Abstract
Ectotherms have evolved preferences for particular body temperatures, but the nutritional and life-history consequences of such temperature preferences are not well understood. We measured thermal preferences in Locusta migratoria (migratory locusts) and used a multi-factorial experimental design to investigate relationships between growth/development and macronutrient utilization (conversion of ingesta to body mass) as a function of temperature. A range of macronutrient intake values for insects at 26, 32 and 38°C was achieved by offering individuals high-protein diets, high-carbohydrate diets or a choice between both. Locusts placed in a thermal gradient selected temperatures near 38°C, maximizing rates of weight gain; however, this enhanced growth rate came at the cost of poor protein and carbohydrate utilization. Protein and carbohydrate were equally digested across temperature treatments, but once digested both macronutrients were converted to growth most efficiently at the intermediate temperature (32°C). Body temperature preference thus yielded maximal growth rates at the expense of efficient nutrient utilization.
Keywords: development, energy budget, geometric framework, phenotypic plasticity, nutrient utilization efficiency, growth rate
1. Introduction
Nearly all physiological and behavioural activities are temperature-sensitive (recently reviewed by Chown & Terblanche 2007), and metabolic rate, feeding, digestion, growth, assimilation and development are often maximized at different temperatures in ectotherms (Damme et al. 1991; Du et al. 2000). Therefore, any given body temperature preference should result in the prioritization of some processes over others, and it is known that ectotherms will adjust their temperature preferences to reflect changing circumstances and physiological priorities (e.g. Bernheim et al. 1978; Beuchat & Ellner 1987; Brett 2001; Elliot et al. 2005). The life-history and performance consequences of temperature preference, however, remain poorly understood.
Two key life-history variables are development time and size at maturity (Stearns 1992). These in turn are affected by the efficiency and rate at which ingested food is converted to growth. In ectotherms, temperature is closely linked to growth, with adult body size and temperature generally inversely related (Atkinson 1994; Kingsolver & Huey 2008). Theoretical work based on the Sharpe–Schoolfield equation predicts that increased temperature leads to a smaller size at maturity in systems where temperature affects cellular differentiation rates more so than growth rates (Schoolfield et al. 1981; van der Have & de Jong 1996). Growth efficiencies are determined in part by the proportion of energy intake diverted away from mass increase to activities such as food acquisition, mating, dispersal, defence and competition (Zera & Denno 1997). Increasing temperatures have been empirically associated with decreased growth efficiency in some systems (e.g. Karl & Fisher 2008), but where interactive effects of diet and temperature upon growth have been investigated, consideration of body temperature preference and nutrient utilization were not the focus (e.g. Stillwell et al. 2007).
Here, the preferred body temperatures of locusts were measured, and the hypothesis that these preferences result in the prioritization of some life-history or physiological processes at the expense of others was tested. We predicted that growth and development rates may relate to efficient resource utilization in a temperature-dependent manner, perhaps with reduced digestive efficiency at high temperatures owing to faster gut passage rates (Yang & Joern 1994). To test this hypothesis, we used a full-factorial design to investigate the ways in which temperature and dietary regime affect ecophysiological factors such as feeding, digestion, activity, growth and development. Locusts are an ideal study system for such an inquiry because they are among the best studied systems in nutritional biology (Simpson & Raubenheimer 2000) and they show active behavioural thermoregulation (e.g. Lactin & Johnson 1996). Furthermore, links between nutrition, individual behaviour, group behaviour, and population dynamics have been derived, offering the potential for understanding processes from individual to environmental levels (Buhl et al. 2006; Clissold et al. 2006; Anstey et al. 2008; Bazazi et al. 2008). In this study, final nymphal stadium migratory locusts (Locusta migratoria) were reared at three temperatures representative of their natural thermal range (26, 32 and 38°C) and given different nutritional challenges. The geometric framework, a graphical method of visualizing nutritional interactions (Simpson & Raubenheimer 1995; Raubenheimer et al. 2009), was used to investigate temperature-dependent relationships between growth, development, and macronutrient-specific consumption (intake) and utilization (conversion of ingesta into body mass). To better understand these relationships, activity levels were assessed as a function of temperature and budgets were generated for energy intake and usage over the final nymphal stadium.
2. Material and methods
(a). Study population
Migratory locusts (L. migratoria) display dramatic density-dependent phenotypic plasticity (Pener & Simpson 2008) and are the most widespread species of locust. Experimental insects (L. migratoria, Taronga Zoo breeding facility, Sydney, NSW, Australia; originally isolated from Central Highlands of Queensland, Australia) were reared at the University of Sydney, Australia, and fed seedling wheat and wheatgerm. Each rearing bin (56 cm × 76 cm × 60 cm) contained 500–1000 insects beneath heat lamps in a room kept at 31–33°C and an LD 14 : 10 h cycle.
(b). Measurement of body temperature preference
A total of 40 fifth-instar insects (eight independent trials, each with five insects, performed on two successive days between 10.00 and 12.00) were placed at random positions in terraria (90 cm × 30 cm) over semi-overlapping heating pads that formed temperature gradients from 25 to 45°C. These insects were distinct from those in other experiments, and were allowed to self-regulate the composition of their diets (analogous to ‘choice’ regimes described below, with ad libitum access to food and water) and freely thermoregulate by varying heat lamp proximity prior to experiments. In the trials, insects were allowed to move unrestricted for 1.5 h, and temperatures at final positions were measured by placement of a 0.5 mm beaded wire type K thermocouple (YC-747D, Yu Ching Technology, Taipei, Taiwan) in the air as close as possible to the vertical midpoint of each insect's thorax (Wilson et al. 2002).
