Abstract
Lysophosphatidylcholine (LPC), a metabolite of membrane phospholipids by phospholipase A2, has been considered responsible for the development of abnormal vascular reactivity during atherosclerosis. Ca2+ influx was shown to be augmented in atherosclerotic artery which might be responsible for abnormal vascular reactivity. However, the mechanism underlying Ca2+ influx change in atherosclerotic artery remains undetermined. The purpose of the present study was to examine the effects of LPC on L-type Ca2+ current (ICa(L)) activity and to elucidate the mechanism of LPC-induced change of ICa(L) in rabbit portal vein smooth muscle cells using whole cell patch clamp. Extracellular application of LPC increased ICa(L) through whole test potentials, and this effect was readily reversed by washout. Steady state voltage dependency of activation or inactivation properties of ICa(L) was not significantly changed by LPC. Staurosporine (100 nM) or chelerythrine (3 µM), which is a potent inhibitor of PKC, significantly decreased basal ICa(L), and LPC-induced increase of ICa(L) was significantly suppressed in the presence of PKC inhibitors. On the other hand, application of PMA, an activator of PKC, increased basal ICa(L) significantly, and LPC-induced enhancement of ICa(L) was abolished by pretreatment of the cells with PMA. These findings suggest that LPC increased ICa(L) in vascular smooth muscle cells by a pathway that involves PKC, and that LPC-induced increase of ICa(L) might be, at least in part, responsible for increased Ca2+ influx in atherosclerotic artery.
Keywords: Lysophosphatidylcholine, Ca2+ current, Protein kinase C, Vascular smooth muscle
INTRODUCTION
Atherosclerosis is an arterial disease with a histopathologic changes, such as fatty streak and atheromatous plaque (Cox & Cohen, 1996a). In addition to this structural changes, the functional abnormalities including impairment of relaxation response and augmentation of contractile response to vasoconstrictors have also been reported in atherosclerotic arteries (Galle et al, 1990; Cox & Tulenko, 1995; Auge et al, 1996; Cox & Cohen, 1996b). These alterations in vascular reactivity are potentially important for the development of acute vasospasm, which is responsible for unstable angina or myocardial infarct. Lysophosphatidylcholine (LPC) is one of the candidates held to be responsible for the development of abnormal vascular reactivity in atherosclerotic artery for the following reasons. First, concentration of LPC in blood and within vessel wall increased profoundly during atherosclerosis (Murohara et al, 1994). Second, application of LPC mimicked abnormal vascular response of atherosclerotic artery and increased intracellular [Ca2+]i in vascular smooth muscle cells which was blocked by L-type Ca2+ channel (ICa(L)) antagonist (Stoll & Spector, 1993; Chen et al, 1995; Eizawa et al, 1995). These findings suggest that LPC increases ICa(L), thereby increasing intracellular [Ca2+]i and vascular contractility which are seen in atherosclerotic artery. However, it is still uncertain whether LPC increases ICa(L) or not.
L-type calcium channels are modulated by distinct vascular agonist known to activate different second messenger systems, including the signaling cascade that activates protein kinase C (PKC) (Keef et al, 2001). Several lines of evidence indicate that PKC activators stimulate ICa(L) in vascular smooth muscle cells obtained from different vascular territories (Lepretre & Mironneau, 1993; Shimamura et al, 1994; Obejero-Paz et al, 1998). In vitro and in vivo studies demonstrated that low concentration (less than 10 µM) of LPC activates PKC in some cell types, including vascular tissue (Sugiyama et al, 1994; Murohara et al, 1996; Watson & Gold, 1997). We also reported previously that LPC decreased delayed rectifier K+ current through activation of PKC in rabbit coronary smooth muscle cells (Yeon et al, 2001). Therefore, it is highly possible that LPC would increase ICa(L) through activation of PKC in vascular smooth muscle cells, although this relationship has not yet been investigated in these cells. The aims of the present study were to examine the LPC-induced modulation of ICa(L) in smooth muscle cells and to investigate the possible role of PKC in this modulation.
