Abstract
Sacral insufficiency fracture is a debilitating injury not easily found in general radiologic examinations and is rarely diagnosed, since its symptoms are obscure. It is known to frequently occur in patients with osteoporosis, but the treatment has not yet been established and various kinds of treatment methods are being attempted. Sacroplasty is sometimes performed by applying percutaneous vertebroplasty which is known to be a less invasive treatment. Since the course of diagnosis of sacral insufficiency fracture is difficult and clear guidelines for treatments have not yet been established, many spine surgeons fail to diagnose patients or speculate on treatment methods. We report our experience in diagnosing a sacral insufficiency fracture in a 54-year-old healthy female patient using MRI and treating her with sacroplasty. From a therapeutic point of view, we then cover the usefulness, effects and characteristics relating to the complications of sacroplasty, along with literature review.
Keywords: Sacroplasty, Sacrum, Insufficiency fracture, Vertebroplasty, Kyphoplasty
INTRODUCTION
Sacral insufficiency fracture (SIF) frequently occurs in relatively old patients with osteoporosis and the pathologic mechanism is a fatigue fracture in most of the cases. However, there is usually no definite trauma history in these cases or the degree of trauma is minimal, and the fracture site is very rarely found in plain X-ray radiography. Thus, this disease is hardly ever diagnosed6). Although the complained pains are very severe and often the patient can't ambulate, the diagnoses are typically not made soon enough, particularly when the symptoms are first reported. Therefore, even if the patients complain the symptoms, they may easily be overlooked16). First introduced in 1987, percutaneous vertebroplasty (PVP) has been used on most malignant or nonmalignant spinal pathologies, except for infection, with increased indications and treatments10). Treatment methods applying PVP to SIF have recently begun to be introduced25). The authors performed the first sacroplasty with a good result, making it reason for this report, which will mainly discuss sacroplasty in terms of its therapeutic value in treating SIF.
CASE REPORT
Climical course
A 54-year-old female patient visited our hospital with the chief complaints of severe lower back pain and buttock pain on both sides, which she had been feeling for the past 3 months. She had no particular past medical history or trauma history. For over 20 years, she had been doing work that required her to sit on a flat floor for 8 hours a day or longer. Various kinds of conservative therapies had been performed for 3 months, with no relief of symptoms. On physical examinations, she complained severe lower back and buttock pain as well as tenderness in the sacral area. In the neurologic examination, there were neither pain nor hypesthesia in either of the lower limbs. There were also no abnormal manifestations in the laboratory findings. The X-ray radiography revealed degenerative spondylolisthesis L5 on S1 (grade I) and no abnormal finding was observed in the sacrum (Fig. 1). T-score of the lower lumbar spine was -2.0 on the bone densitometry, indicating possible osteopenia. The lumbar CT showed a subtle fracture on the sacral alas and the S2 body (Fig. 2). The Lumbosacral MRI, showed evidence of degenerative spondylolisthesis L5 on S1, and a lesion revealing abnormal signal intensity was observed in the S2 body (Fig. 3). This lesion showed low-signal intensity in the T2-weighted sagittal image, and low-signal intensity accompanied with surrounding bone edema in the T1-weighted image which was highly enhanced in the enhancement study (Fig. 3). The axial and coronal MR images showed signal changes in both sacral alas and the S2 body, which had a "classical H- or butterfly shaped appearance," indicating SIF (Fig. 4). She had shown no relief of the symptoms even after approximately 3 months of conservative therapies and she was diagnosed with SIF from the examination of imaging study before the operation. We decided to perform surgical treatment, namely, fluoroscopy-guided percutaneous sacroplasty.
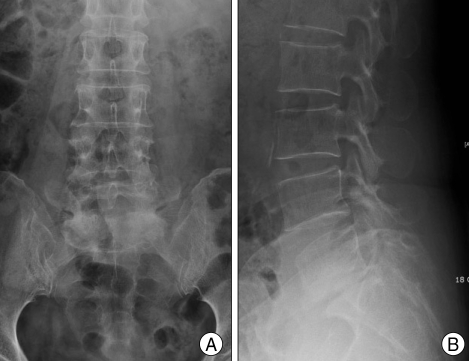
Fig. 1.
