Abstract
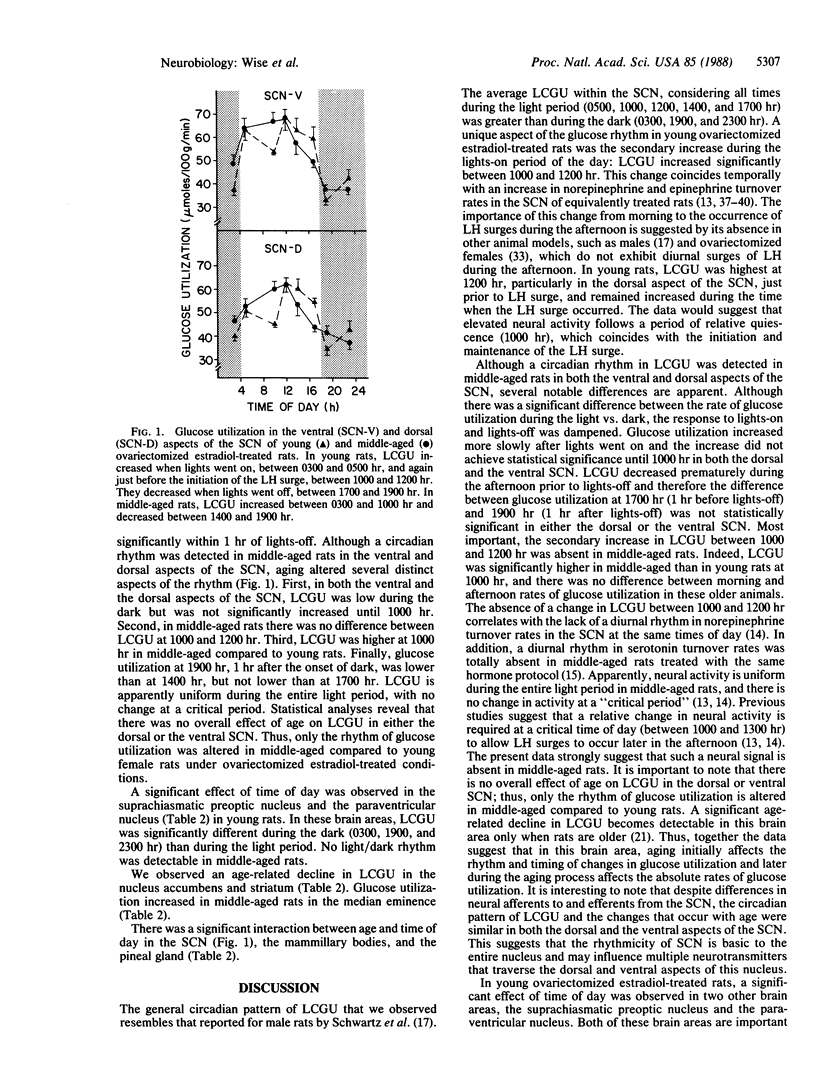
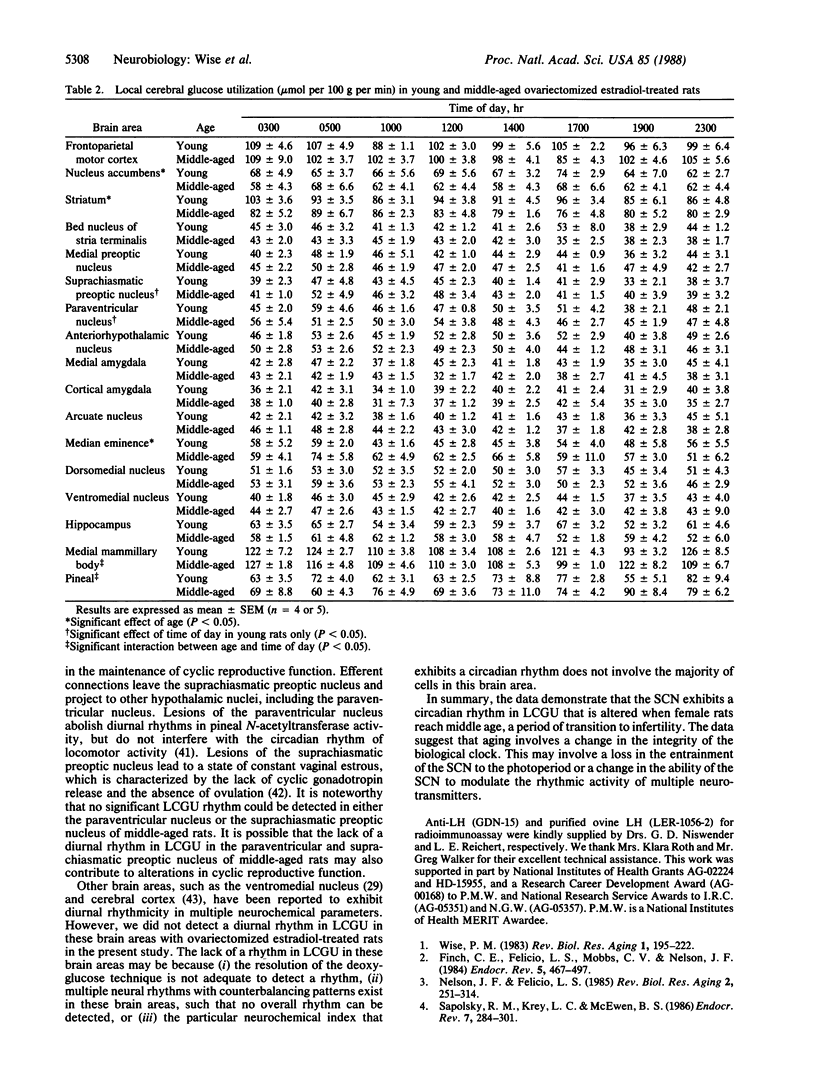
We examined the possibility that alterations in the timing of cyclic luteinizing hormone (LH) release during the middle age transition to infertility reflect differences in the circadian pattern of neural function in pacemaker areas of the hypothalamus, particularly the suprachiasmatic nucleus. We measured local cerebral glucose utilization (LCGU) because this parameter is an index of local brain function. We assessed LCGU in several brain areas of young and middle-aged ovariectomized estradiol-treated rats since LH surges are altered when rats are middle-aged. This alteration is correlated with changes in the diurnal pattern of neurotransmitter turnover in several hypothalamic areas that regulate cyclic LH release. The data demonstrate a circadian rhythm in glucose utilization in the dorsal and ventral suprachiasmatic nucleus. In young rats, LCGU increases within 1 hr of lights-on, increases further just prior to the initiation of the LH surge, and decreases within 1 hr of lights-off. In contrast, middle-aged rats show a more gradual increase in LCGU after lights-on, with no further increase prior to the LH surge, and a premature decrease during the afternoon and evening. The data suggest that changes in the circadian pattern of LCGU may be related to the alteration in timing and amplitude of estradiol-induced LH surges in middle-aged rats. Changes in the integrity of the biological clock or in the ability of the biological clock to entrain other neurochemical events may underlie the onset of altered cyclic reproductive function and the transition to irregular estrous cyclicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B. A., Johnson M. D., Lynch C. O., Crowley W. R. Evidence that norepinephrine and epinephrine systems mediate the stimulatory effects of ovarian hormones on luteinizing hormone and luteinizing hormone-releasing hormone. Endocrinology. 1983 Oct;113(4):1431–1438. doi: 10.1210/endo-113-4-1431. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K., Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci. 1977 Sep 5;198(1132):279–296. doi: 10.1098/rspb.1977.0098. [DOI] [PubMed] [Google Scholar]
- Caligaris L., Astrada J. J., Taleisnik S. Release of luteinizing hormone induced by estrogen injection into ovariectomized rats. Endocrinology. 1971 Apr;88(4):810–815. doi: 10.1210/endo-88-4-810. [DOI] [PubMed] [Google Scholar]
