Abstract
Objective. To evaluate the effectiveness of oxandrolone in improving the nutritional status and linear growth of pediatric patients with cystic fibrosis (CF). Methods. Medical records of patients with CF treated with oxandrolone were reviewed for height z score, height velocity (HV), BMI z score, weight velocity (WV), Tanner stage, pulmonary function, liver enzyme levels, and any reported adverse events. Data were compared before (pre-Ox) and after (Ox) oxandrolone using a paired t-test. Results. 5 subjects (ages 8.5–14.5 years) were treated with oxandrolone 2.5 mg daily for 8–38 months. After 8–12 months of treatment, there was a statistically significant improvement in HV (pre-Ox = 5.3 ± 1.4 cm/yr, Ox = 8.3 ± 1.2 cm/yr, P < .01) and BMI z score (pre-Ox = −0.61 ± 1.04, Ox = −0.30 ± 0.86, P = .02). Both height z score (pre-Ox = −1.64 ± 0.63, Ox = −1.30 ± 0.49, P = .057) and WV (pre-Ox = 4.2 ± 3.7 kg/yr, Ox = 6.8 ± 1.0 kg/yr, P = .072) showed beneficial trends that did not reach statistical significance. No adverse events were reported. Conclusions. In this brief clinical report, oxandrolone improved the HV and BMI z score in patients with CF. Larger studies are needed to determine if oxandrolone is an effective, safe, and affordable option to stimulate appetite, improve weight gain, and promote linear growth in patients with CF.
1. Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder that features chronic pulmonary disease, pancreatic insufficiency, and other organ involvement. Patients with CF often have malnutrition and short stature. According to the Cystic Fibrosis Foundation Patient Registry 2007 Annual Data Report, 21% of patients with CF had weight less than the 10th percentile, while 13% had height below the 5th percentile [1]. Malnutrition and poor growth velocity are associated with declining pulmonary function, morbidity, and mortality [2–5]. Steady improvements in nutritional status are associated with greater pulmonary function [6], and evidence-based practice recommendations strongly advise close monitoring of growth and nutritional status as well as aggressive nutritional intervention for patients with CF [7].
Multiple agents have been used to stimulate appetite and growth in patients with CF, but their utility has been limited due to side effects (such as adrenal suppression with megestrol) or cost (such as growth hormone). Oxandrolone is a relatively weak androgen that stimulates appetite and promotes linear growth and has been safely used to treat a variety of conditions associated with wasting, poor weight gain, or short stature [8]. Furthermore, oxandrolone is an attractive option in growing children because it cannot be aromatized to estrogen, and therefore estrogen-dependent advancement of the bone age is minimized. To our knowledge, there are no reports in the literature of the use of oxandrolone in patients with CF.
For the past 4 years, children with CF referred to our endocrine clinic for persistent growth concerns in spite of other nutrition-augmentation strategies have occasionally been treated with oxandrolone. This retrospective analysis evaluates the effectiveness of oxandrolone in improving the weight gain and linear growth of these patients.
2. Materials and Methods
We reviewed the medical records of all patients in our endocrine clinic with CF who were treated with oxandrolone for at least 8 months between January of 2005 and October of 2008. Medical records were reviewed to determine height z score, height velocity (HV), body mass index (BMI) z score, and weight velocity (WV). Additional information obtained included age, Tanner stage, pulmonary function, liver enzyme levels, and any reported adverse events. This retrospective chart review was approved by the University of Wisconsin Human Subjects Committee.
Patients from the University of Wisconsin CF Clinic are referred to the endocrine clinic due to poor nutritional status and/or poor height gain, particularly when the growth is well below the clinic reference population (see data in results below). There were no predetermined nutritional cutoffs or pubertal stage for the initiation of oxandrolone. All subjects were treated with 2.5 mg of oxandrolone.
Height was measured on a wall-mounted stadiometer to the nearest 0.1 cm. Weight was measured on a calibrated beam balance platform scale to the nearest 0.1 kg. Weight and height measurements were used to calculate the BMI z score, which is the preferred method (as opposed to percentage of ideal body weight) for monitoring nutritional status in patients with cystic fibrosis [7, 9, 10]. Pretreatment height/weight velocities were calculated using the measurement at initiation of oxandrolone and the measurement 4–8 months prior to initiation of oxandrolone. Posttreatment height/weight velocities were calculated using the measurement at initiation of oxandrolone and the measurement 8–12 months after initiation of oxandrolone. Lung function parameters were measured with a Jaeger Masterscreen spirometer in accordance with the American Thoracic Society guidelines [11]. Forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were determined and the results expressed as percentages of the reference values of Wang et al. [12].
