Abstract
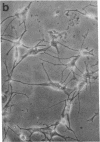
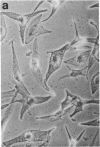
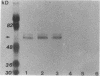
Normal differentiation and malignant transformation of human melanocytes involve a complex series of interactions during which both genetic and environmental factors play roles. At present, the regulation of these processes is poorly understood. We have induced the expression of nerve growth factor (NGF) receptors on cultured human melanocytes with phorbol 12-tetradecanoate 13-acetate and have correlated this event with the appearance of a more differentiated, dendritic morphology. Criteria for NGF receptor expression included protein accumulation and cell-surface immunofluorescent staining with a monoclonal antibody directed against the human receptor and induction of the messenger RNA species as determined by blot-hybridization studies. The presence of the receptor could also be induced by UV irradiation or growth factor deprivation. The NGF receptor is inducible in cultured human melanocytes, and we suggest that NGF may modulate the behavior of this neural crest-derived cell in the skin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977 Sep 16;133(2):358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- Banerjee S. P., Snyder S. H., Cuatrecasas P., Greene L. A. Binding of nerve growth factor receptor in sympathetic ganglia. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2519–2523. doi: 10.1073/pnas.70.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chun L. L., Patterson P. H. Role of nerve growth factor in the development of rat sympathetic neurons in vitro. I. Survival, growth, and differentiation of catecholamine production. J Cell Biol. 1977 Dec;75(3):694–704. doi: 10.1083/jcb.75.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. M., Bandtlow C., Heumann R., Korsching S., Rohrer H., Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. 1987 Mar 26-Apr 1Nature. 326(6111):353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Marko O., Ogata S., Old L. J. Growth regulation of human melanocytes: mitogenic factors in extracts of melanoma, astrocytoma, and fibroblast cell lines. Science. 1985 Sep 6;229(4717):984–986. doi: 10.1126/science.4023718. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood J. M., Lee J. A. Recent data on the epidemiology of malignant melanoma. Semin Oncol. 1975 Jun;2(2):149–154. [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Penman S. Tumor promoters induce a specific morphological signature in the nuclear matrix-intermediate filament scaffold of Madin-Darby canine kidney (MDCK) cell colonies. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4409–4413. doi: 10.1073/pnas.81.14.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Friedmann P. S., Gilchrest B. A. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol. 1987 Oct;133(1):88–94. doi: 10.1002/jcp.1041330111. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A., Karassik R. L., Wilkins L. M., Vrabel M. A., Maciag T. Autocrine and paracrine growth stimulation of cells derived from human skin. J Cell Physiol. 1983 Nov;117(2):235–240. doi: 10.1002/jcp.1041170215. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A., Vrabel M. A., Flynn E., Szabo G. Selective cultivation of human melanocytes from newborn and adult epidermis. J Invest Dermatol. 1984 Nov;83(5):370–376. doi: 10.1111/1523-1747.ep12264638. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature. 1977 Jul 28;268(5618):349–351. doi: 10.1038/268349a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob P. M., Ross A. H., Koprowski H., Bothwell M. Characterization of the human melanoma nerve growth factor receptor. J Biol Chem. 1985 Jul 5;260(13):8044–8049. [PubMed] [Google Scholar]
- Halaban R., Ghosh S., Baird A. bFGF is the putative natural growth factor for human melanocytes. In Vitro Cell Dev Biol. 1987 Jan;23(1):47–52. doi: 10.1007/BF02623492. [DOI] [PubMed] [Google Scholar]
- Hendry I. A. A method to correct adequately for the change in neuronal size when estimating neuronal numbers after nerve growth factor treatment. J Neurocytol. 1976 Jun;5(3):337–349. doi: 10.1007/BF01175119. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Thurin J., Balaban G., Bennicelli J. L., Herlyn D., Elder D. E., Bondi E., Guerry D., Nowell P., Clark W. H. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985 Nov;45(11 Pt 2):5670–5676. [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta nerve growth factor receptor of avian dorsal root ganglia. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3884–3888. doi: 10.1073/pnas.70.12.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner K., Isobe M., Chao M., Bothwell M., Ross A. H., Finan J., Hoxie J. A., Sehgal A., Buck C. R., Lanahan A. The nerve growth factor receptor gene is at human chromosome region 17q12-17q22, distal to the chromosome 17 breakpoint in acute leukemias. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1403–1407. doi: 10.1073/pnas.83.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Lanahan A., Buck C. R., Sehgal A., Morgan C., Mercer E., Bothwell M., Chao M. Expression and structure of the human NGF receptor. Cell. 1986 Nov 21;47(4):545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Klaus S. N. Prospects for growing normal human melanocytes in vitro. Methods Cell Biol. 1980;21A:277–288. doi: 10.1016/s0091-679x(08)60771-2. [DOI] [PubMed] [Google Scholar]