(c). Experimental procedure
A three-by-three multi-factorial design was employed wherein locusts were reared in all combinations of three temperatures on three dietary regimes, creating a total of nine treatment groups. Each group contained 10 naive insects distinct from those used in body temperature preference assessments. Dry, granular, chemically defined diets were prepared as described in Simpson & Abisgold (1985). The three dietary regimes were composed as follows: (i) no-choice high-protein diet (32% protein,10% digestible carbohydrate), (ii) no-choice high-carbohydrate diet (10% protein, 32% digestible carbohydrates), and (iii) a choice dietary regime in which animals had access to diets (i) and (ii) simultaneously. The fifth stadium was selected as the experimental period, since it represents the developmental stage during which the most somatic growth occurs (Uvarov 1966). The 90 experimental locusts were collected within 4 h of ecdysis into the fifth stadium, weighed to an accuracy of 10−5 g (AX205 scales, Mettler-Toledo, Inc., Columbus, OH, USA) and individually housed within clear plastic boxes (11 cm × 17 cm × 5 cm) containing water and pre-weighed synthetic food (representing the assigned dietary regime) in Petri dishes (3 cm diameter). Locusts representing each dietary regime were immediately placed into one of three experimental rooms, maintained at 26, 32 and 38°C (all rooms ±1.5°C), under an LD 12 : 12 h photoregime. Insects were checked twice daily for moulting, and each was weighed along with relevant uneaten food (in randomized order) every second day to determine growth and intake. Faeces (frass) were collected for quantification. All insects were provided with food and water ad libitum.
(d). Carcass measurements and chemical analysis
On the day of ecdysis into adulthood, insects were frozen, lyophilized for 72 h and weighed to yield final dry mass. Lipid body mass was determined using chloroform extraction techniques as previously described (Lee et al. 2002). Lipid-free carcasses were mill-powdered and nitrogen content was determined using a dry combustion analyser (NA2000, Carlo Erba, Milan, Italy). Protein content was estimated by multiplying nitrogen content by 6.25 (a widely used conversion factor; e.g. Slansky & Scriber 1985). Tibia lengths were measured to an accuracy of 0.01 mm using digital microcalipers. Total non-structural carbohydrates in formulated diets (controls) and frass were quantified by phenol/sulfuric acid methods (Dubois et al. 1956; Smith et al. 1964). Protein in formulated diets (included as controls) and frass were assayed by the Bradford method (Bradford 1976).
(e). Measurement of temperature-dependent activity levels
A total of 30 naive insects were taken from the colony and individually housed within clear plastic boxes (11 cm × 17 cm × 5 cm). These insects were distinct from both previous experimental groups. Insects were allowed to self-compose diets from complementary sources (choice regimes) while placed in rooms at 26, 32 and 38°C and allowed to acclimate for 24 h. Each insect was then observed continuously for 20 min each hour between 15.00 and 17.00 (lights on at 07.00), generating a total of 20 observation hours. This time interval was selected to represent the period of peak daytime activity most likely to manifest potential differences between temperatures; activity levels of L. migratoria are very low in the dark (Uvarov 1977). Onset and termination of activity (locomotion or grooming) were recorded, and bouts of activity and active time fraction were computed separately for all temperatures.
(f). Computation of resting metabolic rate and resting energy consumption over the stadium
Insect resting metabolic rate (RMR) is influenced strongly by mass (Chown et al. 2007) and temperature (Keister & Buck 1964), and measurements that control for these factors are usually repeatable (e.g. Nespolo et al. 2003). Furthermore, metabolic rate–temperature relationships seem to be constant despite potential variation in age, gender, temperature acclimation and other insect conditions including feeding and reproductive status (Terblanche et al. 2005). Mass–RMR relationships in L. migratoria have been described, but are limited in scope owing to small sample sizes (Clarke 1957). A temperature-dependent term was therefore introduced into an intraspecific mass–RMR relationship from another orthopteran Acheta domesticus (Hack 1997) to yield:
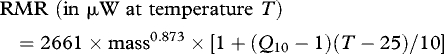 |
2.1 |
In initial calculations, a Q10 of 1.7 was assumed (Chappell 1983) to compute RMRs for a range of locust weights and temperatures using equation (2.1). These rates were integrated through the body mass × temperature space, accounting for both insect mass and time spent in the stadium, to estimate temperature-dependent resting metabolic energy usage during the fifth stadium. Later, the sensitivity of these results was tested using Q10 values ranging from 1.5 to 2.2. A second, simpler mass–RMR relationship (Clarke 1957), based upon a biphasic linear regression in L. migratoria, was used in parallel to check the conclusions of the A. domesticus mass–RMR model. The subsequent energy budget uses an average of the two estimates. Exact quantities of frass protein and carbohydrate were ascertained for determination of macronutrient utilization (as described previously), and non-cellulose frass was subsequently assumed to contain 17 kJ g−1 (as previously measured by Gandar 1982) for energy budgeting.
(g). Statistical analysis
All data were analysed in SPSS 14 (SPSS Inc., Chicago, IL, USA). Where variance-based tests were used, homoscedasticity was confirmed by Levene's tests and spread-level plots and normality of errors was checked by Shapiro–Wilk tests. Appropriate post hoc tests (Sidak-corrected or planned contrasts) were employed. Utilization efficiencies, defined as the fraction of ingested nutrients incorporated into body mass, were compared using ANCOVA as follows (see also Raubenheimer & Simpson 1992, 1994). Growth variables (gain in total dry weight, protein mass or lipid mass) were cast as dependent variables in models, with intake variables (P eaten, C eaten or total nutrients eaten) included as covariates and temperature as a fixed factor. Dietary regime was also included, but was removed in every case because macronutrient intake was far more predictive of dependent variables and was additionally strongly correlated to dietary regime (thus causing computational difficulties associated with collinearity when both variables were included). Temperature × diet interaction terms, as well as variables representing the sex of experimental subjects, were non-significant (p > 0.05) and were removed from the models. The equality of slopes assumption was tested by specific inclusion of covariate by temperature interaction terms; these were also non-significant and were removed from subsequent models. Utilization efficiency data for protein, carbohydrate and protein and carbohydrate together were visualized using utilization plots to avoid numerous pitfalls of traditional ratio-based measures (Raubenheimer & Simpson 1994). ANCOVA-based equivalents of efficiency of conversion of ingested nutrients to growth (ECI) and efficiency of conversion of digested nutrients to growth (ECD) were computed using stadium dry mass gain (total mass gain, lipid gain, protein gain) as a dependent variable, with nutrient eaten or digested, respectively, included as covariates. An ANCOVA-based equivalent of approximate digestibility was computed using frass nutrient content as a dependent variable and nutrient eaten as a covariate. Stadium duration, growth rate and RMR temperature trend data were analysed by the distribution-free Jonckheere–Terpstra test for categorically related groups (Terpstra 1952; Jonckheere 1954). Activity levels (time-fraction active and activity bouts) were analysed by Kruskal–Wallis and Jonckheere–Terpstra tests.