Descriptive statistics are expressed as mean ± SD or frequency with a percentage. Data were compared before (pre-Ox) and after (Ox) initiation of oxandrolone using a paired t-test.
3. Results
Five subjects (3 boys and 2 girls) were identified. The Table 1 lists the baseline characteristics for each subject. The subjects ranged from 8.5 to 14.5 years in age and were treated with oxandrolone for 8–38 months. All subjects were prescribed oral oxandrolone 2.5 mg daily. All subjects had pancreatic insufficiency. At the start of oxandrolone therapy, four subjects had Tanner 1 genitalia or breasts, and one male patient had Tanner 4 genitalia.
Table 1.
Patient characteristics at the initiation of oxandrolone treatment.
| Pancreatic | Height | Weight | FEV/FVC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | Insufficiency | Tanner | Height z | velocity | BMI z | velocity | FEV1 (% | ||
| Patient | (years) | (M/F) | (Y/N) | Stage | score | (cm/yr) | score | (kg/yr) | predicted) | |
| 1 | 8.5 | F | Y | 1 | −2.39 | 5.8 | 0.82 | 6.6 | 118 | 90 |
| 2 | 11.9 | F | Y | 1 | −0.74 | 5.0 | −1.39 | −0.1 | 91 | 90 |
| 3 | 14.5 | M | Y | 4 | −1.97 | 7.4 | −1.03 | 8.8 | 70 | 78 |
| 4 | 12.6 | M | Y | 1 | −1.32 | 4.6 | 0.13 | 4.2 | 95 | 82 |
| 5 | 12.0 | M | Y | 1 | −1.76 | 3.6 | −1.59 | 1.4 | 67 | 78 |
| Mean | 11.9 | −1.64 | 5.3 | −0.61 | 4.18 | 88.2 | 83.6 | |||
| SD | 2.2 | 0.63 | 1.4 | 1.04 | 3.65 | 20.8 | 6.1 | |||
The five subjects had a mean height z score of −1.64 and a mean BMI z score of −0.61. As a comparison, we obtained data for patients between the ages of 11 and 14 seen between January 1, 2004 and December 31, 2008 in our CF Clinic. For females (n = 21), the average height z score was −0.14 and the average BMI z score was 0.04. For males (n = 37), the average height z score was −0.28 and the average BMI z score was −0.19. Therefore, the subjects' poor weight and/or height gain was validated by a comparison to other patients in the CF clinic.
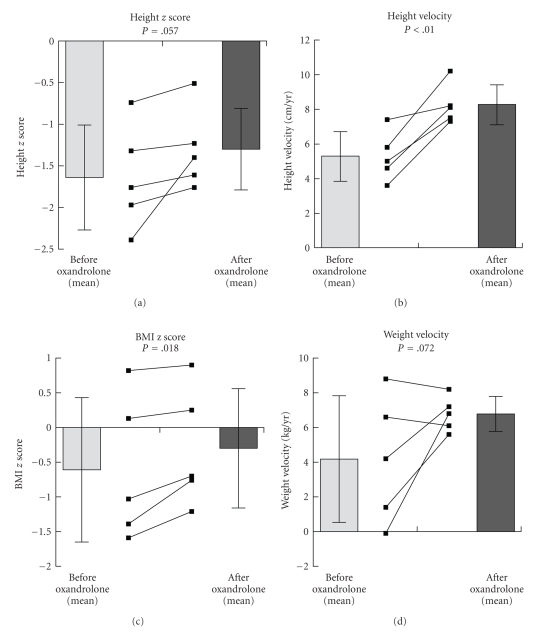
Figure 1 illustrates the anthropometric changes seen with oxandrolone. After 8–12 months of treatment, there was a statistically significant improvement in HV (pre-Ox = 5.3 ± 1.4 cm/yr, Ox = 8.3 ± 1.2 cm/yr, P < .01) and BMI z score (pre-Ox = −0.61 ± 1.04, Ox = −0.30 ± 0.86, P = .02). Both height z score (pre-Ox = −1.64 ± 0.63, Ox = −1.30 ± 0.49, P = .057) and WV (pre-Ox = 4.2 ± 3.7 kg/yr, Ox = 6.8 ± 1.0 kg/yr, P = .072) showed beneficial trends that did not reach statistical significance. When the subject with Tanner 4 genitalia (#3) was removed from the analysis, there was still a significant improvement in HV and BMI z score.