- Chappel S. C., Barraclough C. A. Hypothalamic regulation of pituitary FSH secretion. Endocrinology. 1976 Apr;98(4):927–935. doi: 10.1210/endo-98-4-927. [DOI] [PubMed] [Google Scholar]
- Coen C. W., Coombs M. C. Effects of manipulating catecholamines on the incidence of the preovulatory surge of of luteinizing hormone and ovulation in the rat: evidence for a necessary involvement of hypothalamic adrenaline in the normal or 'midnight' surge. Neuroscience. 1983 Sep;10(1):187–206. doi: 10.1016/0306-4522(83)90092-1. [DOI] [PubMed] [Google Scholar]
- Coen C. W., Coombs M. C., Wilson P. M., Clement E. M., MacKinnon P. C. Possible resolution of a paradox concerning the use of p-chlorophenylalanine and 5-hydroxytryptophan: evidence for a mode of action involving adrenaline in manipulating the surge of luteinizing hormone in rats. Neuroscience. 1983 Mar;8(3):583–591. doi: 10.1016/0306-4522(83)90200-2. [DOI] [PubMed] [Google Scholar]
- Coen C. W., MacKinnon P. C. Lesions of the suprachiasmatic nuclei and the serotonin-dependent phasic release of luteinizing hormone in the rat: effects on drinking rhythmicity and on the consequences of preoptic area stimulation. J Endocrinol. 1980 Feb;84(2):231–236. doi: 10.1677/joe.0.0840231. [DOI] [PubMed] [Google Scholar]
- Crane P. D., Braun L. D., Cornford E. M., Nyerges A. M., Oldendorf W. H. Cerebral cortical glucose utilization in the conscious rat: evidence for a circadian rhythm. J Neurochem. 1980 Jun;34(6):1700–1706. doi: 10.1111/j.1471-4159.1980.tb11263.x. [DOI] [PubMed] [Google Scholar]
- Crowley W. R., Terry L. C. Effects of an epinephrine synthesis inhibitor, SKF64139, on the secretion of luteinizing hormone in ovariectomized female rats. Brain Res. 1981 Jan 5;204(1):231–235. doi: 10.1016/0006-8993(81)90670-3. [DOI] [PubMed] [Google Scholar]
- Dotti C., Taleisnik S. Beta-adrenergic receptors in the premammillary nucleus mediate the inhibition of LH release evoked by locus ceruleus stimulation. Neuroendocrinology. 1984 Jan;38(1):6–11. doi: 10.1159/000123858. [DOI] [PubMed] [Google Scholar]
- Finch C. E., Felicio L. S., Mobbs C. V., Nelson J. F. Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev. 1984 Fall;5(4):467–497. doi: 10.1210/edrv-5-4-467. [DOI] [PubMed] [Google Scholar]
- Gray G. D., Söderstein P., Tallentire D., Davidson J. M. Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology. 1978;25(3):174–191. doi: 10.1159/000122739. [DOI] [PubMed] [Google Scholar]