3. Results
(a). Body temperature preference
Locusts did not choose random positions along the temperature gradient (χ2 = 25.4; d.f. = 5; p < 0.001), suggesting thermoregulation under trial conditions. No effect of trial was detected upon selected temperature (Kruskal–Wallis test, χ2 = 7.983; d.f. = 7; p = 0.334) and the mean preferred body temperature was 38.3 ± 0.6°C s.e. (figure S1, electronic supplementary material).
(b). Temperature-dependent regulation of macronutrient intakes
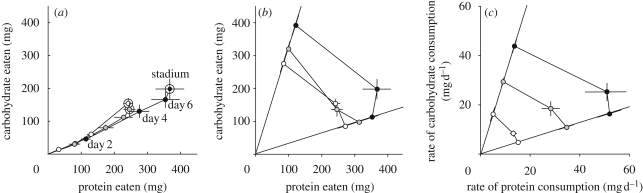
When locusts were allowed to compose their own diet from two unbalanced but complementary food sources, the quantity of nutrients eaten varied significantly by temperature (figure 1a,b): insects at 38°C ate significantly more over the stadium than insects at either 32 or 26°C (mean ± s.e.: 38°C, 565 ± 58 mg; 32°C, 382 ± 11 mg; 26°C, 396 ± 25 mg; F2,51 = 9.1, p < 0.001; Sidák-corrected post hoc p = 0.005 and p = 0.001, respectively), while insects at 26 and 32°C did not differ significantly in their stadium intakes (Sidák-corrected post hoc p = 0.694). The ratio of protein to carbohydrate in the selected diets did not differ significantly by temperature (Kruskal–Wallis test, χ2 = 2.223; d.f. = 2; p = 0.329), as indicated in figure 1a by the overlapping cumulative intake trajectories.
Figure 1.
Temperature-dependent macronutrient intake during the fifth stadium. (a) Self-selected (choice) cumulative intake trajectories (bivariate means with standard errors; corrected for insect initial mass) of insects through protein–carbohydrate (P–C) space at 26, 32 and 38°C. For each time course, points moving away from the origin (labelled in the case of 38°C) represent cumulative intake on days 2, 4, 6 and day-of-molt, respectively. Large white circles, stadium intake target. (b) Comparison of stadium intake between insects restricted to diets (no-choice groups representing P : C, 32 : 10 and 10 : 32; diagonal lines from origin) with intake from insects allowed continuous access to both diets (choice groups self-selecting positions in the intervening P–C space) at 26, 32 and 38°C. (c) Diverse daily mean rates and standard errors of P and C intake by choice and no-choice insects at 26, 32 and 38°C. n = 30 for each temperature; insects failing to moult were excluded from further analysis. Small white circles, 26°C; grey circles, 32°C; black circles, 38°C.
The rate of food intake over the stadium was significantly related to temperature (figure 1c; Kruskal–Wallis test, χ2 = 21.401; d.f. = 2; p < 0.001), and cooler temperatures resulting in slower intake rates (Jonckheere–Terpstra test, p < 0.001). Diet treatment was not a significant predictor of total nutrient intake across the stadium (figure 1b; F2,51 = 0.974, p = 0.384).
(c). Temperature-dependent growth and development
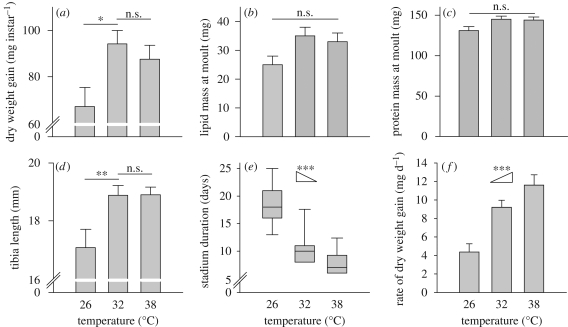
Dry mass gain and tibia length of locusts (figure 2a,d) at 26°C were significantly less than those at 32 and 38°C, whereas the latter two temperatures did not differ significantly (table 1). Protein and lipid masses at the end of the stadium (adjusted for initial mass) did not differ significantly as a function of temperature (figure 2b,c, table 1), although a post hoc power test indicated a relatively low power (0.55) to detect effects of these sizes. However, the sum of protein and lipid masses resulted in significantly less total dry mass gain at 26°C. Further, lipid and protein masses differed as a function of dietary regime (table 1), with insects on high-protein diet having higher protein and lower lipid masses than those on high-carbohydrate diet, and locusts on the choice treatment intermediate for both variables.
Figure 2.
Temperature-dependent growth and development over the fifth stadium. (a–d,f) Growth parameter means and standard errors. Increases in (a–c) mass and (d) size either significantly or strongly tend to be less at 26°C versus warmer temperatures. Insects at 32 and 38°C experience indistinguishable gains over the stadium in (a) total dry weight, (b) lipid mass, (c) lean mass and (d) tibia length. (e) However, because stadium duration significantly decreases with increasing temperature (boxplot whiskers show 10th and 90th percentiles), (f) rates of weight gain increase with temperature. (b,c) Marginal means and standard errors following ANCOVA analysis including dietary regime as a fixed factor and initial mass as a covariate. Tibia length is adjusted for initial mass. Diet did not significantly influence variables in (a), (d), (e) or (f). n = 30 for each temperature; insects failing to moult were excluded from further analysis. ANOVA/ANCOVA results presented in table 1. *p < 0.05; **p < 0.01; ***p < 0.001; n.s.= p > 0.05. Ramp symbols in (e) and (f) indicate Jonckheere–Terpstra trend tests.
