Abstract
Objective
The focus of this study is brain plasticity associated with semantic aspects of language function in patients with medial temporal lobe epilepsy (mTLE).
Methods
Using longitudinal functional magnetic resonance imaging (fMRI), patterns of brain activation were observed in twelve left and seven right unilateral mTLE patients during a word-generation task relative to a pseudo-word reading task before and after anterior temporal section surgery.
Results
No differences were observed in precentral activations in patients relative to normal controls (n = 12), and surgery did not alter the phonological-associated activations. The two mTLE patient groups showed left inferior prefrontal activations associated with semantic processing (word-generation > pseudo-word reading), as did control subjects. The amount of semantic-associated activation in the left inferior prefrontal region was negatively correlated with epilepsy duration in both patient groups. Following temporal resection, semantic-specific activations in inferior prefrontal region became more bilateral in left mTLE patients, but more left-lateralized in right mTLE patients. The longer the duration of epilepsy in the patients, the larger the increase in the left inferior prefrontal semantic-associated activation after surgery in both patient groups. Semantic activation of the intact hippocampus, which had been negatively correlated with seizure frequency, normalized after the epileptic side was removed.
Conclusion
These results indicate alternation of semantic language network related to recruitment of left inferior prefrontal cortex and functional recovery of the hippocampus contralateral to the epileptogenic side, suggesting an intra- and inter-hemispheric reorganization following surgery.
Keywords: fMRI, Language, Epilepsy, Brain plasticity, Hippocampus
INTRODUCTION
Language function is mainly lateralized in the dominant (left) hemisphere in healthy normal subjects6). Epilepsy patients, however, show different patterns of language-associated laterality depending on the side of the lesion as revealed by the Wada test19), and supported by neuroimaging studies20). Here, we examine the semantic-associated brain plasticity in a population with hippocampal lesions-namely those with medial temporal lobe epilepsy (mTLE).
Hippocampal sclerosis (HS), which is thought to result from an initial precipitating injury in early childhood4), may induce subtle, intra-hemispheric neural reorganization of language in mTLE patients20). A recent diffusion tensor imaging (DTI) study of mTLE patients with HS suggests that hippocampal diffusion abnormalities are located bilaterally pre-operatively16), but the hippocampus contralateral to the lesion may recover after epilepsy surgery17). Considering that the hippocampal system is an integral component of the brain circuit responsible for learning and retrieving linguistic facts and conventions15), if the contralateral hippocampus recovered from the active seizure process after successful surgery, we would expect that it would have a key role in inducing brain reorganization for language function in mTLE patients.
Despite a number of neuropsychological studies on outcomes of unilateral temporal lobe resections3,21), there are few published functional brain imaging studies of areas associated with language plasticity after temporal lobectomy13) or hemispherectomy7). A recent functional magnetic resonance imaging (fMRI) study on patients with left mTLE showed that maintenance of effective language capacity in the post-operative state relies on integrating regions from the normal system and recruiting the right hemisphere, which is not involved in language function in normal subjects12). However, this study was conducted on only one type of mTLE (left mTLE), and only after surgery, so it could not be determined if this plastic change occurred pre-operatively or post-operatively, or to what extent the brain plasticity associated with left mTLE existed before surgery.
In this study, our goal was to determine the extent to which brain plasticity modifies this semantic-associated prefrontal language network in mTLE patients, both before and after surgical removal of a dysfunctioning left or right hippocampus.
MATERIALS AND METHODS
Subjects
This study was approved by the Institutional Research Board (number : H-0712-007-227). All subjects agreed to the use of their data for research purposes. Among the approximately 500 patients who underwent epilepsy surgery from 1988 to 2006, nineteen subjects met our inclusion criteria for unilateral mTLE with HS (12 left/7 right mTLE) (Table 1). HS was confirmed pathologically after surgery, and all were either seizure-free or had more than a 90% reduction of seizures (Engel Class I or II) for at least 13 months after surgery. Twelve normal, healthy subjects (5 male and 7 females, 25 ± 1.25 years) served as controls.