Plain X-ray radiographs show no definite fracture lines on sacral body and grade I spondylolithesis L5 on S1 (A : antero-posterior view, B : lateral view).
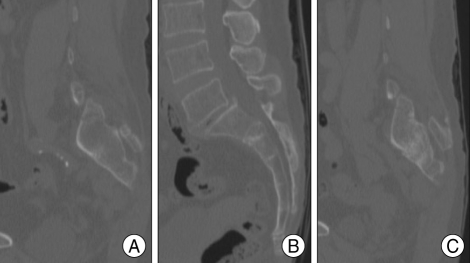
Fig. 2.
Computed tomography shows subtle fracture lines on both sacral alars and the second sacral body (A : right parasagittal, B : mid-sagittal, C : left parasagittal).
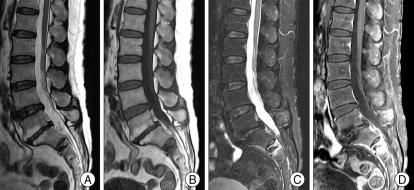
Fig. 3.
Magnetic Resonance Images show abnormal signal intensities on the second sacral body. This lesion shows low signal intensity on T2-weighted image (A), low signal intensity and surrounding bone edema on T1-weighted image (B), high signal intensity on fat-suppression T2-weighted image (C) and highly enhanced after gadolinium infusion(D).
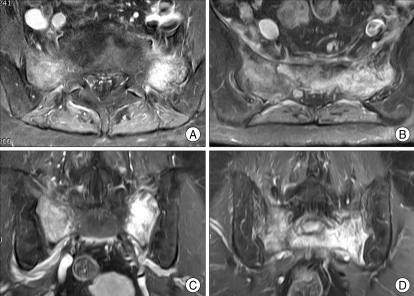
Fig. 4.
Magnetic resonance images show highly enhanced lesions on bilateral sacral alars and the second sacral body after gadolinium infusion. This typical shape and pattern of SIF called "classical H- or butterfly shaped appearance". This is a pathognomic hallmark for sacral insufficiency fracture (A and B : axial views, C and D : coronal views).
Operation
The patient was operated on in a prone position under local anesthesia. The operation area was draped with usual sterile manner. Under C-arm fluoroscopic guidance, needle entry point was identified. This was attempted first on the left side using the "long axis injection technique" (Fig. 5). In the frontal plane, the image intensifier was rotated in a cephalad direction, so that the beam was positioned parallel to the L5-S1 disc space, and was aligned to be perpendicular to the long axis of the upper sacrum (S1-S3). To approach the left sacral ala, the image intensifier was turned to the right by around 25-30 degrees to position the beam parallel to the left sacroiliac joint. However, the overall condition of visualization of the C-arm fluoroscopy was inadequate, due to the pelvic bone. The skin insertion site and entry point of the vertebroplasty needle were decided to be at the mid-point between the inferior border of sacroiliac joint and the lateral border of left S3 neural foramen. The skin and the periosteum were anesthetized with 1% lidocaine and bupivacaine. A small skin incision was made and the vertebroplasty needle was inserted and thrust until it touched the bone. The image intensifier was laterally rotated and the needle was directed to point to the center of the S1 body. In the frontal and lateral projection of the image intensifier, while the tip of the cannula was pointing to the geometrical center of the S1 body, the needle was inserted into the medullary cavity of the sacrum by around 1 cm. While checking the image from the image intensifier, the needle was advanced toward the sacral ala and it was determined that the needle had not penetrated the anterior cortex of the sacral ala. Standard mixture of PMMA cement was prepared and then injected through the cannula, using fluoroscopic visualization in both frontal and lateral projections. When approximately 1 cc of the cement was injected, a cement leak was observed on the side of the L5 neural foramen. The needle was retracted by about 1 cm and after a pause, another 1 cc of cement was injected. In this way, about 4 cc of the cement was injected. When the last cement was being injected, a cement leak occurred toward the sacroiliac joint. Since the state of the C-arm fluoroscopy visualization was not good due to the thick pelvic bone structure and the cement leak which had occurred in the first trial, the operation method for the right side was changed to a posterior approach, where two needles were inserted into the posterior aspect of S1 and S2 levels of the sacral ala. To approach to the right sacral ala, the image intensifier was turned by around 25-30 degrees to the left to position the beam to be parallel to the right sacroiliac joint and the frontal plane to be parallel to the L5-S1 disc space. The needle was inserted from the outside of the S1 extraneural foramen toward the sacral ala on the frontal plane of the image intensifier and positioned to be parallel to the L5-S1 disc space on the lateral plane. The standard PMMA cement mixture was injected first and then about 2 cc of the cement was injected. As soon as the cement was being injected, a cement leak occurred toward the right L5 neural foramen. In the same manner as earlier described, another needle was inserted into the S2 level to inject about 2 cc of the cement (Fig. 5-7). The small skin incision was primarily closed before completion of the operation.