Table 1.
Growth and development ANOVA/ANCOVA and post hoc analysis summary for all treatments. Diet and temp × diet factors and an initial mass covariate were included in each analysis but removed when p > 0.10. Temp × diet factor was not significant in any case. *p < 0.05; **p < 0.01.
| variable | source | d.f. | mean square | F | overall p | post hoc (LSD)a contrasts | contrast p |
|---|---|---|---|---|---|---|---|
| dry weight gain | between groups | 2 | 0.003 | 4.227 | 0.020* | 26 versus 32°C | 0.006** |
| (factor: temperature) | within groups | 53 | 0.001 | 32 versus 38°C | 0.305 | ||
| total | 55 | ||||||
| lipid mass at molt | temperature | 2 | <0.001 | 3.001 | 0.059 | 26 versus 32°C | — |
| diet | 2 | 0.002 | 13.778 | <0.001 | 32 versus 38°C | — | |
| initial mass | 1 | 0.001 | 3.815 | 0.056 | |||
| residual | 50 | ||||||
| protein mass at molt | temperature | 2 | 0.001 | 2.716 | 0.076 | 26 versus 32°C | — |
| diet | 2 | 0.003 | 8.688 | 0.001 | 32 versus 38°C | — | |
| initial mass | 1 | 0.022 | 71.641 | <0.001 | |||
| residual | 50 | ||||||
| tibia length | temperature | 2 | 15.774 | 5.693 | 0.006** | 26 versus 32°C | 0.004** |
| initial mass | 1 | 20.781 | 7.500 | 0.008** | 32 versus 38°C | 0.901 | |
| residual | 52 | 2.771 |
aFisher's least significant difference.
Stadium duration significantly decreased with increasing temperature (median values 7, 10 and 18 days for 26, 32 and 38°C, respectively; figure 2e, Jonckheere–Terpstra test, p < 0.001) and rate of dry mass gain significantly increased with increasing temperature (figure 2f, Jonckheere–Terpstra test, p < 0.001). Ecdysis failure rates, pooled across diet treatments, were 56 per cent at 26°C, 30 per cent at 32°C and 27 per cent at 38°C.
(d). Temperature-dependent utilization efficiencies
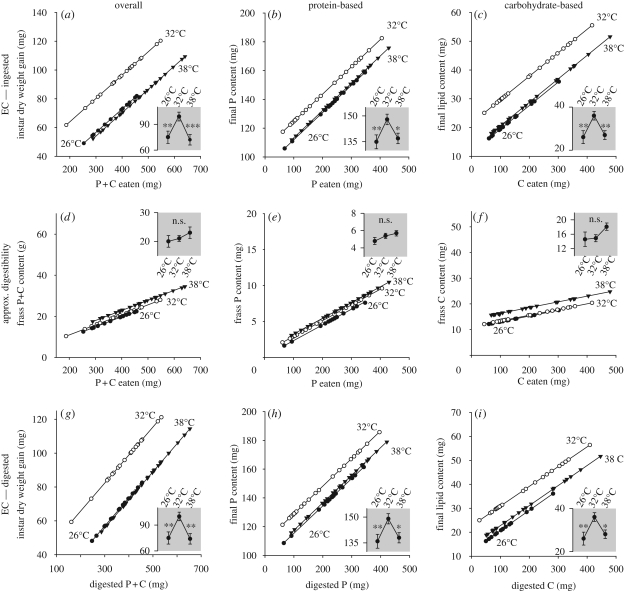
Utilization efficiency of both protein and carbohydrates was greater at 32°C compared with higher (38°C) and lower (26°C) temperatures (figure 3; table S1, electronic supplementary material).
Figure 3.
Temperature-dependent efficiencies of macronutrient conversion and digestion over the fifth stadium showing linear regression lines at 26, 32 and 38°C. An ANCOVA (table S1, electronic supplementary material) removed effects of covariates and factors; insets display resultant estimated marginal means and their standard errors at the three temperatures. The ordinate in each case has the same dimension as that of the parent plot. Insects at 26 and 38°C experience significantly lower utilization efficiency of (b) ingested protein, (c) carbohydrates or (a) both compared with insects at 32°C. This is due to (g–i) inefficiencies in the conversion of digested food rather than (d–f) inefficiencies in digestibility. See text for additional explanation. n = 30 for each temperature; insects failing to molt were excluded from further analysis. *p < 0.05; **p < 0.01; ***p < 0.001.
(e). Temperature, mass and time-dependent energy expenditure
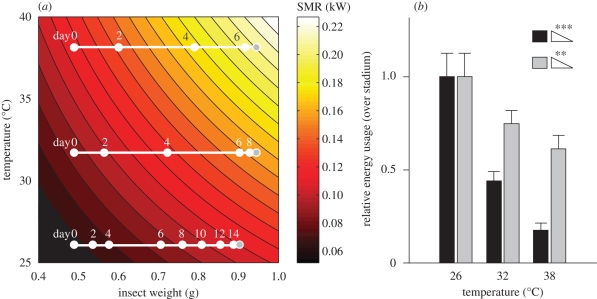
Computed energy usage (for either Clarke or Hack energy relationships; see §2) over the entire stadium varied significantly as a function of temperature. In particular, more energy was required for resting metabolic considerations at lower temperatures owing to longer development times (figure 4; Jonckheere–Terpstra tests, p < 0.001). This trend held over a range of Q10 values from 1.5 to 2.2 (Jonckheere–Terpstra tests, p < 0.0001 and p < 0.05, respectively).
Figure 4.
