Abstract
Previous GCMS methods determining nitrate in biological samples involve either hazardous chemicals or produce multiple isomers that can be difficult to quantitate. Modification of these methods, by the nitration of mesitylene instead of benzene and in the presence of trifluoroacetic anhydride rather than sulphuric acid, should enable simple isotopic quantitation for use in tracer studies, for example, in the measurement of nitric oxide production.
Desiccated urine and saliva samples, in addition to aqueous labelled and unlabelled nitrate standards, were treated with trifluoroacetic anhydride and mesitylene at 70 °C for one hour, cooled, then sequentially washed with deionised water and aqueous sodium bicarbonate. The solution of nitromesitylene in mesitylene was separated, dried and analysed by GCMS.
Full mass spectra exhibited strong signals at m/z 165 and 166 corresponding to the unlabelled and labelled molecular species of nitromesitylene respectively. Selected ion monitoring of these masses for a series of gravimetrically prepared standards indicated good agreement with isotopic enrichments in the range 0.0625 – 5 mole % excess, and at nitrate concentrations within the physiological range of 0.078 - 2 mmol/L. Derivatised samples were stable with respect to isotopic enrichments and nitrate concentrations at −20 °C for up to 21 days and exhibited excellent repeatability.
Nitration of mesitylene proved to be a simple and rapid method for the measurement of isotope ratios in aqueous nitrates by GCMS, which has applications in both tracer studies and concentration determinations by isotope dilution techniques for nitric oxide production.
Keywords: nitrate, mesitylene, urine, saliva
Introduction
Endogenous nitric oxide (NO) is an important modulator of many physiological processes in man. It controls neurotransmission, immune function, hormonal secretion, haemostasis, vascular tone, cardiac contractility and intestinal peristalsis and, as a result, plays a key role in pathological conditions such as obesity, diabetes, septic shock, inflammatory disorders, atherosclerosis and hypertension.1-6 Consequently, the measurement of NO production is important in understanding the mechanisms involved in the development of several diseases.
Nitric oxide is tonically released by endothelial and neuronal cells and is derived endogenously from the conversion of L-arginine and molecular oxygen to L-citrulline and NO in the presence of nitric oxide synthases (NOS). NO is highly reactive and rapidly oxidised to nitrite (NO2−), which in turn is metabolised to the unreactive nitrate (NO3−) ion. The proportions are variable and hence it could be argued that the best indication of NO production is the sum of both nitrite and nitrate. Nitrate is present in most body fluids reflecting the multi-systemic production of NO; the primary pool being plasma with an average concentration of 10 - 60 μmol/L plasma,7-9 and the most accessible pools being urine and saliva in which nitrate is present at levels of between 200 – 2,000 μmol/L.7, 9, 10 Although much of the circulating nitrate is eventually excreted in the urine, up to 25 % is actively taken up by the salivary glands and secreted in saliva.11 The metabolism of nitrate is further complicated by a large dietary-derived component, as exogenous nitrate is a natural part of the diet,12-14 and may be influenced by dietary habits such as vegetarianism, fasting, and dieting. As a consequence, daily intake will fluctuate and influence the systemic nitrate pool, making the measurement of NO production difficult with dietary restriction. To overcome these issues, stable isotope techniques have been used to study NO production kinetics by combining dietary nitrate restriction with administration of 15N-arginine,15, 16 requiring robust methods to measure nitrate isotopic enrichments and ultimately concentrations in biological samples.
