 1999; Van Schaik & Knott 2001; Lefebvre et al. 2002; Van Schaik et al. 2003, 2006; Krützen et al. 2005), defining tools as objects that are used as an extension of the body and that are held directly in the hand or mouth (Lefebvre et al. 2002, see also Beck 1980). However, tool-based innovations in acoustic communication that modify the acoustical properties of a call have been virtually absent, and the only case where such an innovation has been proposed concerns orang-utans (Van Schaik et al. 2003, 2006; Hardus et al. 2009). Nevertheless, the function of this behaviour is not clear (Peters 2001), raising questions about its adaptiveness and dispersal within populations.
Here we report on tool use and its function in one particular call, the kiss squeak, in wild Bornean orang-utans (Pongo pygmaeus wurmbii). The kiss squeak has been described as a sharp intake of air through pursed lips causing a kissing sound (Rijksen 1978; Hardus et al. 2009). This call is produced by all orang-utan age–sex classes in response to disturbance and/or fear towards potential predators (e.g. snakes, clouded leopards, tigers, humans) or other orang-utans upon sight, at times accompanied by display behaviour and most likely to deter the potential predator (Rijksen 1978; Hardus et al. 2009). Because orang-utans are semi-solitary animals living in fission–fusion societies (Delgado & Van Schaik 2000), it often takes hours before conspecifics are attracted to the kiss squeaks (Van Noordwijk & Van Schaik 2009), so its most likely function is to send a signal to the predator or approaching orang-utan and not to attract other conspecifics.
Kiss squeaks can be given in three different forms: unaided, and with either a hand or leaves positioned in front of the lips (Peters 2001; Van Schaik et al. 2003), and these forms are given in the same context and occasionally within the same bout. The leaves function as a tool because they are first stripped off from a twig and then held in one hand as a bundle on the mouth while the kiss squeak is produced (see video file, electronic supplementary material). Leaves used as tools are close at hand and leaf species seem to be chosen indiscriminately. Because the presence or absence of kiss squeaks on leaves in a particular orang-utan population does not appear to be genetically or ecologically determined, this behaviour has been suggested to arise as an innovation and to be culturally transmitted, consistent with its limited geographical distribution and high local prevalence (Van Schaik et al. 2003, 2006; Hardus et al. 2009). Our results indicate that producing kiss squeaks on leaves functions to make the caller sound larger, because it lowers the call's maximum frequency (frequency with the highest power) and because the frequency of unaided kiss squeaks is negatively correlated with body size.
2. Methods
(a). Study site
All kiss squeaks were recorded from wild orang-utans at research station Tuanan (2°09′06″ S; 114°26′26″ E), Central Kalimantan, Borneo, Indonesia. This study area is composed of forest on shallow peat with relatively homogeneous canopy. Orang-utan kiss squeaks were recorded from January 2003 until August 2005.
(b). Data collection and data analysis
All kiss squeaks produced by a focal orang-utan and/or its associates were recorded opportunistically using a Marantz Analogue Recorder PMD222 in combination with a Sennheiser Microphone ME 64 or a Sony Digital Recorder TCD-D100 in combination with a Sony Microphone ECM-M907. All incidents of recorded kiss squeaks occurred throughout the day. We digitized all the recorded kiss squeaks at 44.1 kHz, using Raven Interactive Sound Analysis Software (2003, Cornell Lab of Ornithology, Ithaca, NY, USA). We transformed all recordings into spectrograms (window type = Hanning; spectrogram configuration: time grid spacing = 256; samples/frame overlap = 50%; frequency grid spacing = 86.1; window size = 512 samples; 3 dB bandwidth = 124 Hz, i.e. narrow-band like spectrogram). A call's maximum frequency (Hz), maximum power (dB) and duration (s) were measured from spectrograms to compare the different kiss-squeak forms. Maximum frequency represents the frequency with the highest energy emitted in a call, independently of its location. Maximum power represents the energy of the maximum frequency (i.e. loudness). Duration represents the time period between the beginning and the end of the call. Because kiss squeaks are brief and rather noisy atonal calls (cf. Struhsaker 1967), the number of measurable acoustic parameters was limited. Maximum power was analysed within recording bouts in order to control for distance between recorder and focal, recording volume settings and acoustic environment during recordings. Recordings from adult females were less represented in our sample because these animals were most frequently followed and thus less disturbed by the presence of human observers. Consequently, recordings of kiss squeaks on leaves were biased towards adult males.