Computation of standard energy usage through the fifth stadium accounting for temperature, insect mass and stadium duration. (a) Isometabolic rate curves in temperature–weight space showing the trajectory of average insects on days 0, 2, 4, 6 and bidaily until moult (grey) at each of the three temperatures (26, 32 and 38°C). Curves are computed by combination of intra-specific orthopteran RMR and mass data (Hack 1997) with a Q10 (temperature coefficient) of 1.7 (Chappell 1983). For details see §2. (b) Average energy consumption computed by path integration through (a) for insects at 26, 32 and 38°C using mass-specific oxygen consumption data from either Clarke (1957), black bars, or from Hack (1997), grey bars. The average of these two datasets is used in future computation. Values are plotted relative to stadium energy usage at 26°C. Within a stadium, there is a significant trend towards decreasing cumulative resting metabolic energy usage at higher temperatures (Jonckheere–Terpstra tests, ramp symbols). n = 30 for each temperature; insects failing to molt were excluded from further analysis. **p < 0.01; ***p < 0.001.
(f). Temperature-dependent activity levels
The number of activity bouts did not differ significantly between groups at 26, 32 and 38°C (Kruskal–Wallis test, χ2 = 0.97; d.f. = 2; p = 0.613) or change monotonically with temperature (Jonckheere–Terpstra test, p = 0.345). Likewise, the time fraction spent moving did not significantly differ between temperatures (Kruskal–Wallis test, χ2 = 0.18; d.f. = 2; p = 0.916) or change monotonically with temperature (Jonckheere–Terpstra test, p = 0.840).
4. Discussion
We have shown that locusts with constant access to food choose body temperatures that maximize rapid development and growth at the expense of efficient nutrient utilization. Insects placed in a thermal gradient selected ambient temperatures near 38°C (figure S1, electronic supplementary material), the temperature at which growth rates were highest (figure 2f). In contrast, locusts restricted to 32°C experienced slower growth rates but maximal utilization efficiencies (figure 3).
The observed decrease in macronutrient utilization efficiency at 38°C relative to 32°C corresponds well with a central principle in vertebrate nutrition: as the quantity of metabolizable (assimilated) energy ingested increases, metabolic heat production increases exponentially. Increased energy intake thus leads to a declining efficiency in energy retention (Blaxter & Boyne 1978). The increased growth and development rates at 38°C were fuelled by increased (figure 1b) and more rapid (figure 1c) consumption over the stadium. This corroborates and extends a range of published studies linking growth and development with temperature (e.g. Stamp 1990; Yang & Joern 1994; Petersen et al. 2000; Levesque et al. 2007).
We can infer from our nutrient budgets the source of the change in ECI with temperature. As temperature did not significantly influence the digestibility of nutrients, and because nutrient absorption from the gut was estimated to be nearly complete, it follows that differences in ECD explained differences in the overall efficiency of conversion of food to growth. It should be noted that artificial diets as used in our study, allow higher rates of nutrient digestion and absorption than natural plant foods (Clissold et al. 2006), and may obscure additional utilization inefficiencies at the higher temperature occasioned by more rapid gut passage and absorption rates (Yang & Joern 1994).
The inefficient utilization of conversion of digested nutrients to growth at 38°C cannot be explained by computed trends in energy expenditure owing to RMRs (figure 4). Energy budget schematics were created for the stadium (figure S2, electronic supplementary material) to attempt to localize the nature of the inefficiency. These budgets incorporate measured nutrient intake, faecal content corrected for indigestible cellulose intake and calculated RMR-based energy expenditure through the stadium. Energy assimilated but not used for resting metabolic needs is potentially available for fuelling growth, behavioural activity or the processing of food (diet-induced thermogenesis (DIT); for review in insects, see Trier & Mattson 2003). According to the energy budget (figure S2, electronic supplementary material), the quantity of energy available for growth, activity and DIT increases with temperature as a result of increased food intake and reduced cumulative energy expenditure (over the course of the stadium) for resting metabolism.
As measured activity levels were constant across the tested temperatures (present study; see also Hussein 1937), and measures of total growth at 32 and 38°C were indistinguishable, differences in DIT may have contributed to inefficient conversion efficiencies at 38°C (figures 2 and 3h,i). DIT may be facultative or obligatory. Obligatory DIT represents compulsory energy expenditure directly associated with food consumption and processing, including ingestion, digestion, absorption and post-absorptive metabolic costs for growth and excretion (Trier & Mattson 2003). Obligatory DIT varies with diet composition (being higher for protein diet components than for lipid or carbohydrate; Westerterp-Plantenga et al. 1999) and is expected to be relatively insensitive to temperature. Meanwhile, facultative DIT is a regulatory response by which growth and body composition can be maintained despite excess consumption (Rothwell & Stock 1981), and involves ‘wastage respiration’ of excess caloric intake to produce heat. This process may be temperature dependent in endotherms (Rothwell & Stock 1986), but few studies have undertaken similar work for insects. Processes such as leakage of protons across inner mitochondrial membranes (bypassing ATP synthase) contribute to such thermogenesis (Lowell & Spiegelman 2000), although precise mechanisms in insects are unknown (Zanotto et al. 1997).
Facultative DIT in both mammals and insects is most pronounced in high-carbohydrate, low-protein diets (Zanotto et al. 1997; Stock 1999). Increases in energy expenditure owing to increased DIT at 38°C may explain why, despite greater intake at 38°C, insects at 32 and 38°C have indistinguishable sizes and weights at the moult. However, the possibility that locusts at 38°C were more active during an unmeasured period, or that estimates of cumulative RMR are inaccurate, cannot be ruled out. Further research should investigate whether DIT is temperature dependent in this system.
Inefficiencies in protein and carbohydrate conversion were apparent at 26°C relative to 32°C and may result from the high cumulative metabolic costs of the very long stadium (figures 2e and 4b). Such costs could be met by excessive carbohydrate metabolism and a degree of protein deamination for energy (Thompson et al. 2003). Although intake rates were slowest at 26°C, overall food intake over the stadium was similar to that at 32°C (figure 1a,b; electronic supplementary material, figure S2), suggesting a nutrient intake threshold below which moulting does not occur (Nijhout 1981).