Previous studies quote a variety of techniques for measuring nitrate concentration in biological samples; the most popular of which is the Griess reaction, measuring total nitrate and nitrite concentration after reduction of the nitrate to nitrite. There are however issues with the specificity and the pre-analytical and analytical factors, such as proteins and other plasma constituents interfering with the accuracy of the Griess reaction, which puts this method in doubt.9, 17 Such interference is not seen with GCMS and as a result many groups opt for this technique for measuring nitrate.7, 8, 16, 18, 19
The most commonly applied derivatisation for GCMS analysis of nitrates in biological samples has been the nitration of aromatic compounds such as benzene, toluene and trimethyoxbenzene.7, 8, 16, 18, 19 Tesch et al. initially developed a GC method for nitrite/nitrate after conversion to nitrobenzene,20 which was subsequently adapted for the analysis of isotopic enrichments of 15nitrate by Green and co-workers.7 The accuracy and precision of the method was later improved by opting for trimethoxybenzene in preference to benzene as the substrate thereby reducing the by-products formed in the nitration of benzene, the acid-to-sample ratio and the sample volume.21 One disadvantage of all these methods however is the use of concentrated sulphuric acid as a catalyst for the reaction. Not only is this a hazardous reagent, but studies also suggest that, under derivatisation conditions, nitrated arginine analogs can degrade resulting in artificially high nitrate levels.22, 23 It has been suggested that these interferences can be reduced by replacing benzene with toluene, which is activated towards electrophilic substitution at the ortho- and para- positions. In this case, the less aggressive trifluoroacetic anhydride (TFAA) can be used as a catalyst.24 In the presence of TFAA however, the nitration of toluene produces a mixture of three isomers differing by the relative position of the methyl- and nitro- groups, ortho- (o-), para- (p-) and meta- (m-) nitrotoluene, which can be difficult to separate without the use of specific GC columns. An added complication in the nitration of toluene is the ready loss of an −OH group from o-nitrotoluene under electron ionisation resulting in a strong signal at m/z 120.24 This is known as the ‘ortho-effect’25 and the amount of formation of this bicyclic ion (m/z 120) will be dependant on the ion source conditions. Subsequently, Smythe et al. used only the para- isomer at m/z 137 and ortho-isomer at m/z 120 to detect changes in isotopic enrichment.24
Here, we propose a new derivatisation that uses mesitylene (1,3,5-trimethyl benzene) instead of toluene as the aromatic compound in the presence of TFAA. Unlike toluene, the symmetry of mesitylene ensures that, under conditions of large excess, a single product, 1,3,5-trimethyl-2-nitrobenzene, is formed. The presence of the three methyl groups strongly activates the intermediate ring sites to electrophilic substitution, which is catalysed by trifluoroacetic anhydride. This approach combines the beneficial effects of milder acidic conditions with the formation of just one isomer.
The aim of the present study therefore was to determine the suitability of this derivative for the determination of 15NO3 isotopic enrichments in urine and saliva using GCMS.
Materials and Methods
Chemicals and Reagents
Labelled sodium nitrate (15N, +98 %, Cambridge Isotope Laboratories, Inc., Andover, USA) was obtained from CK Gas Products Ltd, Hook, Hampshire, UK. Mesitylene (puriss. >99 %, GC), trifluoroacetic anhydride, sodium nitrate (99 % min.), sodium bicarbonate and anhydrous sodium sulphate were all obtained from Sigma-Aldrich Co. Ltd, Gillingham, Dorset, UK. Sodium hydroxide was obtained from VWR International Ltd, Lutterworth, UK. Deionised water was used in the preparation of aqueous solutions of nitrite and nitrate and of the internal standards.
Sample and Standards Preparation
Morning and afternoon urine samples were collected from 3 healthy subjects in 25 mL tubes containing 50 μL of 5 mol/L NaOH. Aliquots (1 mL) of the urine were taken and stored at either −20 °C or −80 °C until derivatised. Immediately after each urine sample was collected, the subjects also provided saliva samples in 1.5 mL tubes containing 100 μL 1mol/L NaOH; these were stored at −20 °C. Standard solutions of sodium nitrate (labelled and unlabelled) were prepared from a stock solution (5 mmol/L in deionised water) representing typical urinary nitrate concentrations.
Derivatisation
The derivatisation used was a modification of the method developed by Smythe et al.,24 in which toluene was replaced with mesitylene, which following nitration forms nitromesitylene (1,3,5-trimethyl-2-nitrobenzene).
Desiccated urine samples, saliva samples and aqueous nitrate standards (initial volumes of 100 μL) were treated with 200 μL trifluoroacetic anhydride and 1 mL mesitylene at 70 °C for one hour, cooled to room temperature and then sequentially washed with 1 mL deionised water, 1 mL 1 % aqueous sodium bicarbonate, and finally 1 mL deionised water. The upper layer containing the solution of nitromesitylene in mesitylene was separated, dried over 500 mg anhydrous sodium sulphate and filtered through a Pasteur pipette containing a small wad of tissue paper, into clean 1.5 mL glass GCMS vials and stored at −20 °C until analysed.
An initial comparison of the two derivatisations (toluene vs. mesitylene) was performed on a range of labelled sodium nitrate standards prior to in-depth assessment of the mesitylene method.