- Inouye S. T. Does the ventromedial hypothalamic nucleus contain a self-sustained circadian oscillator associated with periodic feedings? Brain Res. 1983 Nov 21;279(1-2):53–63. doi: 10.1016/0006-8993(83)90162-2. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Arita J., Yoshioka E. Loss of estrogen-induced daily surges of prolactin and gonadotropins by suprachiasmatic nucleus lesions in ovariectomized rats. Endocrinology. 1980 Apr;106(4):1087–1092. doi: 10.1210/endo-106-4-1087. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Hauser H., Krey L. C. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977 Jul 22;197(4301):398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Krieger D. T. Ventromedial hypothalamic lesions abolish food-shifted circadian adrenal and temperature rhythmicity. Endocrinology. 1980 Mar;106(3):649–654. doi: 10.1210/endo-106-3-649. [DOI] [PubMed] [Google Scholar]
- Legan S. J., Karsch F. J. A daily signal for the LH surge in the rat. Endocrinology. 1975 Jan;96(1):57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- London E. D., Nespor S. M., Ohata M., Rapoport S. I. Local cerebral glucose utilization during development and aging of the Fischer-344 rat. J Neurochem. 1981 Jul;37(1):217–221. doi: 10.1111/j.1471-4159.1981.tb05311.x. [DOI] [PubMed] [Google Scholar]
- Lu J. K., Gilman D. P., Meldrum D. R., Judd H. L., Sawyer C. H. Relationship between circulating estrogens and the central mechanisms by which ovarian steroids stimulate luteinizing hormone secretion in aged and young female rats. Endocrinology. 1981 Mar;108(3):836–841. doi: 10.1210/endo-108-3-836. [DOI] [PubMed] [Google Scholar]
- Niswender G. D., Midgley A. R., Jr, Monroe S. E., Reichert L. E., Jr Radioimmunoassay for rat luteinizing hormone with antiovine LH serum and ovine LH-131-I. Proc Soc Exp Biol Med. 1968 Jul;128(3):807–811. doi: 10.3181/00379727-128-33129. [DOI] [PubMed] [Google Scholar]
- Pickard G. E., Turek F. W. The hypothalamic paraventricular nucleus mediates the photoperiodic control of reproduction but not the effects of light on the circadian rhythm of activity. Neurosci Lett. 1983 Dec 23;43(1):67–72. doi: 10.1016/0304-3940(83)90130-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986 Aug;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Davidsen L. C., Smith C. B. In vivo metabolic activity of a putative circadian oscillator, the rat suprachiasmatic nucleus. J Comp Neurol. 1980 Jan 1;189(1):157–167. doi: 10.1002/cne.901890109. [DOI] [PubMed] [Google Scholar]