Fig. 5.
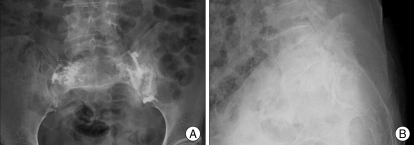
Post-sacroplasty X-Ray radiographs. PMMA cement is well injected into the bilateral sacral alars. Small amount of cement leak is found in both L5 neural foramina and left sacroiliac joint (A : anteroposterior view, B : lateral view).
Fig. 7.
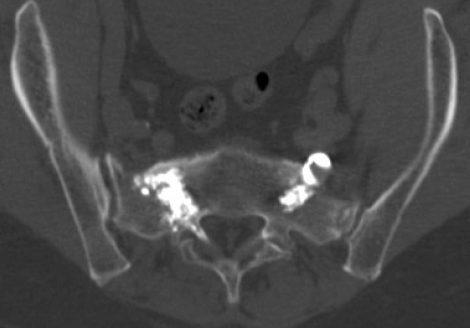
Post-sacroplasty axial CT scan. PMMA injected into sacral vertebra.
Postoperative course
Immediately after the operation, the pain that the patient had complained in both buttocks before the operation had completely subsided. However, she had felt new tingling sensations along the left L5 dermatome. Analgesics and oral steroid had been administered for about 3 weeks until tingling sensation had completely disappeared. She has been followed for 9 months and there has been no recurrent pain or newly occurring pain in the sacrum, where the cement was injected.
DISCUSSION
SIF mainly occurs in female patients, 60 years of age or older and the major symptoms frequently involve severe pain in the areas of the pelvis, lower spine and hip. The predisposing factors include osteoporosis, age, rheumatoid arthritis, previous irradiation of the pelvis, and the use of corticosteroids12). SIF is a sort of stress fracture and, along with fatigue fracture, the injury mechanism is similar. Repetitive, prolonged, and muscular actions against bones induce stress fractures and these stress fractures can be divided into two categories. The first consists of fatigue fractures caused by abnormal stresses imposed on normal bones. The second consists of insufficiency fractures caused by the effects of normal or physiologic stresses on weakened bone with decreased elastic resistance. Eventually, these come about when applied stresses exceed the biomechanical strength of the bone20). The weight of the torso and the upper body is transmitted downward through the central sacrum, and an upward force is applied to the lateral sacrum from the hip joints. The resulting shear stress is concentrated in the sacral ala, and a stress fracture is produced when the applied shear stress exceeds the mechanical strength of the sacral ala16). Most fractures of the sacral ala are vertically oriented and are located in the weak segment of the ala, just lateral to the arcuate lines and S1 articular mass and medial to the posterior iliac crest22).
This concept was first described in 1982 by Lourie et al. under the title of "spontaneous osteoporotic fracture of the sacrum"17). The accurate incidence rate and prevalence of SIF are not well known because SIF is not easily found in general image examinations and is easily overlooked as the symptoms are obscure. Accurate diagnoses are possible only using bone scans or MRI in most cases, and it can be diagnosed if it shows the characteristic "classical H- or butterfly shaped appearance"16).