GCMS Analysis
All standards and biological samples were analysed on an Agilent GCMS 5973N system (Agilent Technologies Ltd, Stockport, UK) comprising a HP 6890 GC with autosampler and split/splitless injector. Unless otherwise stated, injection volumes of 1 μL were used in splitless mode, with the inlet held at 250 °C. Chromatographic separation was performed using a DB-1MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness, Agilent J&W Scientific, Stockport, UK). Oven conditions were 90 °C for 0.5 min, ramped at 30 °C/min up to 270 °C and finally held for 1 minute to clear the column of any interfering compounds. Helium was used as the carrier gas with a flow rate of 1.2 mL/min operating at constant flow. The transfer line to the mass spectrometer was held at 280 °C. The mass spectrometer was operated in electron ionisation (EI) mode, with electron energy of 70 eV, ion source temperature of 250 °C and quadrupole temperature of 120 °C.
Full mass spectral data were obtained by scanning the mass range, m/z 50-180, whilst selected ion monitoring (SIM) at m/z 165 for the molecular ion of unlabelled nitromesitylene and m/z 166 for labelled nitromesitylene was performed with a dwell time of 30 msec. Peak areas were determined for m/z 165 and 166, and the isotopic enrichments calculated. Where necessary, corrections for natural abundance were made for each assay by the analysis of separate labelled or unlabelled nitrate samples.
In order to calculate the nitrate concentration in the urine and saliva, the biological samples were spiked with an internal standard of Na15NO3 (0.1 mmol/L unless specified) prior to derivatisation. The volume of spike added was matched to the volume of sample used. The amount of internal standard added and the resulting enrichments were used to calculate the concentration as follows:
Standard curves
To demonstrate the accuracy of the method, a series of standard serial dilution curves were constructed from a combination of labelled and unlabelled NaNO3 to give the following:
a range of unlabelled nitrate concentrations from a stock of 5 mmol/L, representing typical urinary nitrate values.
a range of enrichments from 20 mole % excess to 0.0625 mole % excess.
a range of unlabelled nitrate concentrations from 5 mmol/L to 0.078 mmol/L, spiked with various concentrations of Na15NO3.
All standards were derivatised as previously described.
Repeatability
The intra-run repeatability was assessed using aqueous standards, urine and saliva samples with 10 injections into the same sample vial. Inter-run repeatability to determine stability post-derivatisation at −20 °C was assessed using urine and saliva samples analysed 6 times at regular intervals over a period of 21 days. The stability of the isotopic ratios with various GCMS injection volumes (0.2, 1, 2, 3, 4, 5 μL) and with a 2-fold serial dilution was also assessed.
Effect of storage temperature and sample volume of urine
The effects of storage temperature and sample volume were assessed using urine samples only. To assess the effects of storage temperature, aliquots of urine from the same subjects were stored at either −20 °C or at −80 °C until analysed. To assess the optimum volume required for derivatisation, sample volumes ranging from 100 μL to 900 μL were used. All samples were thawed and derivatised as previously described prior to analysis.
Effect of spike concentration
To determine whether the concentration of the labelled spike had any effect on the overall nitrate concentration in samples, 500 μL urine and 100 μL saliva samples were spiked with either 500 μL or 100 μL respectively of various concentrations (0.1, 0.5, 1.0 mmol/L) Na15NO3. All samples were derivatised as described above.
Statistical analysis
Standard statistical quantities (mean, standard deviation (SD) and coefficient of variance (CV)) were calculated. Differences between the theoretical and observed isotopic enrichments and nitrate concentrations were plotted against the theoretical values of the respective quantity to determine the extent of agreement. Basic paired T-tests were used to determine any statistical significant differences where appropriate. All data analysis was performed in Excel for Windows (Microsoft, Redmond, WA, USA).
Results and Discussion
Previously, the nitration of an aromatic compound in the presence of concentrated sulphur acid has been used as a method for nitrate analysis on the GCMS. In the present study, a novel derivative is proposed using the nitration of mesitylene in the presence of the catalyst, trifluoroacetic anhydride.
