Abstract
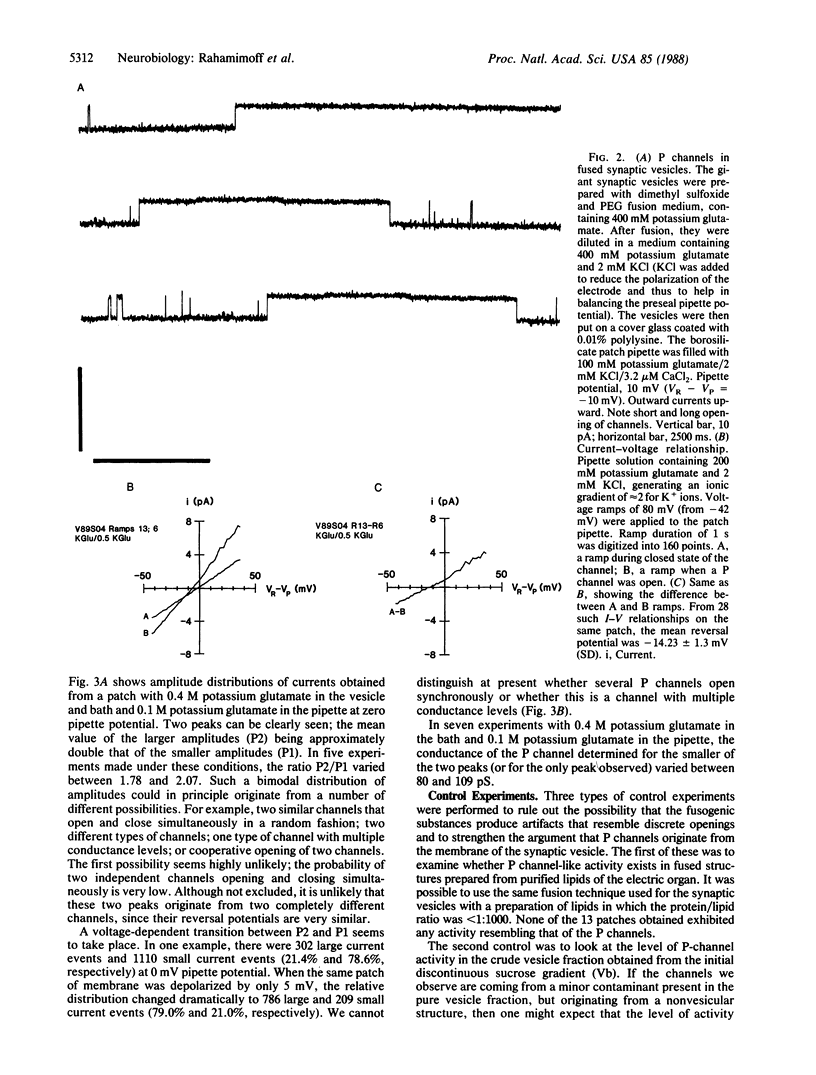
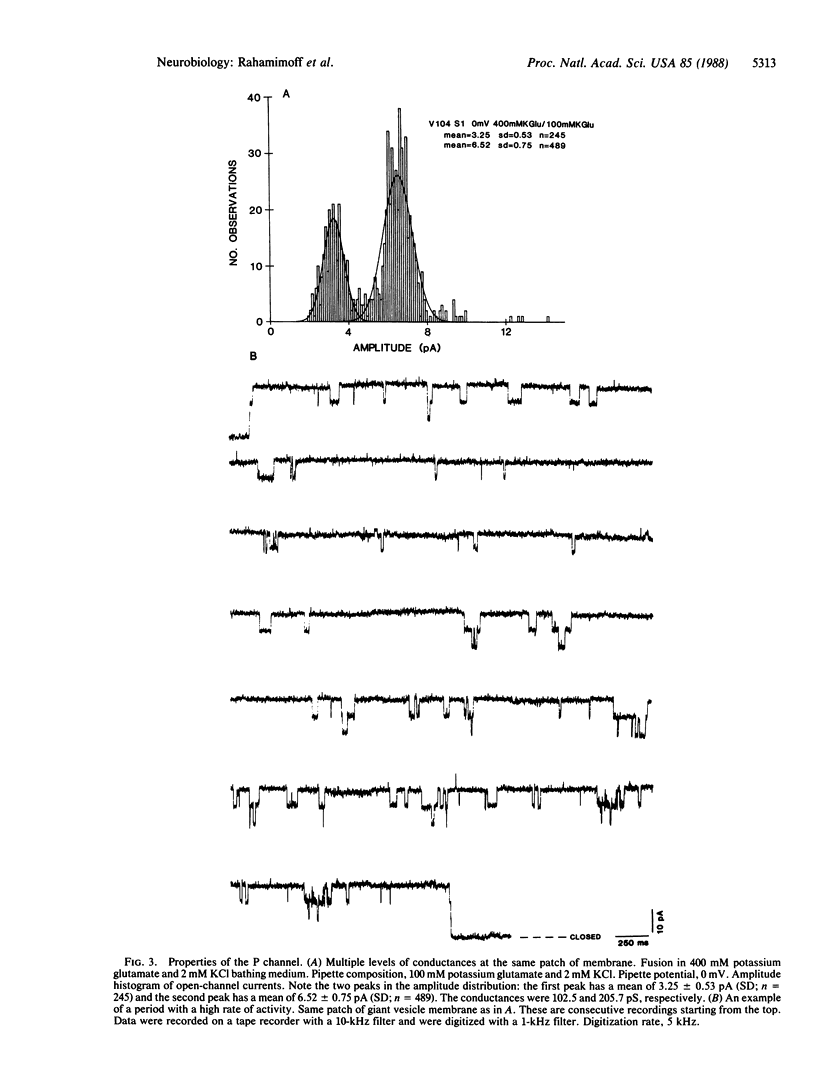
A simple method has been developed for fusing synaptic vesicles into spherical structures 20-50 micron in diameter. The method has been applied to purified cholinergic synaptic vesicles from Torpedo electric organ, and the membrane properties of these fused structures have been studied by the "cell"-attached version of the patch clamp technique. A large conductance potassium-preferring channel, termed the P channel, was consistently observed in preparations of fused synaptic vesicles. The selectivity of the channel for potassium over sodium was approximately equal to 2.8-fold. Two major conductance levels were observed during P-channel activity, and their relative proportion was dependent on the voltage applied to the membrane through the patch pipette. P channels were not seen in fused preparations of purified Torpedo lipids, nor was the frequency of their occurrence increased in preparations enriched with plasma membrane or nonvesicular membranes. We suggest, therefore, that the P channels are components of the synaptic vesicle membrane. Their function in synaptic transmission physiology is still unknown.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel I., Michaelson D. M. Determination of delta psi, delta pH and the proton electrochemical gradient in isolated cholinergic synaptic vesicles. Life Sci. 1981 Jul 27;29(4):411–416. doi: 10.1016/0024-3205(81)90335-0. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. S., Zimmerberg J., Finkelstein A. Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. II. Incorporation of a vesicular membrane marker into the planar membrane. J Gen Physiol. 1980 Mar;75(3):251–270. doi: 10.1085/jgp.75.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harlos P., Lee D. A., Stadler H. Characterization of a Mg2+-ATPase and a proton pump in cholinergic synaptic vesicles from the electric organ of Torpedo marmorata. Eur J Biochem. 1984 Nov 2;144(3):441–446. doi: 10.1111/j.1432-1033.1984.tb08485.x. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michaelson D. M., Sokolovsky M. Induced acetylcholine release from active purely cholinergic Torpedo synaptosomes. J Neurochem. 1978 Jan;30(1):217–230. doi: 10.1111/j.1471-4159.1978.tb07055.x. [DOI] [PubMed] [Google Scholar]