General treatments of SIF can be divided into conservative treatments, iliosacral screw fixation, and percutaneous sacroplasty. In treating SIF, conservative therapies such as long-term bed rest, the administration of analgesics such as NSAIDS, and physiotherapy can first be performed but may not be effective as long-term treatments for more than a year. Conservative therapies have the advantage of being noninvasive, but the effect may not be definitely expected and they are very likely to just consume time. Secondly, the percutaneous placement of augmented iliosacral screw fixation can be considered as a treatment of SIF. The treatment can be used for a sacral fracture accompanied with a pelvic bone fracture, but it has not yet been widely documented and long-term clinical outcomes have not been established21,24). In addition, since the mechanical stability of iliosacral screw fixation has not been systematically investigated and the possibility of potential dislocation of implants in osteoporotic bones can't be eliminated, it may be performed with restriction, only in young patients with no accompanying osteoporosis24). However, this treatment is more invasive than the percutaneous sacroplasty described below. Finally, the most common treatment of SIF is percutaneous sacroplasty. This is an operation method which involves the application of percutaneous vertebroplasty. Introduced for the first time in 1987 by Galibert et al., percutaneous vertebroplasty has been used as a treatment for osteolytic pathologic fractures, aggressive hemangioma, and osteoporotic vertebral compression fractures and its use has gradually increased10). Similar to osteoplasty and kyphoplasty, the application of this treatment method has expanded and thus, many spine surgeons throughout the world are using it. However, most of the treatments are performed on the compression or stable burst fracture of the thorocolumbar area which are cylinder shaped vertebrae. However, from early 2000, the sacroplasty using this vertebroplasty began to be introduced by several researchers as a treatment of sacral vertebrae, which are shield-shaped flat bones11). Persistent pain and dysfunction resulting from an osteoporotic SIF may be related to chronic nonunion of the fracture site from the inability of the osteoporotic bone to heal under repetitive strain. Introduction of PMMA across the sacral fracture site may reduce pain and disability by providing mechanical stabilization preventing painful micromotion8). It is known that there is no difference in the degree of mechanical stabilization produced the different directions of approach4,9).
While the methods used to perform sacroplasty are the same as those used to perform vertebroplasty, they are largely divided into two techniques based on their approaches. There are long and short axis techniques22). Short axis techniques can be further divided into posterior approaches and lateral approaches25). The most commonly used methods are posterior approaches and long axis injection techniques. As described earlier, we used a long axis injection technique for one side and a posterior approach for the other, to inject PMMA. The reason why different techniques were used for one patient was because of the poor visualization of the image intensifier and the leak of PMMA. The biomechanical properties of PMMA include sufficient resistance with regard to compression and strength of the cemented bone, but serious complications may occur. Also, the operation procedures for PVP are relatively simple and have almost no risk but they may sometimes induce very disastrous complications3). The relatively few reported complications associated with sacroplasty are linked to intravasal leakage, pulmonary emboli, infections, and injury to the sacral nerves. These complications are similar to those of vertebroplasty. Among them, the less fatal but more commonly occurring events are cement leaks. When cement leaks occurr, there are usually no symptoms, but sometimes the cement may severely compress the nerve root, causing troubles7). In the past, it was known that the flow of cement could be predicted by performing a intraoperative venography before injecting cement. Although it was frequently performed in the past, it has recently been recognized that venography can't predict cement flow, distribution, and leaks at all, and thus, is not being frequently performed at this time. PMMA cement behaves differently compared to less viscous contrast medium. CT demonstrates fracture lines much better than MR and plain radiographs, but since MR shows secondary bone marrow edema, MRI seems to be crucial in the detection of the condition6).