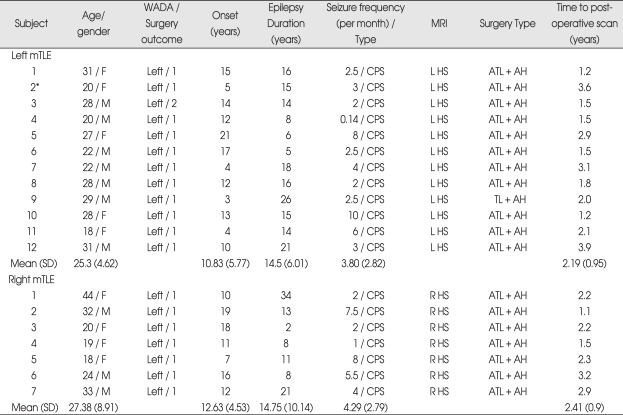
Table 1.
Clinical information for mTLE patients
Seizure outcome is measured based on the engels outcome : 1. completely seizure-free, 2. rare seizure. *The preference of language for patient no.2 in left TLE was determined based on the fMRI result (Laterality index = 0.78), because of equivocal language preference by Wada test. AH : amygdalohippocampectomy, ATL : anterior temporal lobectomy, CPS : complex partial seizure, F : female, L HS : left hippocampal sclerosis, M : male, mTLE : medial temporal lobe epilepsy, R HS : right hippocampal sclerosis, SD : standard deviation, TL : temporal lobectomy
Surgery
All subjects had undergone both standard en bloc anteromesial temporal lobectomy (ATL) and selective amygdalohippocampectomy (AH) by a single neurosurgeon. The superior temporal lobe was included in resection, with the central sulcus as the landmark of the posterior limit. The amygdala was removed en bloc and at least the first 3 cm of the head of the hippocampus was removed with reference to the midbrain as a landmark.
Image acquisition
Pre-operative patients were scanned with a GE Signa 1.5-T scanner and post-operative patients were scanned with a GE Signa 3.0-T scanner equipped with a standard head coil (General Electric Medical System, Milwaukee, WI, USA). Each underwent four fMRI runs for each fMRI session. In each run, four conditions (three tasks and one fixation) were repeated three times. Patients underwent two fMRI sessions, a pre-operative and a post-operative; control subjects participated only in one fMRI session.
For each run, we acquired 96 T2*-weighted, gradientecho (GRE) planar imaging (EPI) scans in axial direction (thickness, 6 mm with no gap; TR, 3,000 ms; TE, 50 ms; resolution 64×64; FOV 240 mm). A high-resolution structural T1-weighted image was acquired using a flow-sensitive conventional gradient echo sequence with 120 slices (thickness, 1.4 mm; interslice gap, 0 mm; TR, 50 ms; TE, 4 ms; flip angle, 60°). A neck-holder (MJ-200, USA) reduced movement, and earplugs dampened scanner noise.
Language task paradigms
Three different language tasks in Korean were used : sentence reading, pseudo-word reading, and word generation9). During the sentence reading task, patients read covertly a simple sentence ['(I) opened (the) window.']. During the pseudo-word reading task, subjects read covertly two clusters of phonologically Korean-like, pronounceable non-words as if they formed a simple sentence. During the word generation task, patients were required to covertly generate a word that semantically and grammatically filled a blank in the sentences ['Sharp ( ).' for a noun; '( ) a radio.' for a verb; 'Sugar is ( ).' for an adjective; 'Bird is ( ) singing.' for an adverb]. A sentence was presented every 3 sec for each trial, and a total of eight trials were given in a 24-sec task condition block. Prior to the experiment, subjects were given practice outside the scanner to ensure they fully understood the instructions and had the ability to competently perform each task. Later, outside the scanner, patients repeated the tasks overtly to verify that they performed them appropriately.
fMRI analysis
fMRI data were analyzed using Analysis of Functional NeuroImages (AFNI)5). Pre-processing steps included discarding first 4 volumes, slice timing correction, head motion correction, co-registration, spatial normalization, spatial smoothing (8 mm full-width half-maximum), and signal normalization9). To avoid possible suboptimal results of spatial normalization in brains with structural damage following temporal lobe resection, a mask for the resected region was semi-automatically created using ITK-SNAP (http://www.itksnap.org/) for the post-operative structural image. We derived the correspondence between anatomical pre- and post-operative images using linear transformations with lesion masks, and then aligned the pre-operative anatomical image, instead of the post-operative image, for spatial transformations such as coregistration and normalization of post-operative EPI data.