3. Results and discussion
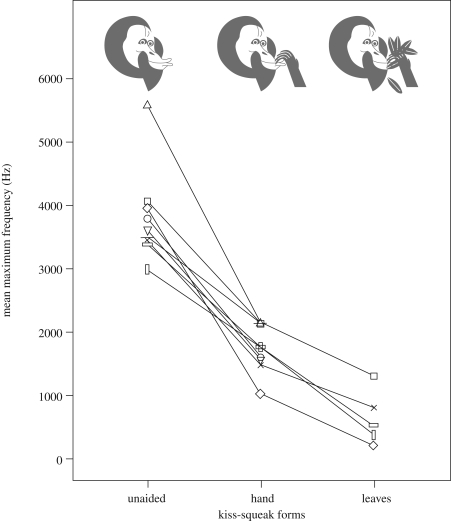
The maximum acoustic frequency of the three different kiss-squeak forms are significantly different from each other within individual orang-utans (figure 1; Friedman test: n = 813 calls emitted in disturbance contexts by eight adults and one adolescent individual; χ2 = 1596.0, p < 0.001; followed by a multiple comparisons post hoc test: p < 0.001 between all three forms). Specifically, the maximum frequency decreased progressively from the unaided kiss squeak to kiss squeak with hand, to kiss squeak on leaves (figure 1). Maximum power did not differ between kiss-squeak forms within recording bouts (paired-samples t-test: unaided/hand, n = 62 call pairs, p = 0.848; unaided/leaves, n = 14 call pairs, p = 0.357). Duration also did not differ among the three kiss-squeak forms (Kruskall–Wallis test, n = 536 calls emitted towards observers by 17 adult individuals, χ2 = 3.813, p = 0.149). Hence, these results do not support the suggestion by Peters (2001) that kiss-squeak forms with hand and on leaves function to increase the volume, and ‘somewhat’ the frequency of the call. Ambient factors, e.g. branches, leaves, trunks or atmospheric factors, produce rapid attenuation of high acoustic frequencies and a shift to lower frequencies (Lameira & Wich 2008), thus a decrease in a call's frequency generated by positioning some item in front of the mouth during emission supports this prediction that is well-supported by various studies (e.g. Wiley 1991; Brown 2003).
Figure 1.
Mean maximum frequency (Hz) of each kiss-squeak form for nine orang-utans. Triangle, Kondor (adolescent female); circle, Mindi (parous female); vertical rectangle, Rambo (flanged male); straight line, Kay (flanged male); inverted triangle, Fugit (flanged male); cross, Sultan (flanged male); diamonds, Preman (unflanged male); horizontal rectangle, SAM1 (unflanged male); square, SAM2 (unflanged male). Illustrations by A. R. Lameira.