Figure 1.
Anthropometric data for subjects (n = 5) before and after initiation of oxandrolone.
There was no significant change in pulmonary function or liver enzyme levels, and no adverse events were reported. Neither female subject experienced hirsutism, clitoromegaly, or any evidence of hyperandrogenism. One female subject (#2) remained on oxandrolone for a total of 38 months (beyond the time frame used to calculate the anthropometric data shown above) and had normal pubertal progression and menarche. One subject (#4) had CF-related liver disease with mildly elevated liver enzyme tests at the initiation of oxandrolone, and his liver enzyme tests improved slightly while on oxandrolone. Two subjects (#4 and #5) had previously been on mirtazapine to stimulate appetite.
4. Discussion
In this brief clinical report, we describe 5 children with CF who received oxandrolone for at least 8–12 months. All 5 children had height z scores well below the clinic average, and 3 of the subjects had BMI z scores below the clinic average. Oxandrolone improved the height velocity and BMI z score in these patients with CF and showed a trend towards significance in weight velocity and height z score. No significant changes in pulmonary function or liver enzyme tests were appreciated.
Optimizing linear growth and weight gain is critically important in the care of children with CF. A comprehensive practice recommendation found significant evidence that weight-for-age, height-for-age, and weight-for-height percentiles are associated with improved pulmonary function and survival [7]. It is important to emphasize that a “weight only” approach will not necessarily result in improved height and/or lung function. Lai et al. noted that poor linear growth can occur independent of weight [13], and some children with CF have a linear height that is more adversely affected than would be suggested by the degree of malnutrition [14]. Therefore, increasing weight alone may not fully address the short stature that is commonly seen in patients with CF. Ideally, a growth-promoting agent in patients with CF would improve both weight and height.
Multiple appetite stimulants have been studied in patients with CF, including megestrol, cyproheptadine, atypical antipsychotics, and antidepressants [15]. Megestrol has been shown to improve weight in patients with CF, but side effects include adrenal suppression, glucose intolerance, and diabetes [16–18]. More importantly, the nonanabolic glucocorticoid actions of megestrol promote accumulation of fat mass in excess of lean body mass.
In order to stimulate appetite but to also improve body composition, anabolic agents have been used in patients with CF. Growth hormone (GH) is a potent anabolic agent that has the capacity to improve the nutritional status of patients with CF. Several studies have shown that GH in children with CF leads to improved height and weight [19–23], while one study found only an improvement in height [24]. While there were theoretical concerns of glucose intolerance with the use of GH, no study of GH in patients with CF has described this complication. There are a few disadvantages of GH. A minor concern is the use of daily injections, while a major concern is cost. The mean average wholesale price for GH, based on 6 different GH preparations, is $66.60 per mg, and therefore at an expected dose of 0.30 mg/kg/week, the estimated annual cost of GH for the 5 subjects in this paper would range from $28,500 (subject #1) to $43,900 (subject #3).
Insulin is an anabolic hormone that can improve BMI in patients with CF-related diabetes, but insulin has not been studied in patients without CF-related diabetes and carries the obvious risk of hypoglycemia.
Several articles from the 1960s describe the use of older anabolic steroids (stanozolol, methandrostenolone, and norethandrolone) in patients with CF [25–27]. While these studies reported some modest improvements in weight and height gain (as well as an improvement in “cheerfulness” [27]), in general these early anabolic steroids had very high androgenic side effect profiles that limited their utility.
Oxandrolone is a weak oral androgen taken once daily that has marked anabolic properties with minimal androgenic effects [28]. Oxandrolone is clinically efficacious across a variety of diseases associated with catabolism and wasting and is FDA-approved as adjunctive therapy for weight loss due to catabolic conditions in both adults and children [8]. Oxandrolone has also been used as a height-promoting agent. Prior to the availability of recombinant human GH, oxandrolone was commonly used to increase growth velocity in girls with Turner Syndrome [29, 30]. Oxandrolone has also been shown to increase growth velocity (but not eventual adult height) in boys with constitutional delay of growth and puberty [31]. Oxandrolone cannot be aromatized to estrogen, which minimizes advancement of the bone age. Oxandrolone is also relatively cheap. The average wholesale price of a 2.5 mg tablet of oxandrolone is $5.53, which translates to an annual cost of approximately $2,000, substantially less than the cost of GH.