To remove motion artifacts, realignment parameters went into the analysis as covariates of no interest. In a first level analysis, condition-specific effects of interest were computed for each subject (3dDeconvolve in AFNI). In a second level analysis, a random-effect model was used to create group activation maps. A two-way mixed-effect analysis of variance (ANOVA) was performed on each voxel for within- and between-group analysis. Planned contrasts (word generation vs. pseudo-word reading) were undertaken (fixed effect), with each individual subject serving as the repeated measure (random effect). Using AlphaSim (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim), corrections for multiple comparisons were carried out at the cluster level (min p < 0.001; cluster significant: p < 0.05, corrected). Between-group effects were reported at p < 0.001, uncorrected for multiple comparisons.
Region of interest analysis
To delineate the inferior prefrontal region of interest (ROI), we used both functional and anatomical ROI definitions1). The functional ROI was defined in the left prefrontal regions (p < 0.05 (uncorrected), and at least 80% of patients (WG > Pseudo). The anatomical ROI was defined in a brain atlas map, which is available in AFNI. Only voxels common to both the functional ROI and the anatomical ROI were included as the left inferior prefrontal ROI. The right inferior prefrontal ROI was generated by using the mirror image of the left inferior prefrontal ROI.
A Laterality Index (LI) was calculated for the inferior prefrontal ROI as follows :
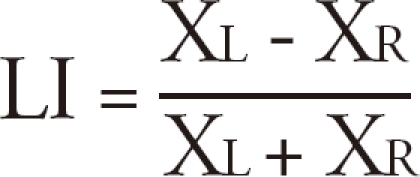
where XL = the mean of parameter estimate in the left ROI, and XR = the mean of parameter estimate in the right ROI. LI scores between +.20 and -.20 were considered bilateralized activations. Any ROI mean parameter (XL or XR) less than zero (Word-generation < Pseudo-word reading) was taken as zero.
In most patients, the map based on functional activations in the medial temporal regions was not reliable enough to delineate a functional hippocampal ROI. Thus, delineation of the hippocampal ROI was based entirely on the anatomical brain atlas map available in AFNI.
RESULTS
Phonological language processing (Pseudo-Fix)
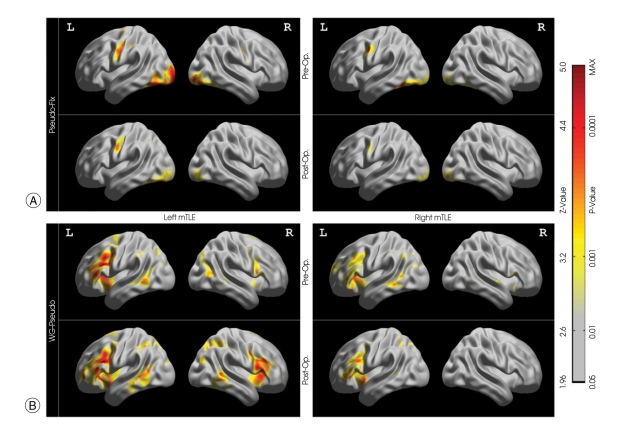
For the pseudo-word reading task relative to the fixation condition, activations were found mainly in the left hemisphere (Fig. 1A). No significant difference was found between left and right mTLE groups. No significant change was found in the post-operative session relative to the preoperative session, either in the left or the right mTLE groups. Thus, temporal resection surgery did not alter the phonological-associated activation pattern.
Fig. 1.
Group activation map in the medial temporal lobe epilepsy (mTLE) patients. A : Activation maps during phonetic processing. B : Activation maps during semantic-specific language processing. Color bars represent Z-values, and corresponding p-values.