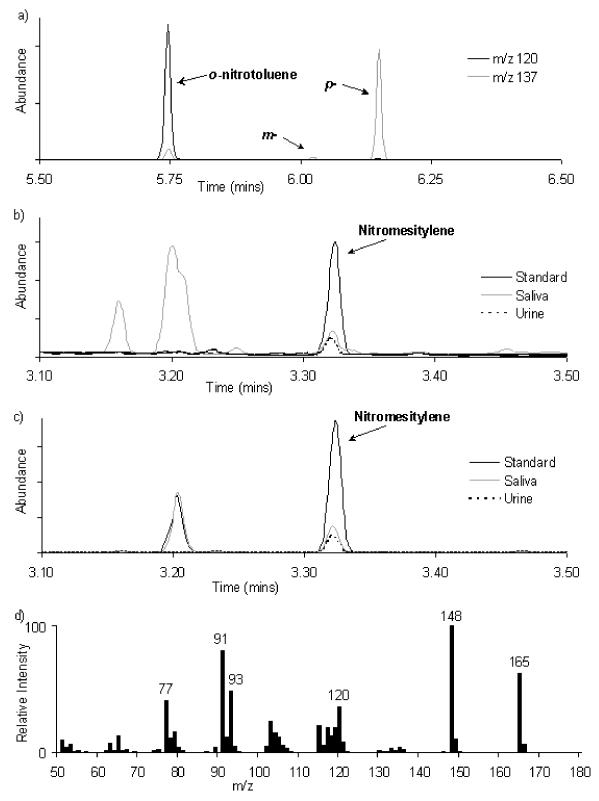
Unlike nitrotoluene (Figure 1a), the nitration of mesitylene in the presence of TFAA produces only a single product. A typical GCMS chromatogram and spectrum of nitromesitylene obtained from standards and biological samples is shown in Figures 1b, 1c and 1d. The retention temperature and retention time were approximately 172 °C and 3.32 minutes respectively. The relatively short retention time and clean separation from other urine and saliva constituents meant that up to 48 samples could be analysed in any 8-hour period. A preliminary comparison of a range of Na15NO3 standards, derivatised using either toluene or mesitylene, showed good agreement between the two methods in determining isotopic enrichment (r =0.996 ± 0.003) and nitrate concentration (r =0.998 ± 0.001). Nitrotoluene, with the molecular ion at m/z 137, was identified at the retention times of 5.75, 6.02 and 6.15 minutes and in the proportions 56 %: 2 %: 42 % for o-, m- and p-nitrotoluene respectively (Figure 1a). This was in support of the findings of Smythe and co-workers where all three isomers were resolved in the proportions 57 %: 3 %: 40 % respectively for o-, m- and p-nitrotoluene.24 No significant difference was found when comparing nitromesitylene at m/z 165 to either o-nitrotoluene at m/z 120 or p-nitrotoluene at m/z 137 (m-nitrotoluene being too small a signal to use for comparison purposes) signifying that mesitylene was a suitable replacement for toluene and worthy of further investigation.
Figure 1.
Typical partial GCMS chromatograms of a) nitrotoluene; b) nitromesitylene from saliva, urine and standards performed in scan; c) nitromesitylene from saliva, urine and standards performed in selected ion monitoring; and d) a typical mass spectrum of unlabelled nitromesitylene showing strong signals at m/z 165, 148 and 120.
There was a strong signal for the molecular ion of nitromesitylene at m/z 165 with an intensity of 63 % compared to the base peak at m/z 148, indicating that this ion could be suitable for isotope work (Figure 1d). The base peak at m/z 148 could possibly be due to the loss of an −OH group from the molecular ion in a similar manner to the ‘ortho’ effect seen in nitrotoluene, whilst the subsequent expulsion of CO and re-arrangement to a five-membered ring could result in the fragment at m/z 120. Although the fragment at m/z 120 has a good intensity (37 % with respect to base peak) and may be of use, the availability of the molecular ion at a much higher intensity would render this pointless.
Comparison of the isotopomer ratios, expressed as a percentage of the molecular ion m/z165, obtained from selected ion monitoring of the m/z 165 cluster with those expected theoretically for natural abundances are given in Table 1. The close agreement between experimental and theoretical values indicates that there is little interference, and that this cluster is suitable for isotope work. All subsequent validation was therefore performed on the molecular ion at m/z 165.
Table 1.