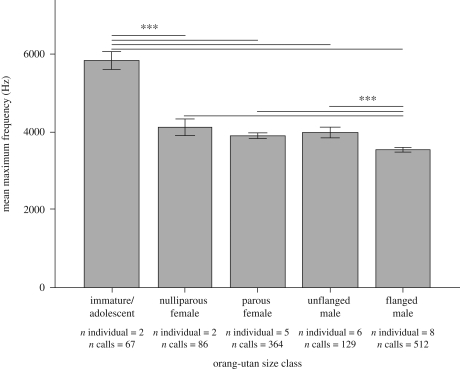
We hypothesize that lowering the maximum frequency functions to mislead the receiver that the producer has a larger body size than it actually has. Body size enlargement behaviour occurs throughout the animal kingdom in situations in which an individual is disturbed (e.g. fur-bristle in cats, air-swallowing and rising-on-legs in frogs, pilo-erection in chimpanzees), but it is rare in mammalian-calling behaviour (e.g. Fitch & Reby 2001; Matrosova et al. 2007). In accordance with this hypothesis, unaided kiss squeaks decreased significantly in their maximum frequency with age and thus with increased body size (Kruskal–Wallis test: KW = 238.7, d.f. = 4, p < 0.001; followed by a multiple comparisons post hoc test: p < 0.001 between immatures and all the other size classes and between flanged males and all the other size classes, figure 2). To our knowledge this is the first evidence of how and why non-human primates affect their own call variables through the use of hand and tools.
Figure 2.
Mean maximum frequency (Hz) per size class of the unaided kiss squeak. Error bars: ±2 s.e. ***p < 0.001.
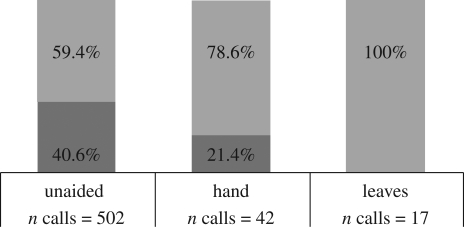
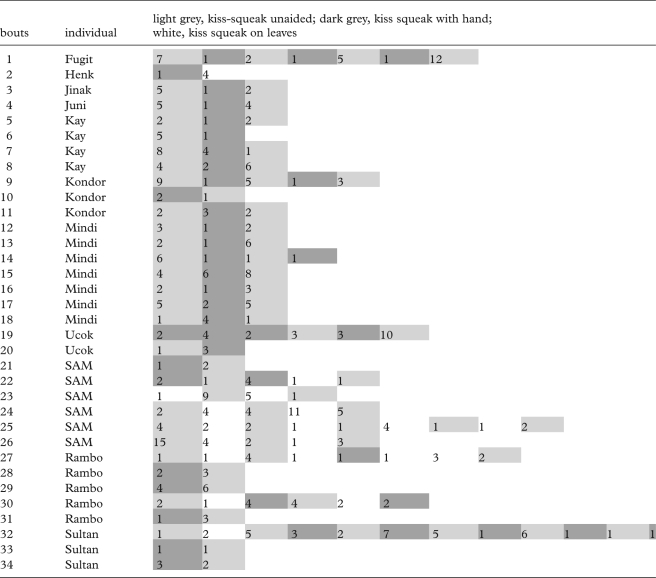
Because dishonestly signalling larger body size might yield the greatest advantage in highly dangerous situations, we expect that the use of kiss squeaks on leaves would increase in such conditions. It is likely that for unhabituated orang-utans, encounters (i.e. when parties detect each other) with human observers qualify as dangerous. Unhabituated orang-utans were those that could not be followed for several days without showing signs of disturbance, expressed in flight, displays and the emission of disturbance calls. Indeed, kiss squeaks on leaves were only produced by unhabituated orang-utans (n = 5) during encounters with human observers (n = 17 calls, figure 3). Kiss squeaks with the hand towards humans also made up a significantly higher proportion for unhabituated orang-utans (n = 10) than habituated orang-utans (n = 12) (χ2 = 5.46, d.f. = 1, p = 0.019, n = 42 calls, figure 3). On the other hand, unaided kiss squeaks were not emitted significantly more commonly among unhabituated (n = 15) than habituated orang-utans (n = 10) (χ2 = 0.44, d.f. = 1, p = 0.5, n = 502 calls, figure 3). When unhabituated orang-utans encountered humans, although it would be beneficial to modify all emitted kiss squeaks, orang-utans emitted kiss squeaks on leaves/with hand in combination with unaided kiss squeaks (table 1). It seems that this way orang-utans free their hands' for other beneficial purposes during an encounter with a potential predator, such as display, branch-missiles, movement through the canopy or escape. To refrain from emitting unaided kiss squeaks could prove deleterious by drastically decreasing the alarm call rate by the orang-utan facing a predator (see Zuberbühler et al. 1999).