Other differences between vertebroplasty performed on the thorocolumbar vertebrae and sacroplasty are in the direction of the image intensifier, when it is used, and the degree of visualization12,14,23). When vertebroplasty is performed on the thorocolumbar area, the surgeon just needs to see the frontal and lateral planes of the image intensifier for the operation. The cement leak particularly requires attention to the posterior direction only. Thus, the surgeon can simply place the image intensifier on the lateral plane when injecting cement to prevent a leak. However, unlike the vertebroplasty which is done with these familiar methods, the sacrum has completely different features. Its shape is not the simple, cylindrical shape but a long and wide shield shape in a crooked form12). Also, it is enclosed by the pelvic bone and the volume of the muscles supporting it is high. Thus, many structures have to be penetrated by the X-ray beam. In many cases where sacroplasty is actually performed, the accurate structure in the sacrum, sacral foramina are not clearly visible23). This kind of situation occurs more frequently when the image intensifier is placed on its lateral plane, making it impossible to identify the accurate contour of the sacrum and the distribution state of the injected cement. Such circumstances may put the surgeons into more difficult situations when the sacroplasty is performed using the long axis injection technique. If the accurate state of the distribution of cement and cement leaks are not quickly detected, a disastrous situation may occur18). Additionally, the various angles and direction of the image intensifier provide unfamiliar images to surgeons. Thus, the initial experience in sacroplasty may cause surgeons embarrassment, thereby increasing the possibility of cement leaks.
To overcome these situations, several alternative measures may be considered. One of them is CT-guided sacroplasty. Sacroplasty using CT makes it possible to identify the accurate structure of the sacrum and makes it possible to allocate accurate cannula. For these reasons, many clinicians prefer CT-guided sacroplasty3,12,14,23). However, since there is a time gap between the injection of the cement and identifying the images of cement distribution, the cement flow can't be observed in real-time, and so it can't be claimed that CT-guided sacroplasty can absolutely prevent cement leaks. However, since its resolution is better than that of C-arm fluoroscopy and has the advantage of being able to provide images 3-dimensionally, CT-guided sacroplasty may be recommended to those surgeons who are performing sacroplasty for the first time2,3,13,23). Another method is to perform short axis rather than long axis techniques. The advantage of long axis techniques is that an operation can be completed with one cannula insertion. But, short axis techniques, like posterior approaches, require 2 or more cannulas to be inserted for the desired amount of cement to be injected into the desired area22). However, the posterior approaches make cannula inserts relatively easy, thus enabling more accurate allocations of cannulas than in long axis techniques, even if the resolution of the image intensifier is lower. In general, the most important thing in performing vertebroplasty is the resolution of the image intensifier, and the resolution for the sacrum is definitely lower than that of the vertebroplasty for the thoracic or lumbar area. Addtionally, it is known that leaks around the cement are common in the case of sacroplasty where about 4 cc of cement is injected22,27). The reason for this has not yet been clarified. Finally, the method of using balloon kyphoplasty can be considered. In balloon kyphoplasty, a balloon is inserted inside the fractured vertebrae and does ballooning to artificially create a space on the inside of the bone marrow. The space is then filled with cement and the risk of cement leaks is much smaller than in the case of simple vertebroplasty. Therefore, it is considered that performing balloon kyphoplasty may reduce the risk of cement leaks in the sacral ala. However, without many examples of such attempts or many reports on the limitation of the pressure of the balloon when ballooning as of yet, these attempts can still be considered experimental1,5).
Recently, sacroplasty is being attempted on hemangiomas and metastatic tumors occurring in the sacrum with good outcomes on pain control being reported1,24,26).
CONCLUSION
Although the authors could not find fracture evidence in X-ray radiography, they could diagnose sacral insufficiency fracture from MRIs and succeeded in treating it with sacroplasty. Although this was authors' first case, it was possible to provide complete pain relief despite the fact that PMMA cement leaks occurred. It is recognized that with this treatment, more attention should be given to accurate cannula allocations and cement leaks than is required with thorocolumbar vertebroplasty.
Fig. 6.