Isotopomer ratios as a percentage of the molecular ion obtained from selected ion monitoring of the m/z 165 cluster.
| Molecular ions (m/z) |
Experimental Ratios (%) |
Theoretical Ratios (%) |
|---|---|---|
| 165 | 100.00 | 100.00 |
| 166 | 10.318 ± 0.223 | 10.341 |
| 167 | 1.068 ± 0.130 | 0.887 |
| 168 | 0.065 ± 0.014 | 0.055 |
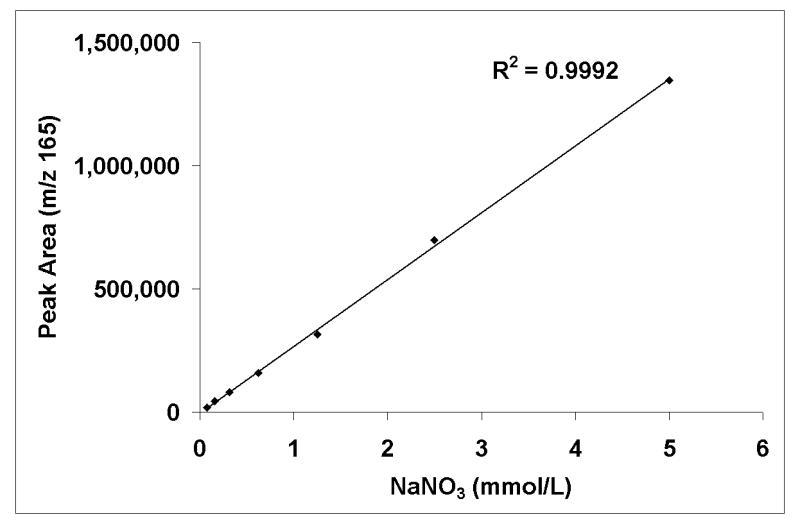
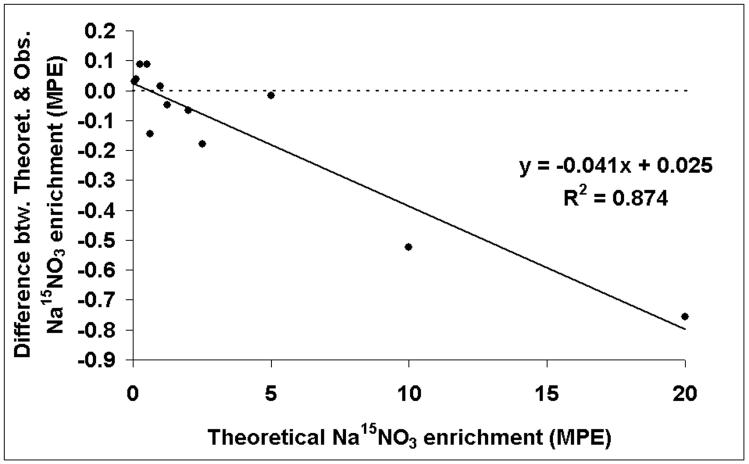
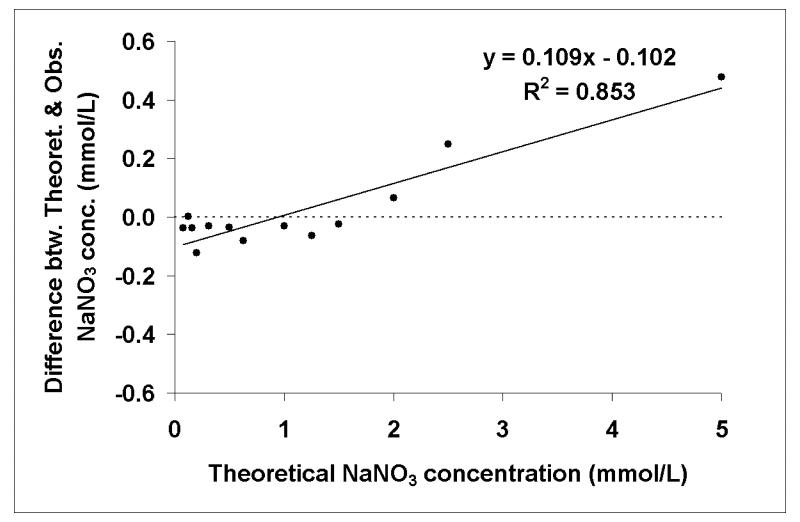
Many studies have attempted to determine normal ranges for nitrate concentration in biological fluids by a variety of methods either under free-living conditions or as part of nitrate controlled diet interventions.26-28 Typical nitrate concentrations are found to be between 200 and 2,000 μmol/L for urine and saliva7, 9, 10 but much lower, 10 - 60 μmol/L, for plasma.7-9 In order to determine the suitability of GCMS analysis of nitromesitylene in urine and saliva, we looked at standard dilution and calibration curves containing nitrate concentrations indicative of physiological concentrations. Dilution curves of unlabelled NaNO3 indicated good linearity between peak area at m/z 165 and nitrate concentrations down to 0.078 mmol/L, with correlation coefficients, r, in excess of 0.999 (Figure 2). Calibration curves (not shown) containing a combination of 15N-labelled and unlabelled NaNO3 showed good accuracy and recovery with good agreement between observed and theoretical values in ranges of enrichments from 20 mole % excess down to 0.0625 mole % excess (r = 0.999), and of unlabelled nitrate concentrations from 5 mmol/L to 0.078 mmol/L, spiked with various known volumes and concentrations of Na15NO3. Further analysis comparing the difference between the observed and theoretical enrichments with the theoretical revealed little bias at enrichments of 5 mole % excess and below however this increased significantly (p < 0.001) as enrichment increased (Figure 3). Similarly for nitrate concentration, the bias increased significantly (p < 0.001) as concentration increased but at physiological concentrations of 2 mmol/L nitrate and less, there was little bias and good agreement between theoretical and observed values (Figure 4).