Semantic-specific language processing (WG-Pseudo)
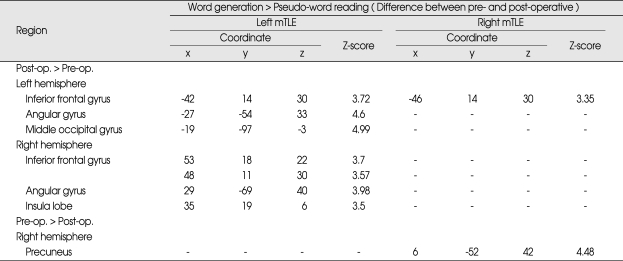
The pre- and post-operative semantic-specific language activations were summarized in Table 2 and Fig. 1B. For the left mTLE group, post-operative activations were significantly increasing in the bilateral inferior frontal regions, bilateral angular gyrus, right insula, and left middle occipital gyrus when compared with pre-operative activations; no significant decreases of activation were found. For the right mTLE group, post-operative activations were found only in left inferior frontal regions when compared with pre-operative activations. The right precuneus of the right mTLE showed a decrease of activation post-operatively relative pre-operatively. Changes in semantic-specific activation after surgery are summarized in Table 3.
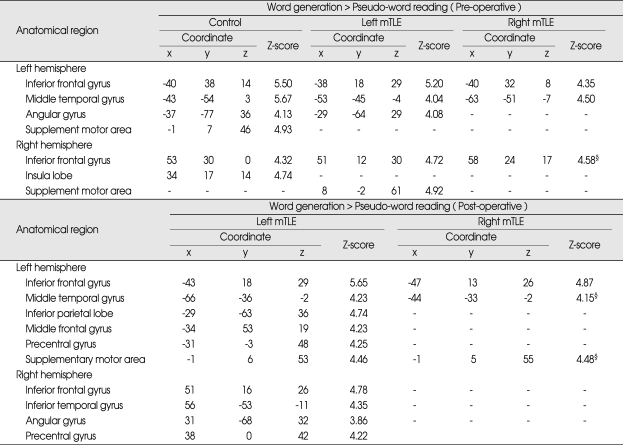
Table 2.
Localization and z statistic of peak voxel for semantic-specific language activations
All coordinates are reported using the Montreal Neurological Institute system. Coordinates represent voxels significant at p < 0.05 (corrected). §p < 0.001 (uncorrected). mTLE : medial temporal lobe epilepsy
Table 3.
Comparison of pre- and post-operative semantic-specific language activation in left or right mTLE patient groups
All coordinates are reported using the Montreal Neurological Institute system. Coordinates represent voxels significant at p < 0.001 (uncorrected). mTLE : medial temporal lobe epilepsy
Change of semantic-specific language dominance (Laterality)
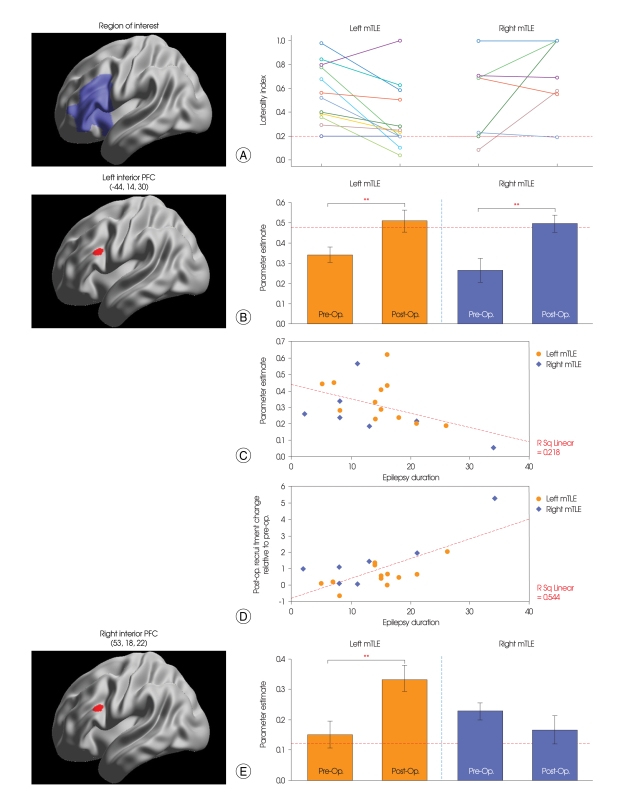
The pre-operative LI (Fig. 2A) showed good agreement with individual Wada tests, with the exception of two patients (patient #1 of the left and patient #3 of the right mTLE groups). After resection of the left anterior temporal lobe, 5 of 12 left mTLE patients showed bilateralized language dominance. The difference between the average of the pre-operative LI (0.57) and post-operative LI (0.35) was significant for the left mTLE patients (p < 0.01). No significant was observed in the right mTLE group.