While oxandrolone is generally well-tolerated, there are important potential side effects to consider. Oxandrolone can cause transient elevations in liver transaminases [8], which could be a factor in patients with or at risk for CF-related liver disease. Importantly, however, oxandrolone does not appear to cause the severe hepatotoxic effects associated with other anabolic steroids [8]. Secondly, while oxandrolone has a high anabolic to androgenic ratio [28], girls in particular may experience dose-dependent androgenic side effects, such as increase in body hair, deepened voice, or clitoral hypertrophy [30]. In a review of the efficacy and safety of oxandrolone used at modest dosages (e.g., usually 2.5–3.75 mg/day), very few studies report detectable androgenic side effects, even in studies that included women and girls [8]. Nonetheless, it would be prudent to monitor closely for signs of excess androgen in girls treated with oxandrolone.
This retrospective, noncontrolled report of a small number of subjects has intrinsic limitations. Changes in clinical and anthropometric outcomes may be confounded by the disease itself (which waxes and wanes) or by early puberty. The duration of the study was also relatively short, which makes it difficult to determine if oxandrolone had a positive effect on pulmonary function tests or a negative effect on liver enzyme tests. A randomized controlled trial would help determine if oxandrolone, compared to an appropriate control group, improves the nutritional status and/or lung function in patients with CF.
5. Conclusion
In this small retrospective exploratory study, oxandrolone safely and effectively improved the height velocity and BMI z score in patients with CF. Larger prospective studies are needed to determine more conclusively whether oxandrolone should be considered an effective, safe, and affordable option to stimulate appetite and promote growth in patients with CF.
References
- 1.Cystic Fibrosis Foundation. Annual Data Report to the Center Directors. Bethesda, Md, USA: Cystic Fibrosis Foundation; 2008. Patient Registry 2007. [Google Scholar]
- 2.Sharma R, Florea VG, Bolger AP, et al. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax. 2001;56(10):746–750. doi: 10.1136/thorax.56.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. Journal of Clinical Epidemiology. 1988;41(6):583–591. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 4.Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. Journal of the American Dietetic Association. 2001;101(4):438–442. doi: 10.1016/S0002-8223(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 5.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. Journal of Pediatrics. 2000;137(3):374–380. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 6.Peterson ML, Jacobs DR, Jr., Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics. 2003;112(3):588–592. doi: 10.1542/peds.112.3.588. [DOI] [PubMed] [Google Scholar]
- 7.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. Journal of the American Dietetic Association. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Orr R, Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64(7):725–750. doi: 10.2165/00003495-200464070-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lai HJ. Classification of nutritional status in cystic fibrosis. Current Opinion in Pulmonary Medicine. 2006;12(6):422–427. doi: 10.1097/01.mcp.0000245709.66762.f9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Lai HJ. Comparison of the use of body mass index percentiles and percentage of ideal body weight to screen for malnutrition in children with cystic fibrosis. American Journal of Clinical Nutrition. 2004;80(4):982–991. doi: 10.1093/ajcn/80.4.982. [DOI] [PubMed] [Google Scholar]
- 11.Crapo RO, Hankinson JL, Irvin C, et al. Standardization of spirometry: 1994 update. American Journal of Respiratory and Critical Care Medicine. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr. Pulmonary function between 6 and 18 years of age. Pediatric Pulmonology. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 13.Lai H-C, Kosorok MR, Sondel SA, et al. Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: evaluation of various criteria used to identify malnutrition. Journal of Pediatrics. 1998;132(3):478–485. doi: 10.1016/s0022-3476(98)70024-1. [DOI] [PubMed] [Google Scholar]
- 14.Hardin DS. Growth problems and growth hormone treatment in children with cystic fibrosis. Journal of Pediatric Endocrinology and Metabolism. 2002;15(supplement 2):731–735. [PubMed] [Google Scholar]