Figure 2.
Standard serial dilution curve indicating good linearity between unlabelled NaNO3 concentration, from 5mmol/L down to 0.078 mmol/L, and peak area at m/z 165.
Figure 3.
Correlation analysis comparing the difference between the theoretical and observed isotopic enrichments with theoretical enrichments in range of 20 mole % excess (MPE) down to 0.0625 mole % excess Na15NO3.
Figure 4.
Correlation analysis comparing the difference between theoretical and observed nitrate concentrations with theoretical concentrations for a range of unlabelled nitrate standards from 5 mmol/L down to 0.078 mmol/L, spiked with Na15NO3.
There seems to be little consensus in how samples should be stored; previous studies quote various storage temperatures for biological samples but whether this is purely for practical reasons rather than any analytical criteria is unclear.10, 16, 29, 30 The present study found no statistically significant difference in isotopic enrichments (−20 °C vs. −80 °C; 38.95 ± 19.27 % vs. 39.77 ± 19.50 %, p = 0.17) or nitrate concentrations (−20 °C vs. −80 °C; 1.29 ± 0.65 mmol/L vs. 1.26 ± 0.65 mmol/L, p = 0.19) in urine samples when stored at either −20 °C or −80 °C over a short period of time (< 1 month). The standard practice within the authors’ laboratory is to store urine and saliva samples at −20 °C and this would appear to be adequate for this type of analysis. It should be noted that the large standard deviations observed were due to the variation of urinary nitrate concentrations between subjects; the conditions under which the subjects provided the samples were not controlled i.e. the subjects were not fasted or fed a diet controlled for nitrate composition therefore reflecting normal variations in the nitrate concentration due to the everyday routines and diets of the subjects.
The nitromesitylene derivative proved to be stable and reproducible not only over repeated injections within batch analysis but also over time after storage. The intra- and inter-run repeatability for isotopic enrichments and nitrate concentrations of standards and samples is shown in Table 2. The percentage coefficient of variation (CV) was less than 1.6 % for all standards and samples. Peak area was positively correlated with injection volume (n = 5, r = 0.999 ± 0.0005) and negatively correlated with dilution (n = 6, r = 0.9998 ± 0.0001). Neither injection volume nor a 2-fold serial dilution of the sample affected isotopic enrichments or nitrate concentrations (Table 2).
Table 2.
Repeatability of isotopic enrichments (isotope ratios and mole % excess) and nitrate concentrations from samples of urine, saliva and standards within a run, across runs, following various volumes of injection on to the GCMS and a 2-fold serial dilution.