Fig. 2.
Changes of semantic-specific language activation. A : Language lateralization in each medial temporal lobe epilepsy group. Dotted line represents the critical value (LI = 0.2) for determining bilateralized language dominance. B : Changes of activations in the left PFC. C : Scatter plot depicts the correlation of epilepsy duration and individuals' regional activation during the pre-operative session. D : Scatter plot depicts the correlation of epilepsy duration and individuals' regional recruitment change. E : Changes of activations in the right PFC. Dotted line represents the normal activation level for semantic-specific language processing, obtained from 12 right-handed healthy controls. The error bars represent the standard error of the means. **p < 0.01.
Change of semantic-specific language activation (left and right PFC)
For further analysis, mean activation levels were extracted from 12-mm diameter circle centered on previously-identified local maximum of left inferior PFC activation [-44, 14, 30]. Paired T-test showed a increase of semantic-specific activation in the left inferior PFC after surgery (p < 0.01 for left mTLE, p < 0.01 for right mTLE)(Fig. 2B). Epilepsy duration correlated negatively with activation of the left inferior PFC pre-operatively (r = -0.467, R2 = 0.218, p < 0.05)(Fig. 2C), and there was a strong positive correlation between epilepsy duration and recruitment of the left inferior PFC (r = 0.738, R2 = 0.544, p < 0.0005)(Fig. 2D).
There was a significant increase in the right inferior PFC after surgery in the left mTLE group (p < 0.01)(Fig. 2E). There was no significant in the right mTLE group.
Recovery of hippocampus from epileptic seizures
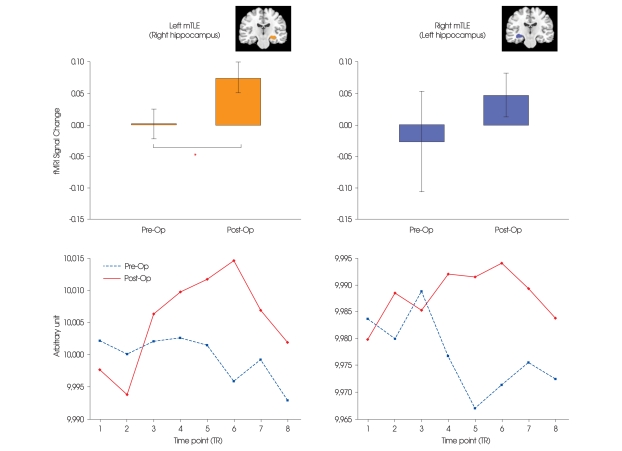
There was a significant post-operative increase in activation in the right hippocampus in left mTLE patients (p < 0.05) (Fig. 3A). An increased activation of the left hippocampus in the right mTLE patients post-operatively was not significant. Unlike the pre-operative session, the post-operative hemodynamic response during the word-generation block was well matched to the canonical hemodynamic response function (Fig. 3B). A negative correlation was found between epileptic seizure frequency and semantic-specific language activation of the right hippocampus in left mTLE group pre-operatively (r = -0.58, R2 = 0.336, p < 0.05).
Fig. 3.
Semantic-related hippocampus profile, which is contralateral to the epilepsy lesion. A : Percent signal change in the hippocampus within the semantic related network. B : Average time profiles of the hippocampus during word generation task. The individual blocks of the word generation task have been averaged into a representative time series. The values are in arbitrary units. Blue dotted line represents average time series during pre-operative session, and red solid line during post-operative session. *p < 0.05.
DISCUSSION
Phonetic language processing
Our results indicate that dysfunctions of the medial temporal area do not affect phonological language processing in the left frontal area. This result is not surprising since, unlike long-term memory, orthographical reading doesn't require intact function of medial temporal cortex in well-practiced readers.