What are the relative costs and benefits of selecting a particular body temperature? At 26°C (versus warmer temperatures), both growth and development are slowed, final adult size is smaller and nutrient utilization is poorer. There would therefore seem to be no benefits in selecting such cool temperatures unless constrained to do so. In contrast, insects reared at 32°C attain similar final adult size and body composition to those reared at 38°C, and additionally experience higher utilization efficiencies.
The fact that locusts selected temperatures near 38°C in the thermal gradient, despite reduced nutrient utilization efficiency, suggests that the benefits outweighed the costs of doing so. One potential cost is an increase in general levels of activity or other risky behaviours at higher temperature, leading to an increased exposure to predation (Terblanche & Chown 2007), although the time-dependent risk can still be decreased compared with cooler temperatures if development time is shortened significantly. The most obvious cost of rapid development and low utilization efficiency at 38°C is the increased need for food. In our experiments, food was available in unlimited supply; hence, the costs of low utilization efficiency were irrelevant, leading to the interesting prediction that insects might select lower temperatures when food is limited, or perhaps when greater food intake incurs costs associated with ingestion of toxic secondary plant compounds (Slansky 1992; Simpson & Raubenheimer 2001; Angilletta et al. 2006).
Despite possible costs, there are several potential benefits of maintaining higher body temperatures. First, as adulthood is reached more quickly, reproduction can occur sooner; therefore, lifetime fecundity may increase; albeit there is the possibility that increased temperatures may reduce fecundity owing to decreased resources devoted to reproduction. Second, if development occurs rapidly, time-dependent mortality risks such as predation, cannibalism and disease are reduced (Clancy & Price 1987). A counterpoint here, of course, is that in some situations rapid growth itself may carry a cost (Gotthard et al. 1994; Gotthard 2000). Third, many insects may only have a ‘small window of opportunity’ during which growth and reproduction are possible; this window may be defined by food availability or quality (e.g. Feeny 1970). Shorter development times, therefore, may provide greater fitness benefits than any costs of poor nutrient utilization and high RMR.
Although trade-offs are an intensively studied subject in life-history evolution, the consequences of thermal strategies on key life-history traits and their underlying physiological mechanisms remain a fertile area for investigation. New avenues for research arise from the present study, including testing the prediction that locusts may change their thermal preferences in response to the availability of food or other circumstances that increase the importance of nutrient utilization efficiency and/or devalue rapid development.
Acknowledgements
The authors would like to thank John S. Terblanche for many helpful discussions, Tim Dodgson and Naz Soran for expert technical assistance, Scott van Barneveld for support with temperature gradients and several anonymous reviewers for improving the manuscript. G.A.M. was funded by an Oxford Clarendon Award (UK) and grants from the Orthopterists’ Society (USA) and Worcester College (UK).
References
- Angilletta M. J., Jr, Lee V., Silva A. C.2006Energetics of lizard embryos are not canalized by thermal acclimation. Physiol. Biochem. Zoolog. 79, 573–580 (doi:10.1086/501062) [DOI] [PubMed] [Google Scholar]
- Anstey M. L., Rogers S. M., Ott S. R., Burrows M., Simpson S. J.2008Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323, 627–630 (doi:10.1126/science.1165939) [DOI] [PubMed] [Google Scholar]
- Atkinson D.1994Temperature and organism size: a biological law for ectotherms? Adv. Ecol. Res. 25, 1–58 (doi:10.1016/S0065-2504(08)60212-3) [Google Scholar]
- Bazazi S., Buhl J., Hale J. J., Anstey M. L., Sword G. A., Simpson S. J., Couzin I. D.2008Collective motion and cannibalism in locust migratory bands. Curr. Biol. 18, 735–739 (doi:10.1016/j.cub.2008.04.035) [DOI] [PubMed] [Google Scholar]
- Bernheim H. A., Bodel P. T., Askenase P. W., Atkins E.1978Effects of fever on host defense mechanisms after infection in the lizard Dipsosaurus dorsalis. Br. J. Exp. Pathol. 59, 76–84 [PMC free article] [PubMed] [Google Scholar]
- Beuchat C. A., Ellner S.1987A quantitative test of life history theory: thermoregulation by a viviparous lizard. Ecol. Monogr. 57, 45–60 (doi:10.2307/1942638) [Google Scholar]
- Blaxter K. L., Boyne A. W.1978The estimation of the nutritive value of feeds as energy sources for ruminants and the derivation of feeding systems. J. Agric. Sci. Camb. 90, 47–68 (doi:10.1017/S0021859600048589) [Google Scholar]
- Bradford M. M.1976A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (doi:10.1016/0003-2697(76)90527-3) [DOI] [PubMed] [Google Scholar]
- Brett J. R.2001Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerkd). Integr. Comp. Biol. 11, 99–113 (doi:10.1093/icb/11.1.99) [Google Scholar]
- Buhl J., Sumpter D. J., Couzin I. D., Hale J. J., Despland E., Miller E. R., Simpson S. J.2006From disorder to order in marching locusts. Science 312, 1402–1406 (doi:10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- Chappell M. A.1983Metabolism and thermoregulation in desert and montane grasshoppers. Oecologia 56, 126–131 (doi:10.1007/BF00378228) [DOI] [PubMed] [Google Scholar]