- 15.Nasr SZ, Drury D. Appetite stimulants use in cystic fibrosis. Pediatric Pulmonology. 2008;43(3):209–219. doi: 10.1002/ppul.20766. [DOI] [PubMed] [Google Scholar]
- 16.Eubanks V, Koppersmith N, Wooldridge N, et al. Effects of megestrol acetate on weight gain, body composition, and pulmonary function in patients with cystic fibrosis. Journal of Pediatrics. 2002;140(4):439–444. doi: 10.1067/mpd.2002.121936. [DOI] [PubMed] [Google Scholar]
- 17.Marchand V, Baker SS, Stark TJ, Baker RD. Randomized, double-blind, placebo-controlled pilot trial of megestrol acetate in malnourished children with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition. 2000;31(3):264–269. doi: 10.1097/00005176-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Nasr SZ, Hurwitz ME, Brown RW, Elghoroury M, Rosen D. Treatment of anorexia and weight loss with megestrol acetate in patients with cystic fibrosis. Pediatric Pulmonology. 1999;28(5):380–387. doi: 10.1002/(sici)1099-0496(199911)28:5<380::aid-ppul11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Hardin DS, Sy JP. Effects of growth hormone treatment in children with cystic fibrosis: the National Cooperative Growth Study experience. Journal of Pediatrics. 1997;131(1, supplement 1):S65–S69. doi: 10.1016/s0022-3476(97)70015-5. [DOI] [PubMed] [Google Scholar]
- 20.Hardin DS, Stratton R, Kramer JC, Reyes de la Rocha S, Govaerts K, Wilson DP. Growth hormone improves weight velocity and height velocity in prepubertal children cystic fibrosis. Hormone and Metabolic Research. 1998;30(10):636–641. doi: 10.1055/s-2007-978949. [DOI] [PubMed] [Google Scholar]
- 21.Hardin DS, Ferkol T, Ahn C, et al. A retrospective study of growth hormone use in adolescents with cystic fibrosis. Clinical Endocrinology. 2005;62(5):560–566. doi: 10.1111/j.1365-2265.2005.02259.x. [DOI] [PubMed] [Google Scholar]
- 22.Hardin DS, Ellis KJ, Dyson M, Rice J, McConnell R, Seilheimer DK. Growth hormone improves clinical status in prepubertal children with cystic fibrosis: results of a randomized controlled trial. Journal of Pediatrics. 2001;139(5):636–642. doi: 10.1067/mpd.2001.117578. [DOI] [PubMed] [Google Scholar]
- 23.Hardin DS, Adams-Huet B, Brown D, et al. Growth hormone treatment improves growth and clinical status in prepubertal children with cystic fibrosis: results of a multicenter randomized controlled trial. Journal of Clinical Endocrinology and Metabolism. 2006;91(12):4925–4929. doi: 10.1210/jc.2006-1101. [DOI] [PubMed] [Google Scholar]
- 24.Schnabel D, Grasemann C, Staab D, Wollmann H, Ratjen F. A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the metabolic and respiratory effects of growth hormone in children with cystic fibrosis. Pediatrics. 2007;119(6):e1230–e1238. doi: 10.1542/peds.2006-2783. [DOI] [PubMed] [Google Scholar]
- 25.Dooley RR, Moss AJ, Wright PM, Hassakis PC. Norethandrolone in cystic fibrosis of the pancreas. Journal of Pediatrics. 1969;74(1):95–102. doi: 10.1016/s0022-3476(69)80013-2. [DOI] [PubMed] [Google Scholar]
- 26.Good TA, Bessman SP. Anabolic steroids in cystic fibrosis of the pancreas. American Journal of Diseases of Children. 1966;111(3):272–277. doi: 10.1001/archpedi.1966.02090060082007. [DOI] [PubMed] [Google Scholar]
- 27.Dennis JL, Panos TC. Growth and bone-age retardation in cystic fibrosis. Response to an anabolic steroid. Journal of the American Medical Association. 1965;194(8):855–858. [PubMed] [Google Scholar]
- 28.Fox M, Minot AS, Liddle GW. Oxandrolone: a potent anabolic steroid of novel chemical configuration. Journal of Clinical Endocrinology & Metabolism. 1962;22:921–924. doi: 10.1210/jcem-22-9-921. [DOI] [PubMed] [Google Scholar]
- 29.Naeraa RW, Nielsen J, Pedersen IL, Sorensen K. Effect of oxandrolone on growth and final height in Turner’s syndrome. Acta Paediatrica Scandinavica. 1990;79(8-9):784–789. doi: 10.1111/j.1651-2227.1990.tb11555.x. [DOI] [PubMed] [Google Scholar]
- 30.Crock P, Werther GA, Wettenhall HNB. Oxandrolone increases final height in Turner syndrome. Journal of Paediatrics and Child Health. 1990;26(4):221–224. doi: 10.1111/j.1440-1754.1990.tb02434.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilson DM, McCauley E, Brown DR, et al. Oxandrolone therapy in constitutionally delayed growth and puberty. Bio-Technology General Corporation Cooperative Study Group. Pediatrics. 1995;96(6):1095–1100. [PubMed] [Google Scholar]