Dynamic shifts of semantic-specific language processing in mTLE patients after surgery
Functional imaging studies of post-operative brain plasticity for language networks have been previously reported. Using a series of pre- and post-operative fMRI maps, Hertz-Pannier et al.7) reported a right hemispheric shift of language function after left hemispherectomy in a 9-year-old patient with Rasmussen's encephalitis. Using PET, Müller et al.11) observed a left hemispheric activation after right hemispherectomy in a 6-year-old patient with Rasmussen's encephalitis. Consistent with previous reports7,11), we observed plasticity in the language network after surgery, in particular in the semantic processing network, but not in the phonological processing network. In particular, there was a shift in semantic-associated inferior prefrontal activation from left-lateralized to bi-lateralized following left temporal resection in left mTLE patients. Additionally, a shift in semantic activation in prefrontal regions from moderately left lateralized to predominantly left-lateralized occurred following right temporal resection in right mTLE patients.
Increase of PFC activations associated with semantic processing in mTLE patients after surgery
We found that left PFC activations increased in both patient groups following removal of the pathological hippocampus, relative to activations before surgery. Interestingly, the left mTLE group also showed increased PFC activation in the right hemisphere. These post-operative changes might have resulted from the surgical removal of a dysfunctional hippocampus that, in the preoperative condition, interfered with the contralateral hippocampus. Considering the parallel speech processing8,14), where the left inferior PCF seems to functionally connect to the parietal lobe via a strongly left-hemisphere dominated dorsal language pathway, and to also connect to the temporal lobe via a largely bilaterally-organized ventral language pathway, someone could postulate that surgical removal of the pathological (left) hippocampus may eliminate its deleterious influences on the ipsilateral (left) language pathway and the contralateral (right) hippocampus, resulting in 'normalization' of left PCF function (via ipsilateral dorsal language pathway) and a 'functioning' right hippocampus. Additionally, permanent loss of the pathological (left) hippocampus may lead to development of an alternative right PFC network (via a contralateral ventral language pathway), which is possibly homologous to the original left PFC network for compensation of semantic language processing.
Functional reversible change in mTLE patients after surgery
Recent studies of a computer simulation10) and DTI study17) of TLE patents suggest that it may be possible to reverse some of the deleterious effect of epileptic seizures by surgical intervention. For left and right mTLE patients, we found that BOLD (blood-oxygenation level dependent) activation of the left PFC was abnormal pre-operatively, that the magnitude of the activation was negatively correlated with epileptic duration pre-operatively, and that the BOLD activation was normalized and recovered from the deleterious effects of epileptic seizures after surgery. We also found that the BOLD response of the right hippocampus was abnormal pre-operatively in the left mTLE patients, that the magnitude of the response was negatively correlated with epileptic seizure frequency pre-operatively, and that the BOLD response was normalized in the post-operative state. These results suggest that the functional abnormality in left inferior PFC and right hippocampus within semanticspecific language network may recover from epileptic pathology after successful surgery, meaning that pathological effects on the left inferior PFC and the right hippocampus must be reversible after successful surgery.
Interpretation on changes of the semantic-specific language processing after surgery
Several factors could explain our observation of changes of the semantic-specific language processing after ATL. First, the temporal confounds such as time between the two fMRI sessions, might have induced a change of activation between repeated fMRI measurements. A second possible confound is that stimulus repetition may have resulted in a learning/practice effect for the patients. This is unlikely because word retrieval learning seems to be accompanied by functional response decrement, not increment, in the left inferior frontal gyrus, at least during relatively short-intervals2,18). A third possibility is that the loss of the anterior temporal lobe may have caused the change in fMRI activation of the PFC. This is also unlikely because we observed the change of the left inferior PFC activation in both the left mTLE and the right mTLE patients independent of the side of resection. A fourth possibility is that the antiepileptic drugs, taken by refractory mTLE patients, can affect language networks in pre-operative state. In this study, all confounding factors cannot be well controlled due to the complexity of the study design and absence of longitudinal control subjects. Although all four mechanisms may have some merit in explaining the differences observed between pre-operative and post-operative language network in mTLE patients, we carefully conclude that the change of functional activity in inferior PFC was most likely related to reorganization of language network including effect of release from functional disturbance by epileptic activity.