- Chown S. L., Terblanche J. S.2007Physiological diversity in insects: ecological and evolutionary contexts. In Advances in insect physiology, vol. 33 (ed. Simpson S. J.), pp. 50–152 San Diego, CA: Academic Press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S. L., Marais E., Terblanche J. S., Klok C. J., Lighton J. R. B., Blackburn T. M.2007Scaling of insect metabolic rate is inconsistent with the nutrient supply network model. Funct. Ecol. 21, 282–290 (doi:10.1111/j.1365-2435.2007.01245.x) [Google Scholar]
- Clancy K. M., Price P. W.1987Rapid herbivore growth enhances enemy attack: sublethal plant defenses remain a paradox. Ecology 68, 733–737 (doi:10.2307/1938479) [Google Scholar]
- Clarke K. U.1957The relationship of oxygen consumption to age and weight during the post-embryonic growth of Locusta migratoria L. J. Exp. Biol. 34, 29–41 [Google Scholar]
- Clissold F. J., Sanson G. D., Read J.2006The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust. J. Anim. Ecol. 75, 1000–1013 (doi:10.1111/j.1365-2656.2006.01122.x) [DOI] [PubMed] [Google Scholar]
- Damme R. V., Bauwens D., Verheyen R. F.1991The thermal dependence of feeding behaviour, food consumption and gut-passage time in the lizard Lacerta vivipara Jacquin. Funct. Ecol. 5, 507–517 (doi:10.2307/2389633) [Google Scholar]
- Du W. G., Yan S. J., Ji X.2000Selected body temperature, thermal tolerance and thermal dependence of food assimilation and locomotor performance in adult blue-tailed skinks Eumeces elegans. J. Thermal Biol. 25, 197–202 (doi:10.1016/S0306-4565(99)00022-4) [Google Scholar]
- Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F.1956Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (doi:10.1021/ac60111a017) [Google Scholar]
- Elliot S. L., Horton C. M., Blanford S., Thomas M. B.2005Impacts of fever on locust life-history traits: costs or benefits? Biol. Lett. 1, 181–184 (doi:10.1098/rsbl.2004.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny P.1970Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51, 565–581 (doi:10.2307/1934037) [Google Scholar]
- Gandar M. V.1982The dynamics and trophic ecology of grasshoppers (Acridoidea) in a South African savanna. Oecologia 54, 370–378 (doi:10.1007/BF00380006) [DOI] [PubMed] [Google Scholar]
- Gotthard K.2000Increased risk of predation as a cost of high growth rate: an experimental test in a butterfly. J. Anim. Ecol. 896–902 (doi:10.1046/j.1365-2656.2000.00432.x) [DOI] [PubMed] [Google Scholar]
- Gotthard K., Nylin S., Wiklund C.1994Adaptive variation in growth rate: life history costs and consequences in the speckled wood butterfly Pararge aegeria. Oecologia 99, 281–289 (doi:10.1007/BF00627740) [DOI] [PubMed] [Google Scholar]
- Hack M. A.1997The effects of mass and age on standard metabolic rate in house crickets. Physiol. Entomol. 22, 325–331 (doi:10.1111/j.1365-3032.1997.tb01176.x) [Google Scholar]
- Hussein M.1937The effect of temperature on locust activity. Bull. Minist. Agric. Egypt Tech. Sci. Ser. 184 [Google Scholar]
- Jonckheere A. R.1954A distribution-free FC-sample test against ordered alternatives. Biometrika 41, 133–145 [Google Scholar]
- Karl I., Fischer K.2008Why get big in the cold? Towards a solution to a life-history puzzle. Oecologia 155, 215–225 (doi:10.1007/s00442-007-0902-0) [DOI] [PubMed] [Google Scholar]
- Keister M., Buck J.1964Some endogenous and exogenous effects on rate of respiration. In Physiology of insecta, vol. 3 (ed. Rockstein M.), pp. 617–658 New York, NY: Academic Press [Google Scholar]
- Kingsolver J. G., Huey R. B.2008Size, temperature, and fitness: three rules. Evol. Ecol. Res. 10, 251–268 [Google Scholar]
- Lactin D. J., Johnson D. L.1996Behavioural optimization of body temperature by nymphal grasshoppers (Melanoplus sanguinipes, Orthoptera: Acrididae) in temperature gradients established using incandescent bulbs. J. Thermal Biol. 21, 231–238 (doi:10.1016/0306-4565(96)00007-1) [Google Scholar]
- Lee K. P., Behmer S. T., Simpson S. J., Raubenheimer D.2002A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval). J. Insect Physiol. 48, 655–665 (doi:10.1016/S0022-1910(02)00088-4) [DOI] [PubMed] [Google Scholar]
- Levesque K. R., Fortin M., Mauffette Y.2007Temperature and food quality effects on growth, consumption and post-ingestive utilization efficiencies of the forest tent caterpillar Malacosoma disstria (Lepidoptera: Lasiocampidae). Bull. Entomol. Res. 92, 127–136 [DOI] [PubMed] [Google Scholar]
- Lowell B. B., Spiegelman B. M.2000Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 (doi:10.1038/35007527) [DOI] [PubMed] [Google Scholar]
- Nespolo R. F., Lardies M. A., Bozinovic F.2003Intrapopulational variation in the standard metabolic rate of insects: repeatability, thermal dependence and sensitivity (Q10) of oxygen consumption in a cricket. J. Exp. Biol. 206, 4309–4315 (doi:10.1242/jeb.00687) [DOI] [PubMed] [Google Scholar]
- Nijhout H. F.1981Physiological control of molting in insects. Am. Zool. 21, 631–640 [Google Scholar]
- Pener M. P., Simpson S. J.2008Locust phase polyphenism: an update. Adv. Insect Physiol. 35 [Google Scholar]
- Petersen C. H., Woods H. A., Kingsolver J.2000Stage-specific effects of temperature and dietary protein on growth and survival of Manduca sexta caterpillars. Physiol. Entomol. 25, 35–40 (doi:10.1046/j.1365-3032.2000.00163.x) [Google Scholar]
- Raubenheimer D., Simpson S. J.1992Analysis of covariance: an alternative to nutritional indices. Entomol. Exp. Appl. 62, 221–231 (doi:10.1007/BF00353441) [Google Scholar]
- Raubenheimer D., Simpson S. J.1994The analysis of nutrient budgets. Funct. Ecol. 8, 783–791 (doi:10.2307/2390238) [Google Scholar]