Figure 3.
Percentage emitted by habituated and unhabituated individuals for each kiss-squeak form. Light grey, unhabituated; dark grey, habituated.
Table 1.
Kiss-squeak bouts comprising two or three forms.
 |
This pattern indicates that kiss squeaks with the hand and on leaves, i.e. with lower frequencies, were emitted in circumstances assumed to be more dangerous. This functional use is plausible because orang-utans are arboreal apes living in dense forests, where visibility is usually poor and rarely sufficient to make accurate visual assessments of body size, particularly in disturbing encounters when orang-utans use displays and missiles towards potential predators. This way, the visual salience hypothesis (Peters 2001) for the function of the kiss-squeak forms also seems unlikely owing to poor visibility and because the dropping of leaves is not executed in isolation, but in combination with branch-shaking, breaking and throwing for example. Although one might argue that individuals of bigger body size classes (e.g. flanged males) would not deploy kiss squeaks with the hand and on leaves for the purpose of functional deception, this interpretation ignores that all individuals may benefit from appearing larger in highly disturbing contexts. Thus, it is reasonable to assume that kiss-squeak forms function to deceptively convey to the predator a larger body size. Indeed, as expected, orang-utans were never observed or reported to emit kiss squeaks with the hand and on leaves towards other orang-utans, since producer and receiver will generally be familiar with one another and/or with the deceptive technique. At the same time, low-sound frequencies travel farther than high-sound frequencies (Lameira & Wich 2008), and thus the use of hands and leaves during the production of kiss squeaks could be meant for the purpose of conspecifics' recruitment. However, this is unlikely since recruitment of conspecifics via the emission of kiss-squeak forms, while not uncommon, usually takes much longer than the duration of an encounter (Van Noordwijk & Van Schaik 2009).
To confirm that kiss squeaks with hands and on leaves are innovations that spread within populations owing to their putative deceptive function, it is important that the behaviour is voluntary and not under strict emotional or motivational control. Although we cannot test this directly, the different kiss-squeak forms were given within the same bout per individual (34 bouts recorded for 11 individuals; table 1). No patterns were seen per bout that could indicate escalating irritation. Hence, they were used interchangeably within the same context showing no particular relationship to apparent changes in the underlying emotional/motivational state (Owren & Rendall 2001). Moreover, immature individuals are known to emit uncoordinated kiss squeaks with the hand and on leaves in playful situations (Hardus et al. 2009) (not considered in this study), which indicates that practice is important in the acquisition of these techniques. Orang-utans in captivity also use kiss squeaks to capture the attention of otherwise inattentive humans (Cartmill & Byrne 2007). Such flexible use of kiss squeaks suggests that the use of hands and leaves during the production of kiss squeaks and its functional use is, to some extent, under volitional control and thus both forms are suitable candidates for innovative call variants. Supporting this view, Hopkins et al. (2007) have reported the novel and functional use of certain calls by captive chimpanzees, implying that these behaviours are voluntary in some measure, and argued that this represents a form of social innovation that parallels those associated with tool use in other contexts (Leavens et al. 2005). Moreover, the spontaneous acquisition of human whistling by a captive orang-utan without prior training (Wich et al. 2009) demonstrates that orang-utans have particular flexibility and control over calls produced by the lips.