Methodological considerations
A limitation of this study concerns the different magnetic field strength between pre- and post-operative state. Although we cannot exclude the possibility of the beneficial effect of 3 T in post-operative activation, we thought that the changes of post-operative activation may have resulted from the surgical removal of a pathological hippocampus. First, there was strong increase of percent signal change (96% for WG) at post-operative fMRI (3 T) compared with preoperative fMRI (1.5 T). Second, we found that the preoperative percent signal change and post-operative recruitment change was correlated with pathological variables. Another limitation of this study is the absence of a behavioral measure of language function inside the scanner. Because the subjects performed covert reading or wordgeneration, the task did not allow monitoring of performance criteria such as accuracy or reaction time. However, we made sure that subjects were given enough practice outside the scanner in order to ensure that they fully understood the instructions and demonstrated the ability to competently perform each task prior to the experiment.
CONCLUSION
Our results provide evidence for reversible recovery of hippocampus and PFC from epilepsy after successful surgery for semantic-specific language processing, but not for phonological language processing, suggesting that these reversible changes could be important factors in brain reorganization. This understanding of brain plasticity may provide therapeutic strategies in the treatment and neurorehabilitation of chronic mTLE patients.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) NRL program grant funded by the Korea government (MEST) (R0A-2007-000-20068-0).
References
- 1.Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 2.Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36:159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- 3.Bonilha L, Yasuda CL, Rorden C, Li LM, Tedeschi H, de Oliveira E, et al. Does resection of the medial temporal lobe improve the outcome of temporal lobe epilepsy surgery? Epilepsia. 2007;48:571–578. doi: 10.1111/j.1528-1167.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox RW. Afni: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 6.Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertz-Pannier L, Chiron C, Jambaque I, Renaux-Kieffer V, Van de Moortele PF, Delalande O, et al. Late plasticity for language in a child's non-dominant hemisphere: a pre- and post-surgery fMRI study. Brain. 2002;125:361–372. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- 8.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 9.Kim CH, Kim JH, Chung CK, Kim JS, Lee JM, Lee SK. Localization of broca's area using functional MR imaging : quantitative evaluation of paradigms. J Korean Neurosurg Soc. 2009;45:219–223. doi: 10.3340/jkns.2009.45.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci U S A. 2008;105:6179–6184. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller RA, Chugani HT, Muzik O, Mangner TJ. Brain organization of motor and language functions following hemispherectomy : a [(15)o]-water positron emission tomography study. J Child Neurol. 1998;13:16–22. doi: 10.1177/088307389801300103. [DOI] [PubMed] [Google Scholar]
- 12.Noppeney U, Price CJ, Duncan JS, Koepp MJ. Reading skills after left anterior temporal lobe resection : an fMRI study. Brain. 2005;128:1377–1385. doi: 10.1093/brain/awh414. [DOI] [PubMed] [Google Scholar]
- 13.Pataraia E, Billingsley-Marshall RL, Castillo EM, Breier JI, Simos PG, Sarkari S, et al. Organization of receptive language-specific cortex before and after left temporal lobectomy. Neurology. 2005;64:481–487. doi: 10.1212/01.WNL.0000150900.71773.E6. [DOI] [PubMed] [Google Scholar]
- 14.Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 15.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 16.Thivard L, Lehericy S, Krainik A, Adam C, Dormont D, Chiras J, et al. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005;28:682–690. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Thivard L, Tanguy ML, Adam C, Clémenceau S, Dezamis E, Lehéricy S, et al. Postoperative recovery of hippocampal contralateral diffusivity in medial temporal lobe epilepsy. Epilepsia. 2007;48:599–604. doi: 10.1111/j.1528-1167.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- 18.Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 19.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. 1960. J Neurosurg. 2007;106:1117–1133. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- 20.Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, et al. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- 21.Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy : a randomized prospective study. Neurosurgery. 1995;37:982–990. doi: 10.1227/00006123-199511000-00019. discussion 990-991. [DOI] [PubMed] [Google Scholar]