METHODS
Cell Preparation
Single rabbit portal vein smooth muscle cells were prepared using enzymatic digestion as previously described (Lepretre et al, 1994). Briefly, portal vein segments were incubated at 37℃ for 60 min in a Ca2+-free buffer solution of the following composition (in mmol/L): NaCl 140, KCl 5, MgCl2 1, N-2-hydroxyethylpiperazine-N'-2-ethansulfonic aid (HEPES) 10, glucose 10, with pH adjusted to 7.4 with Tris. The tissue was then incubated in a buffer with collagenase (1 mg/ml, Wako) and papain (0.1 mg/ml, Sigma) for 15~20 min. The tissue was gently agitated with Pasteur pipette, and released cells were kept at 4℃.
Electrophysiological recording
An aliquot of cells was placed in a perfusion chamber mounted on an inverted microscope (Nikon Diaphot), allowed to adhere to the bottom of the glass, then superfused with HEPES-buffered solution. Cells were sealed to pipettes with gentle suction, and the patch membrane was ruptured with further negative pressure at a holding potential of -50 mV. All experiments were performed at room temperature. Membrane currents were recorded by whole cell clamp technique with voltage clamp amplifier (Axopatch 1-D, Axon) using pipettes with a resistance from 2 to 3 MΩ. Voltages were corrected for the liquid junction potential. Voltage command and data collections were controlled by pClamp 6.0 software (Axon Instruments). The data were filtered by an 8-pole low-pass Bessel filter at 5 KHz, digitized at a sampling frequency of 25 KHz and then stored at hard disk for further analysis.
Experimental solutions
The pipette solution contained (mmol/L) Cs-aspartate 100, CsCl 30, MgATP 5, NaGTP 1, BAPTA 10, HEPES 10, adjusted to pH 7.2 with CsOH. The extracellular solution contained (mmol/L) NaCl 130, TEA-Cl 5.6, MgCl2 1.2, BaCl2 10, glucose 10, HEPES 10, adjusted to pH 7.4 with Tris. Nifedipine, staurosporine, chelerythrine, phorbol myristic acid (PMA), and LPC were dissolved in dimethylsulfoxide (DMSO) in concentrations that allowed DMSO concentration to remain at <1:10,000 in all experiments. At these concentrations, DMSO had no effect on ICa(L) compared with ICa(L) amplitudes prior to DMSO application. All the chemicals listed above were added directly to the bath solution from a freshly made stock solution.
Statistics
The results of the experiments are expressed as means ± S.E.M. Statistical evaluation of the data was performed by Student's t-test for comparison between two groups. A value of p less than 0.05 was taken as significant. The number of preparations taken from separate animals was indicated by n.
RESULTS
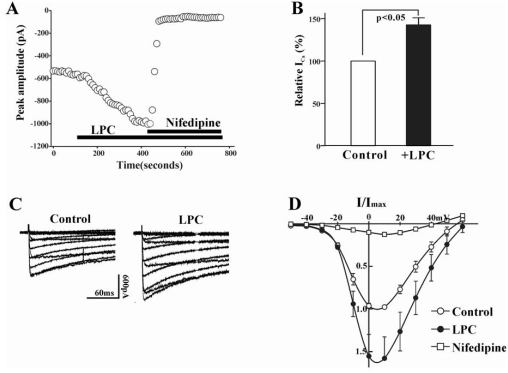
In the first series of experiment, we determined the effect of LPC on ICa(L) in rabbit portal vein smooth muscle cells. The inward current activating with depolarizing command steps under our experimental conditions was due to activation of ICa(L), because the application of nifedipine (1 µM), a specific blocker of ICa(L), abolished the inward current (Fig. 1A). LPC produced a significant increase in ICa(L) at 0 mV test pulse (p<0.01, 142.6±8.4%, n=19). However, LPC had no apparent effect on ICa(L) in some portal vein smooth muscle cells, which might be due to damage of cells during preparation of single cells or rundown of Ca2+ current. We excluded these LPC-unresponsive cells from analysis. Average values of ICa(L) from LPC-responsive portal vein smooth muscle cells were significantly larger than those from controls for voltages positive to -40 mV. As shown in Fig. 1D, this was most apparent above the threshold for activation of ICa(L). The reversal potential of ICa(L) was 54.5±1.5 mV and 56.9±0.9 mV in control and LPC-treated group, respectively (n=8).
Fig. 1.