Because kiss squeaks with the hand are almost certainly a universal behaviour across orang-utan wild populations, while kiss squeaks on leaves are only present at certain sites, where they are highly prevalent (Van Schaik et al. 2003, 2006; Hardus et al. 2009), the results presented here suggest that kiss squeaks on leaves represent a functional innovation that spread locally and became cultural. Degree of habituation also determines whether or not orang-utans display this behaviour, but highly unhabituated individuals in some survey areas have failed to perform this behaviour. Thus, by further decreasing the kiss squeak's maximum frequency, the use of leaves functions to increase the caller's perceived body size, potentially intimidating a potential predator and possibly improving chances of survival.
In the end, the proposed deceptive function of kiss-squeak forms can only be evaluated by examining which potential predators elicit the kiss squeaks on leaves and how they respond to them. By following a radio-collared African leopard, Zuberbühler et al. (1999) found that after higher alarm call rates by primates, the leopard gave up its hiding location and left the hunt significantly faster than would be expected by chance. Schaller (1967) observed similar responses for Asian tigers. Because natural cases of predation on orang-utans are rare, playback experiments with potential predators may be needed to elucidate this function.
These results indicate that non-human primate tool-use innovations are more extensive than hitherto appreciated and actually include acoustic communication, where they may play a functional role. This opens the possibility for cultural evolution in non-human primate communication.
Acknowledgements
This study complied with the current laws of Indonesia.
We are grateful to LIPI for authorization to carry out research in Indonesia; BOS for permission to work at Mawas; UNAS for acting as a sponsor; Jo Kolk Foundation, Netherlands Organisation for Scientific Research (NWO), The Leakey Foundation, the National Geographical Society and Utrechts Universiteitsfonds for financial support; Han de Vries and Liesbeth Sterck for (statistical) advice and Tine Geurts and Maria Van Noordwijk for the video data in the electronic supplementary material; three anonymous reviewers for suggestions on a previous version of this manuscript. The authors have declared that no competing interests exist.
References
- Beck B. B.1980Animal tool behavior: the use and manufacture of tools by animals New York, USA: Garland, STPM Press [Google Scholar]
- Brown C. H.2003Ecological and physiological constraints for primate vocal communication. In Primate audition: ethology and neurobiology (ed. Ghazanfar A. A.), pp. 127–149 Boca Raton, FL: CRC Press [Google Scholar]
- Cartmill E. A., Byrne R. W.2007Orangutans modify their gestural signaling according to their audience's comprehension. Curr. Biol. 17, 1345–1348 (doi:10.1016/j.cub.2007.06.069) [DOI] [PubMed] [Google Scholar]
- Delgado R. A., Van Schaik C. P.2000The behavioral ecology and conservation of the orangutan (Pongo pygmaeus): a tale of two islands. Evol. Anthropol. 9, 201–218 (doi:10.1002/1520-6505(2000)9:5<201::AID-EVAN2>3.0.CO;2-Y) [Google Scholar]
- Fitch W. T., Reby D.2001The descended larynx is not uniquely human. Proc. R. Soc. Lond. B 268, 1669–1675 (doi:10.1098/rspb.2001.1704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardus M. E., Lameira A., Singleton I., Knott C. D., Morrogh-Bernard H., Ancrenaz M., Utami-Atmoko S. S., Wich S. A.2009A description of the orangutan's vocal and sound repertoire, with a focus on geographic variation. In Orangutans (eds Wich S., Utami S., Setia T., Van Schaik C.), pp. 49–64 Oxford, UK: Oxford University Press [Google Scholar]