| Sample | n | Isotope Ratio (% ± SD) |
Mole % Excess (% ± SD) |
Nitrate Concentration (mmol/L ± SD) |
CV (%) |
|---|---|---|---|---|---|
| Intra-run repeatability | |||||
| Aqueous standard* | 10 | 10.34 ± 0.30 | - | - | 1.13 |
| Aqueous standard 10.5 mole % excess |
10 | - | 10.79 ± 0.45 | - | 1.59 |
| Saliva* | 10 | 10.71 ± 0.03 | - | - | 0.30 |
| Saliva (a) | 10 | - | - | 0.88 ± 0.004 | 0.46 |
| Urine (a) | 10 | - | - | 1.98 ± 0.008 | 0.44 |
| Inter-run repeatability | |||||
| Saliva* | 6 | 10.64 ± 0.09 | - | - | 0.83 |
| Saliva (a) | 6 | 67.15 ± 0.51 | 36.12 ± 0.24 | 0.88 ± 0.008 | 0.90 |
| Urine (a) | 6 | 35.52 ± 0.22 | 19.95 ± 0.20 | 2.01 ± 0.017 | 0.87 |
| Urine (b) | 6 | 45.58 ± 0.33 | 25.91 ± 0.26 | 1.43 ± 0.013 | 0.94 |
| Injection volume | |||||
| Aqueous standard* | 6 | 10.38 ± 0.29 | |||
| Saliva (a) | 6 | 67.7 ± 0.51 | 36.46 ± 0.20 | 0.87 ± 0.008 | |
| Saliva (b) | 6 | 56.16 ± 0.58 | 31.43 ± 0.27 | 1.09 ± 0.014 | |
| Urine (a) | 6 | 35.58 ± 0.73 | 20.17 ± 0.47 | 1.98 ± 0.057 | |
| Urine (b) | 6 | 48.50 ± 3.24 | 27.60 ± 1.68 | 1.32 ± 0.108 | |
| 2-fold serial dilution | |||||
| Aqueous standard* | 15 | 10.45 ± 0.05 | - | - | |
| 2mmol/l Aqueous standard |
15 | 61.59 ± 0.20 | 33.89 ± 0.09 | 1.95 ± 0.01 | |
| Saliva (a) | 15 | 67.18 ± 0.33 | 36.25 ± 0.13 | 0.88 ± 0.005 | |
| Saliva (b) | 15 | 55.60 ± 0.18 | 31.17 ± 0.08 | 1.11 ± 0.008 | |
| Urine (a) | 15 | 35.81 ± 0.21 | 20.32 ± 0.65 | 1.96 ± 0.014 | |
| Urine (b) | 15 | 45.00 ± 0.14 | 25.76 ± 0.08 | 1.44 ± 0.013 |
All values are expressed as Mean ± SD; all samples and standards were spiked with 0.1mmol/L Na15NO3 unless stated
natural abundance.
In optimising the derivatisation, we confirmed that concentration of the internal standard did not affect isotopic enrichment or nitrate concentration of the sample and, for the purpose of all future experiments, a concentration of 0.1 mmol/L Na15NO3 was used in volumes matching that of the sample volume. Equally, it was concluded that the optimum volume of urine needed to provide a peak of sufficient intensity for the determination of isotope ratios, and hence to establish the nitrate concentration of that sample, was 500 μL. This is in line with sample volumes used by other research groups ranging from 100 μL to 1 mL.7, 10, 16, 31 A smaller sample volume of 100 μL was found to be adequate for saliva.
Overall our data, with regards to nitrate concentrations in urine and saliva, is comparable with published data elsewhere7, 10 despite doubts that analyses by methods such as the Griess assay are accurate.9
Conclusion
Mesitylene proved to be a suitable and effective reagent for the nitration of biological samples in the presence of the catalyst, trifluoroacetic anhydride, producing only a single end-product, nitromesitylene, which could be well characterised using a standard DB-1MS column on the GCMS. The derivatisation is simple and rapid and could be of potential in tracer studies assessing the kinetics of NO production.
Acknowledgements
We would like to thank Dr Derek Macallan (Centre for Infection, St George’s, University of London) for his discussions relating to the methodology.