- Miller C., Arvan P., Telford J. N., Racker E. Ca++-induced fusion of proteoliposomes: dependence on transmembrane osmotic gradient. J Membr Biol. 1976;30(3):271–282. doi: 10.1007/BF01869672. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Parsons S. M., Koenigsberger R. Specific stimulated uptake of acetylcholine by Torpedo electric organ synaptic vesicles. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6234–6238. doi: 10.1073/pnas.77.10.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazoles C. J., Pollard H. B. Evidence for stimulation of anion transport in ATP-evoked transmitter release from isolated secretory vesicles. J Biol Chem. 1978 Jun 10;253(11):3962–3969. [PubMed] [Google Scholar]
- Sorgato M. C., Keller B. U., Stühmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987 Dec 3;330(6147):498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- Stadler H., Kiene M. L. Synaptic vesicles in electromotoneurones. II. Heterogeneity of populations is expressed in uptake properties; exocytosis and insertion of a core proteoglycan into the extracellular matrix. EMBO J. 1987 Aug;6(8):2217–2221. doi: 10.1002/j.1460-2075.1987.tb02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler H., Tashiro T. Isolation of synaptosomal plasma membranes from cholinergic nerve terminals and a comparison of their proteins with those of synaptic vesicles. Eur J Biochem. 1979 Nov 1;101(1):171–178. doi: 10.1111/j.1432-1033.1979.tb04229.x. [DOI] [PubMed] [Google Scholar]
- Stanley E. F., Ehrenstein G. A model for exocytosis based on the opening of calcium-activated potassium channels in vesicles. Life Sci. 1985 Nov 25;37(21):1985–1995. doi: 10.1016/0024-3205(85)90029-3. [DOI] [PubMed] [Google Scholar]
- Tashiro T., Stadler H. Chemical composition of cholinergic synaptic vesicles from Torpedo marmorata based on improved purification. Eur J Biochem. 1978 Oct 16;90(3):479–487. doi: 10.1111/j.1432-1033.1978.tb12627.x. [DOI] [PubMed] [Google Scholar]