- Hopkins W. D., Taglialatela J. P., Leavens D. A.2007Chimpanzees differentially produce novel vocalizations to capture the attention of a human. Anim. Behav. 73, 281–286 (doi:10.1016/j.anbehav.2006.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krützen M., Mann J., Heithaus M. R., Connor R. C., Bejder L., Sherwin W.2005Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. 102, 8939–8943 (doi:10.1073/pnas.0500232102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameira A. R., Wich S. A.2008Orangutan long call degradation and individuality over distance: a playback approach. Int. J. Primatol. 29, 615–625 (doi:10.1007/s10764-008-9253-x) [Google Scholar]
- Leavens D. A., Hopkins W. D., Bard K. A.2005Understanding the point of chimpanzee pointing: epigenesis and ecological validity. Am. Psychol. Soc. 14, 185–189 (doi:10.1111/j.0963-7214.2005.00361.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L., Nicolakakis N., Boire D.2002Tools and brains in birds. Behaviour 139, 939–973 (doi:10.1163/156853902320387918) [Google Scholar]
- Matrosova V. A., Volodin I. A., Volodina E. V., Babitsky A. F.2007Pups crying bass: vocal adaptation for avoidance of age-dependent predation risk in ground squirrels? Behav. Ecol. Sociobiol. 62, 181–191 (doi:10.1007/s00265-007-0452-9) [Google Scholar]
- Owren M., Rendall D.2001Sound on the rebound: bringing form and function back to the forefront in understanding non-human primate vocal signalling. Evol. Anthropol. 10, 58–71 (doi:10.1002/evan.1014) [Google Scholar]
- Peters H. H.2001Tool use to modify calls by wild orangutans. Folia Primatol. 72, 242–244 (doi:10.1159/000049943) [DOI] [PubMed] [Google Scholar]
- Rijksen H. D.1978A field study on Sumatran orangutans (Pongo pygmaeus abelli Lesson 1872): ecology, behaviour and conservation Wageningen, The Netherlands: Veenman & Zonen [Google Scholar]
- Schaller G. B.1967The deer and the tiger Chicago, IL: University of Chicago Press [Google Scholar]
- Struhsaker T. T.1967Auditory communication among vervet monkeys (Cercopithecus aethiops). In Social communication among primates (ed. Altmann S. A.), pp. 128–161 Chicago, IL: University of Chicago Press [Google Scholar]
- Van Noordwijk M. A., Van Schaik C. P. Intersexual food transfer among orangutans: do females test males for coercive tendency? Behav. Ecol. Soc. Biol. 2009 ( doi:10.1007/s00265-009-0728-3) [Google Scholar]
- Van Schaik C. P., Knott C. D.2001Geographic variation in tool use on Neesia fruits in orangutans. Am. J. Phys. Anthropol. 114, 331–342 (doi:10.1002/ajpa.1045) [DOI] [PubMed] [Google Scholar]
- Van Schaik C. P., Ancrenaz M., Borgen G., Galdikas B., Knott C. D., Singleton I., Suzuki A., Utami S. S., Merrill M. Y.2003Orangutan cultures and the evolution of material culture. Science 299, 102–105 (doi:10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- Van Schaik C. P., Van Noordwijk M., Wich S. A.2006Innovation in wild Bornean orangutans (Pongo pygmaeus wurmbii). Behaviour 143, 839–876 (doi:10.1163/156853906778017944) [Google Scholar]
- Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V., Sugiyama Y., Tutin C. E. G., Wrangham R. W., Boesch C.1999Cultures in chimpanzees. Nature 399, 682–685 (doi:10.1038/21415) [DOI] [PubMed] [Google Scholar]
- Wich S. A., Swartz K., Hardus M. E., Lameira A. R., Stromberg E., Shumaker R. W.2009A case of spontaneous acquisition of a human sound by an orangutan. Primates 50, 56–64 (doi:10.1007/s10329-008-0117-y) [DOI] [PubMed] [Google Scholar]
- Wiley R. H.1991Associations of song properties with habitats for territorial oscine birds of eastern North America. Am. Nat. 138, 973–993 (doi:0003-0147/91/3804-001) [Google Scholar]
- Zuberbühler K., Jenny D., Bshary R.1999The predator deterrence function of primate alarm calls. Ethology 105, 477–490 (doi:10.1046/j.1439-0310.1999.04??.x) [Google Scholar]