- Landreth G. E., Shooter E. M. Nerve growth factor receptors on PC12 cells: ligand-induced conversion from low- to high-affinity states. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4751–4755. doi: 10.1073/pnas.77.8.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi A., Foppen F. H., Kopin I. J. Stimulation and maintenance by nerve growth factor of phenylethanolamine-N-methyltransferase in superior cervical ganglia of adult rats. Brain Res. 1977 Dec 16;138(2):309–315. doi: 10.1016/0006-8993(77)90748-x. [DOI] [PubMed] [Google Scholar]
- MacDonnell P. C., Nagaiah K., Lakshmanan J., Guroff G. Nerve growth factor increases activity of ornithine decarboxylase in superior cervical ganglia of young rats. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4681–4684. doi: 10.1073/pnas.74.10.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Lapetina E. G., Cuatrecasas P. Intracellular activation of protein kinase C and regulation of the surface transferrin receptor by diacylglycerol is a spontaneously reversible process that is associated with rapid formation of phosphatidic acid. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1281–1284. doi: 10.1073/pnas.83.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. I., Don P. Culture of normal adult human melanocytes. Br J Dermatol. 1984 May;110(5):569–580. doi: 10.1111/j.1365-2133.1984.tb04680.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Rieger F., Shelanski M. L., Greene L. A. The effects of nerve growth factor on acetylcholinesterase and its multiple forms in cultures of rat PC12 pheochromocytoma cells: increased total specific activity and appearance of the 16 S molecular form. Dev Biol. 1980 Apr;76(1):238–243. doi: 10.1016/0012-1606(80)90376-0. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Sommer I. Simultaneous expression of neuronal and glial properties by chick ciliary ganglion cells during development. J Neurosci. 1983 Aug;3(8):1683–1693. doi: 10.1523/JNEUROSCI.03-08-01683.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. H., Grob P., Bothwell M., Elder D. E., Ernst C. S., Marano N., Ghrist B. F., Slemp C. C., Herlyn M., Atkinson B. Characterization of nerve growth factor receptor in neural crest tumors using monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6681–6685. doi: 10.1073/pnas.81.21.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Hawley-Nelson P., Poirier M., Katz S. I., Yuspa S. H. Detection of pemphigoid antigen, pemphigus antigen, and keratin filaments by indirect immunofluorescence in cultured human epidermal cells. J Invest Dermatol. 1980 Aug;75(2):183–186. doi: 10.1111/1523-1747.ep12522615. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Hawley-Nelson P., Yaar M., Martin G. R., Katz S. I. Laminin and bullous pemphigoid antigen are distinct basement membrane proteins synthesized by epidermal cells. J Invest Dermatol. 1982 Jun;78(6):456–459. doi: 10.1111/1523-1747.ep12510132. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Woodley D. T., Katz S. I. Identification and partial characterization of pemphigoid antigen extracted from normal human skin. J Invest Dermatol. 1984 Jan;82(1):108–111. doi: 10.1111/1523-1747.ep12259224. [DOI] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Johnson E. M., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Angeletti P. U., Levi-Montalcini R., Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Lee L. S., Fisher P. B., Mufson A., Yamasaki H. Action of phorbol esters in cell culture: mimicry of transformation, altered differentiation, and effects on cell membranes. J Supramol Struct. 1979;12(2):195–208. doi: 10.1002/jss.400120206. [DOI] [PubMed] [Google Scholar]
- Wilkins L., Gilchrest B. A., Szabo G., Weinstein R., Maciag T. The stimulation of normal human melanocyte proliferation in vitro by melanocyte growth factor from bovine brain. J Cell Physiol. 1985 Mar;122(3):350–361. doi: 10.1002/jcp.1041220304. [DOI] [PubMed] [Google Scholar]
- Zimmermann A., Sutter A. beta-Nerve growth factor (beta NGF) receptors on glial cells. Cell-cell interaction between neurones and Schwann cells in cultures of chick sensory ganglia. EMBO J. 1983;2(6):879–885. doi: 10.1002/j.1460-2075.1983.tb01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]