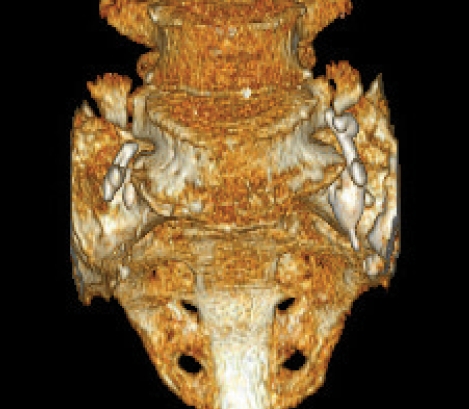
Three-dimentional CT shows PMMA cement leak identified along the both L5 nerve roots.
References
- 1.Atalay B, Caner H, Yilmaz C, Altinors N. Sacral kyphoplasty for relieving pain caused by sacral hemangioma. Spinal Cord. 2006;44:196–199. doi: 10.1038/sj.sc.3101829. [DOI] [PubMed] [Google Scholar]
- 2.Binaghi S, Guntern D, Schnyder P, Theumann N. A new, easy, fast, and safe method for CT-guided sacroplasty. Eur Radiol. 2006;16:2875–2878. doi: 10.1007/s00330-006-0467-z. [DOI] [PubMed] [Google Scholar]
- 3.Brook AL, Mirsky DM, Bello JA. Computerized tomography guided sacroplasty : a practical treatment for sacral insufficiency fracture : case report. Spine. 2005;30:E450–E454. doi: 10.1097/01.brs.0000172182.35619.d1. [DOI] [PubMed] [Google Scholar]
- 4.Butler CL, Given CA, 2nd, Michel SJ, Tibbs PA. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol. 2005;184:1956–1959. doi: 10.2214/ajr.184.6.01841956. [DOI] [PubMed] [Google Scholar]
- 5.Deen HG, Nottmeier EW. Balloon kyphoplasty for treatment of sacral insufficiency fractures. Report of three cases. Neurosurg Focus. 2005;18:e7. [PubMed] [Google Scholar]
- 6.Ehara S. Percutaneous sacroplasty for osteoporotic insufficiency fractures. AJR Am J Roentgenol. 2006;186:580. doi: 10.2214/AJR.06.5009. [DOI] [PubMed] [Google Scholar]
- 7.Frey ME, DePalma MJ, Cifu DX, Bhagia SM, Daitch JS. Efficacy and safety of percutaneous sacroplasty for painful osteoporotic sacral insufficiency fractures : a prospective, multicenter trial. Spine. 2007;32:1635–1640. doi: 10.1097/BRS.0b013e318074d4e1. [DOI] [PubMed] [Google Scholar]
- 8.Frey ME, Depalma MJ, Cifu DX, Bhagia SM, Carne W, Daitch JS. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures : a prospective, multicenter, observational pilot study. Spine J. 2008;8:367–373. doi: 10.1016/j.spinee.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Galibert P, Deramond H, Rosat P, Le Gars D. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty] Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 10.Garant M. Sacroplasty : a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol. 2002;13:1265–1267. doi: 10.1016/s1051-0443(07)61976-9. [DOI] [PubMed] [Google Scholar]
- 11.Gjertsen O, Schellhorn T, Nakstad PH. Fluoroscopy-guided sacroplasty : special focus on preoperative planning from three-dimensional computed tomography. Acta Radiol. 2008;49:1042–1048. doi: 10.1080/02841850802350659. [DOI] [PubMed] [Google Scholar]
- 12.Heron J, Connell DA, James SL. CT-guided sacroplasty for the treatment of sacral insufficiency fractures. Clin Radiol. 2007;62:1094–1100. doi: 10.1016/j.crad.2007.04.017. discussion 1101-1103. [DOI] [PubMed] [Google Scholar]
- 13.Layton KF, Thielen KR, Wald JT. Percutaneous sacroplasty using CT fluoroscopy. AJNR Am J Neuroradiol. 2006;27:356–358. [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YJ, Bong HJ, Kim JT, Chung DS. Sacral insufficiency fracture, usually overlooked cause of lumbosacral pain. J Korean Neurosurg Soc. 2008;44:166–169. doi: 10.3340/jkns.2008.44.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lourie H. Spontaneous osteoporotic fracture of the sacrum: An unrecognized syndrome of the elderly. JAMA. 1982;248:715–717. [PubMed] [Google Scholar]