- Raubenheimer D., Simpson S., Mayntz D.2009Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 23, 4–16 (doi:10.1111/j.1365-2435.2009.01522.x) [Google Scholar]
- Rothwell N. J., Stock M. J.1981Regulation of energy balance. Annu. Rev. Nutr. 1, 235–256 (doi:10.1146/annurev.nu.01.070181.001315) [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J.1986Influence of environmental temperature on energy balance, diet-induced thermogenesis and brown fat activity in ‘cafeteria’-fed rats. Br. J. Nutr. 56, 123–129 (doi:10.1079/BJN19860092) [DOI] [PubMed] [Google Scholar]
- Schoolfield R., Sharpe P., Magnuson C.1981Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J. Theor. Biol. 88, 719 (doi:10.1016/0022-5193(81)90246-0) [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Abisgold J. D.1985Compensation by locusts for changes in dietary nutrients—behavioral mechanisms. Physiol. Entomol. 10, 443–452 (doi:10.1111/j.1365-3032.1985.tb00066.x) [Google Scholar]
- Simpson S. J., Raubenheimer D.1995The geometric analysis of feeding and nutrition—a users guide. J. Insect Physiol. 41, 545–553 (doi:10.1016/0022-1910(95)00006-G) [Google Scholar]
- Simpson S. J., Raubenheimer D.2000The hungry locust. Adv. Study Behav. 29, 1–44 (doi:10.1016/S0065-3454(08)60102-3) [Google Scholar]
- Simpson S. J., Raubenheimer D.2001The geometric analysis of nutrient-allelochemical interactions: a case study using locusts. Ecology 82, 422–439 [Google Scholar]
- Slansky F.1992Allelochemical–nutrient interactions in herbivore nutritional ecology. In Herbivores: their interactions with secondary plant metabolites, vol. 2 (eds Rosenthal G. A., Berenbaum M. R.), pp. 135–174 San Diego, CA: Academic Press [Google Scholar]
- Slansky F., Scriber J. M.1985Food consumption and utilization. In Comprehensive insect physiology, biochemistry and pharmacology, vol. 4 (eds Kerkut G. A., Gilbert L. I.), pp. 87–163 Oxford, UK: Pergamon Press [Google Scholar]
- Smith D., Paulsen G. M., Raguse C. A.1964Extraction of total available carbohydrates from grass and legume tissue. Plant Physiol. 39, 960–962 (doi:10.1104/pp.39.6.960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp N. E.1990Growth versus molting time of caterpillars as a function of temperature, nutrient concentration and the phenolic rutin. Oecologia 82, 107–113 (doi:10.1007/BF00318541) [DOI] [PubMed] [Google Scholar]
- Stearns S. C.1992. In The evolution of life histories Oxford, UK: Oxford University Press [Google Scholar]
- Stillwell R. C., Wallin W. G., Hitchcock L. J., Fox C. W.2007Phenotypic plasticity in a complex world: interactive effects of food and temperature on fitness components of a seed beetle. Oecologia 153, 309–321 (doi:10.1007/s00442-007-0748-5) [DOI] [PubMed] [Google Scholar]
- Stock M. J.1999Gluttony and thermogenesis revisited. Int. J. Obes. 23, 1105–1117 (doi:10.1038/sj.ijo.0801108) [DOI] [PubMed] [Google Scholar]
- Terblanche J. S., Chown S. L.2007The effects of temperature, body mass and feeding on metabolic rate in the tsetse fly Glossina morsitans centralis. Physiol. Entomol. 32, 175–180 (doi:10.1111/j.1365-3032.2006.00549.x) [Google Scholar]
- Terblanche J. S., Jaco Klok C., Chown S. L.2005Temperature-dependence of metabolic rate in Glossina morsitans morsitans (Diptera, Glossinidae) does not vary with gender, age, feeding, pregnancy or acclimation. J. Insect Physiol. 51, 861–870 (doi:10.1016/j.jinsphys.2005.03.017) [DOI] [PubMed] [Google Scholar]
- Terpstra T. J.1952The asymptotic normality and consistency of Kendall's test against trend, when ties are present in one ranking. Indagationes Math. 14, 327–333 [Google Scholar]
- Thompson S. N., Borchardt D. B., Wang L. W.2003Dietary nutrient levels regulate protein and carbohydrate intake, gluconeogenic/glycolytic flux and blood trehalose level in the insect Manduca sexta L. J. Comp. Physiol. B Biochem. Systemic Environ. Physiol. 173, 149–163 [DOI] [PubMed] [Google Scholar]
- Trier T. M., Mattson W. J.2003Diet-induced thermogenesis in insects: A developing concept in nutritional ecology. Environ. Entomol. 32, 1–8 [Google Scholar]
- Uvarov B. P.1966Grasshoppers and locusts: a handbook of general acridology, vol. 1 London, UK: Cambridge University Press [Google Scholar]
- Uvarov B. P.1977. In Grasshoppers and locusts: a handbook of general acridology, vol. 2 London, UK: Cambridge University Press [Google Scholar]
- van der Have T. M., de Jong G.1996Adult size in ectotherms: temperature effects on growth and differentiation. J. Theor. Biol. 183, 329–340 (doi:10.1006/jtbi.1996.0224) [Google Scholar]
- Westerterp-Plantenga M. S., Rolland V., Wilson S. A. J., Westerterp K. R.1999Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs. high fat diets measured in a respiration chamber. Eur. J. Clin. Nutr. 53, 495–502 (doi:10.1038/sj.ejcn.1600782) [DOI] [PubMed] [Google Scholar]
- Wilson K., Thomas M. B., Blanford S., Doggett M., Simpson S. J., Moore S. L.2002Coping with crowds: density-dependent disease resistance in desert locusts. Proc. Natl Acad. Sci. USA 99, 5471–5475 (doi:10.1073/pnas.082461999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Joern A.1994Influence of diet quality, developmental stage, and temperature on food residence time in the grasshopper Melanoplus differentialis. Physiol. Zool. 67, 598–616 [Google Scholar]
- Zanotto F., Gouveia S., Simpson S., Calder D.1997Nutritional homeostasis in locusts: is there a mechanism for increased energy expenditure during carbohydrate overfeeding? J. Exp. Biol. 200, 2437–2448 [DOI] [PubMed] [Google Scholar]
- Zera A., Denno R.1997Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 42, 207–230 (doi:10.1146/annurev.ento.42.1.207) [DOI] [PubMed] [Google Scholar]