References
- 1.Choi JW, Pai SH, Kim SK, Ito M, Park CS, Cha YN. Clin. Chem. 2001;47:1106–1109. [PubMed] [Google Scholar]
- 2.Ignarro LJ. J. Physiol. Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 3.Maxwell AJ. Nitric Oxide. 2002;6:101–124. doi: 10.1006/niox.2001.0394. [DOI] [PubMed] [Google Scholar]
- 4.Yki-Jarvinen H. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17:411–430. doi: 10.1016/s1521-690x(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 5.Naseem KM. Mol. Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric Oxide. 2006;15:265–279. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes PM, Leone AM, Francis PL, Struthers AD, Moncada S. Biochem. Biophys. Res. Commun. 1995;209:590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- 9.Tsikas D, Gutzki FM, Rossa S, Bauer H, Neumann C, Dockendorff K, et al. Anal. Biochem. 1997;244:208–220. doi: 10.1006/abio.1996.9880. [DOI] [PubMed] [Google Scholar]
- 10.Tsikas D, Boger RH, Bode-Boger SM, Gutzki FM, Frolich JC. J. Chromatogr. B. 1994;661:185–191. doi: 10.1016/0378-4347(94)00374-2. [DOI] [PubMed] [Google Scholar]
- 11.l’Hirondel J, l’Hirondel JL. Nitrate and Man: Toxic, Harmless or Beneficial? CABI Publishing; Oxford: 2002. [Google Scholar]
- 12.Ministry of Agriculture Fisheries and Food . Food Surveillance Information Sheet. London: 1998. MAFF UK 1997 Total Diet Study - Nitrate and Nitrite. [Google Scholar]
- 13.McKnight GM, Duncan CW, Leifert C, Golden MH. Br. J. Nutr. 1999;84:349–358. doi: 10.1017/s000711459900063x. [DOI] [PubMed] [Google Scholar]
- 14.Jakszyn PG, Ibáñez R, Pera G, Agudo A, García-Closas R, Amiano P, et al. Food Content of Potential Carcinogens: Nitrates, nitrites, nitrosamines, heterocyclic amines and polycyclic aromatic hydrocarbons. Catalan Institute of Oncolgy; Barcelona: 2004. [DOI] [PubMed] [Google Scholar]
- 15.Luiking YC, Deutz NE. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:103–108. doi: 10.1097/00075197-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Avogaro A, Toffolo G, Kiwanuka E, de Kreutzenberg SV, Tessari P, Cobelli C. Diabetes. 2003;52:795–802. doi: 10.2337/diabetes.52.3.795. [DOI] [PubMed] [Google Scholar]
- 17.Tsikas D. J. Chromatogr. B. 2007;851:51–70. doi: 10.1016/j.jchromb.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 18.Green LC, de Luzuriaga K Ruiz, Wagner DA, Rand W, Istfan N, Young VR, et al. Proc. Natl. Acad. Sci. U.S.A. 1981;78:7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Cancer Research. 1983;43:1921–1925. [PubMed] [Google Scholar]
- 20.Tesch JW, Rehg WR, Sievers RE. J. Chromatogr. 1976;126:743–755. doi: 10.1016/s0021-9673(01)84117-0. [DOI] [PubMed] [Google Scholar]
- 21.Gutzki FM, Tsikas D, Alheid U, Frölich JC. Biol. Mass Spectrom. 1992;21:97–102. doi: 10.1002/bms.1200210207. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg SS, Xie J, Spitzer JJ, Wang JF, Lancaster J, Grisham MB, et al. Life Sci. 1995;57:1949–1961. doi: 10.1016/0024-3205(95)02181-h. [DOI] [PubMed] [Google Scholar]
- 23.Tsikas D, Fuchs I, Gutzki FM, Frölich JC. J. Chromatogr. B. 1998;715:441–444. [PubMed] [Google Scholar]
- 24.Smythe GA, Matanovic G, Yi D, Duncan MW. Nitric Oxide. 1999;3:67–74. doi: 10.1006/niox.1999.0210. [DOI] [PubMed] [Google Scholar]
- 25.Beynon JH, Saunders RA, Tophan A, Williams AE. J. Chem. Soc. 1965:6403–6405. [Google Scholar]
- 26.Lee K, Greger JL, Consaul JR, Graham KL, Chinn BL. Am. J. Clin. Nutr. 1986;44:188–194. doi: 10.1093/ajcn/44.2.188. [DOI] [PubMed] [Google Scholar]
- 27.Jungersten L, Edlund A, Petersson AS, Wennmalm A. Clin. Physiol. 1996;16:369–379. doi: 10.1111/j.1475-097x.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki S, Toyota E, Hiramatsu O, Kajita T, Shigeto F, Takemoto M, et al. Heart Vessels. 2000;15:274–279. doi: 10.1007/s003800070005. [DOI] [PubMed] [Google Scholar]
- 29.Castillo L, deRojas TC, Chapman TE, Vogt J, Burke JF, Tannenbaum SR, et al. Proc. Natl. Acad. Sci. U.S.A. 1993;90:193–197. doi: 10.1073/pnas.90.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forte P, Copland M, Smith LM, Milne E, Sutherland J, Benjamin N. Lancet. 1997;349:837–842. doi: 10.1016/S0140-6736(96)07631-3. [DOI] [PubMed] [Google Scholar]
- 31.Tsikas D. Anal. Chem. 2000;72:4064–4072. doi: 10.1021/ac9913255. [DOI] [PubMed] [Google Scholar]