- 16.Masala S, Konda D, Massari F, Simonetti G. Sacroplasty and iliac osteoplasty under combined CT and fluoroscopic guidance. Spine. 2006;31:E667–E669. doi: 10.1097/01.brs.0000231962.04739.ac. [DOI] [PubMed] [Google Scholar]
- 17.Pommersheim W, Huang-Hellinger F, Baker M, Morris P. Sacroplasty : a treatment for sacral insufficiency fractures. AJNR Am J Neuroradiol. 2003;24:1003–1007. [PMC free article] [PubMed] [Google Scholar]
- 18.Schizas C, Theumann N. An unusual natural history of a L5-S1 spondylolisthesis presenting with a sacral insufficiency fracture. Eur Spine J. 2006;15:506–509. doi: 10.1007/s00586-005-1011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sciubba DM, Wolinsky JP, Than KD, Gokaslan ZL, Witham TF, Murphy KP. CT fluoroscopically guided percutaneous placement of transiliosacral rod for sacral insufficiency fracture : case report and technique. AJNR Am J Neuroradiol. 2007;28:1451–1454. doi: 10.3174/ajnr.A0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DK, Dix JE. Percutaneous sacroplasty : long-axis injection technique. AJR Am J Roentgenol. 2006;186:1252–1255. doi: 10.2214/AJR.05.0823. [DOI] [PubMed] [Google Scholar]
- 21.Strub WM, Hoffmann M, Ernst RJ, Bulas RV. Sacroplasty by CT and fluoroscopic guidance : is the procedure right for your patient? AJNR Am J Neuroradiol. 2007;28:38–41. [PMC free article] [PubMed] [Google Scholar]
- 22.Tjardes T, Paffrath T, Baethis H, Shafizadeh S, Steinhausen E, Steinbuechel T, et al. Computer assisted percutaneous placement of augmented iliosacral screws : a reasonable alternative to sacroplasty. Spine. 2008;33:1497–1500. doi: 10.1097/BRS.0b013e318175c25c. [DOI] [PubMed] [Google Scholar]
- 23.Tsiridis E, Upadhyay N, Giannoudis PV. Sacral insufficiency fractures : current concepts of management. Osteoporos Int. 2006;17:1716–1725. doi: 10.1007/s00198-006-0175-1. [DOI] [PubMed] [Google Scholar]
- 24.Uemura A, Matsusako M, Numaguchi Y, Oka M, Kobayashi N, Niinami C, et al. Percutaneous sacroplasty for hemorrhagic metastases from hepatocellular carcinoma. AJNR Am J Neuroradiol. 2005;26:493–495. [PMC free article] [PubMed] [Google Scholar]
- 25.Waites MD, Mears SC, Richards AM, Mathis JM, Belkoff SM. A biomechanical comparison of lateral and posterior approaches to sacroplasty. Spine. 2008;15:E735–E738. doi: 10.1097/BRS.0b013e31817ecc22. [DOI] [PubMed] [Google Scholar]
- 26.Wee B, Shimal A, Stirling AJ, James SL. CT-guided sacroplasty in advanced sacral destruction secondary to tumour infiltration. Clin Radiol. 2008;63:906–912. doi: 10.1016/j.crad.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Whitlow CT, Mussat-Whitlow BJ, Mattern CW, Baker MD, Morris PP. Sacroplasty versus vertebroplasty : comparable clinical outcomes for the treatment of fracture-related pain. AJNR Am J Neuroradiol. 2007;28:1266–1270. doi: 10.3174/ajnr.A0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitlow CT, Yazdani SK, Reedy ML, Kaminsky SE, Berry JL, Morris PP. Investigating sacroplasty : technical considerations and finite element analysis of polymethylmethacrylate infusion into cadaveric sacrum. AJNR Am J Neuroradiol. 2007;28:1036–1041. doi: 10.3174/ajnr.A0500. [DOI] [PMC free article] [PubMed] [Google Scholar]