- Seibel M. M., Shine W., Smith D. M., Taymor M. L. Biological rhythm of the luteinizing hormone surge in women. Fertil Steril. 1982 May;37(5):709–711. doi: 10.1016/s0015-0282(16)46288-6. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Goochee C., Rapoport S. I., Sokoloff L. Effects of ageing on local rates of cerebral glucose utilization in the rat. Brain. 1980 Jun;103(2):351–365. doi: 10.1093/brain/103.2.351. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stephan F. K., Berkley K. J., Moss R. L. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6(12):2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Stetson M. H., Watson-Whitmyre M. Nucleus suprachiasmaticus: the biological clock in the hamster? Science. 1976 Jan 16;191(4223):197–199. doi: 10.1126/science.942799. [DOI] [PubMed] [Google Scholar]
- Takei H., Fredericks W. R., Rapoport S. I. The lumped constant in the deoxyglucose procedure declines with age in Fischer-344 rats. J Neurochem. 1986 Mar;46(3):931–938. doi: 10.1111/j.1471-4159.1986.tb13059.x. [DOI] [PubMed] [Google Scholar]
- Testart J., Frydman R., Roger M. Seasonal influence of diurnal rhythms in the onset of the plasma luteinizing hormone surge in women. J Clin Endocrinol Metab. 1982 Aug;55(2):374–377. doi: 10.1210/jcem-55-2-374. [DOI] [PubMed] [Google Scholar]
- Turek F. W. Circadian neural rhythms in mammals. Annu Rev Physiol. 1985;47:49–64. doi: 10.1146/annurev.ph.47.030185.000405. [DOI] [PubMed] [Google Scholar]
- Weiland N. G., Wise P. M. Estrogen alters the diurnal rhythm of alpha 1-adrenergic receptor densities in selected brain regions. Endocrinology. 1987 Nov;121(5):1751–1758. doi: 10.1210/endo-121-5-1751. [DOI] [PubMed] [Google Scholar]
- Wiegand S. J., Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982 Jun;34(6):395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wise P. M. Estradiol-induced daily luteinizing hormone and prolactin surges in young and middle-aged rats: correlations with age-related changes in pituitary responsiveness and catecholamine turnover rates in microdissected brain areas. Endocrinology. 1984 Aug;115(2):801–809. doi: 10.1210/endo-115-2-801. [DOI] [PubMed] [Google Scholar]
- Wise P. M. Norepinephrine and dopamine activity in microdissected brain areas of the middle-aged and young rat on proestrus. Biol Reprod. 1982 Oct;27(3):562–574. doi: 10.1095/biolreprod27.3.562. [DOI] [PubMed] [Google Scholar]
- Wise P. M., Walovitch R. C., Cohen I. R., Weiland N. G., London E. D. Diurnal rhythmicity and hypothalamic deficits in glucose utilization in aged ovariectomized rats. J Neurosci. 1987 Nov;7(11):3469–3473. doi: 10.1523/JNEUROSCI.07-11-03469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