(A) Plot of Ca2+ currents evoked by repetitive step depolarization from -80 mV to 0 mV versus time. Horizontal bar indicates the time of LPC (1 µM), and nifedipine (1 µM) application in the bath. (B) Summary of the LPC-induced change of ICa(L). The value of LPC-induced change was expressed as % of control. Bath application of LPC increased the current amplitude to 142.6±8.4% (n=19). (C) Effect of LPC on current-voltage relations of ICa(L). ICa(L) was recorded at 10 mV increments between -50 and +60 mV from a holding potential of -80 mV. (D) Summary of voltage dependence of ICa(L) for control (○), 1 µM LPC (●), and 1 µM nifedipine. At each voltage steps, peak values of current were determined, and averaged (n=8).
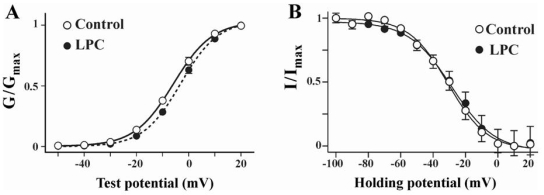
To investigate how LPC modulates ICa(L), we examined the effects of LPC on the voltage dependence of steady state activation and inactivation of ICa(L). The conductance values of ICa(L) were calculated using estimated reversal potential and were normalized by peak conductance to produce activation curves. The voltage dependent activation curves of control and LPC treatment were constructed using Boltzman fitting. As shown in Fig. 2, the half maximal activation potential (V1/2) was -5.8±0.2 mV and -3.1±0.1 mV in control and LPC-treated group, respectively (n=8). Average values of half-maximal activation potentials in LPC treated groups slightly positively shifted compared to control. The voltage dependence of ICa(L) availability was determined with 10 sec conditioning voltage pulses stepped from -100 mV to 20 mV in 10 mV increments, followed by a 500 ms test pulse to the voltage of 10 mV. Conditioning potentials positive to -70 mV produced a decrease of ICa(L), reaching full inactivation near 0mV. No difference was observed in half maximal steady state inactivation potential (-31.4±1.2 mV and -30.2±1.6 mV in control and LPC-treated group, n=6). These data suggest that, although LPC significantly increased the activity of ICa(L), it did not depend on the change in the voltage-dependent characteristics of ICa(L).
Fig. 2.
Steady state activation and inactivation curves of ICa(L) obtained before and after LPC application. (A) Steady state activation curves of ICa(L) in control and LPC. Chord conductance (G) was measured at different membrane potentials and normalized to the maximal chord conductance (Gmax). Each data points were the means of 8 experiments and fitted using a following form of Boltzman equation; Y={1+exp[(V1/2 - V)/k]}-1, where V1/2 represents half maximal activation potential and k is slope factor. Measured V1/2 was -5.8±0.2 mV and -3.1±0.1 mV in control and LPC, respectively. (B) Steady state inactivation curves of ICa(L) in control and LPC. Current amplitudes (I) of test potential to 0 mV from different pretest potentials were normalized to the maximal current (Imax). Each data points were the means of 6 experiments. Curves were obtained from following form of Boltzman equation; Y={1+exp[(V - V1/2)/k]}-1, where V1/2 represents half maximal inactivation potential and k is slope factor. V1/2 was -31.4±1.2 mV and -30.2±1.6 mV in control and LPC, respectively.
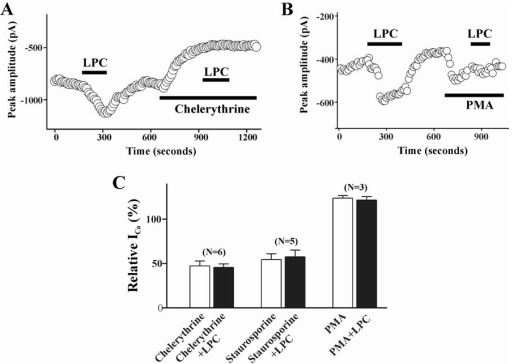
To test the possibility that LPC stimulates ICa(L) through activation of a protein kinase C, we examined the effect of PKC inhibitor or activator on LPC-induced increase of ICa(L). Thus, staurosporine (100 nM) was applied to the bath after acquiring a control ICa(L). Staurosporine produced a significant inhibition of basal ICa (57.6±7.5% of control), suggesting that a basal activity of protein kinase C is critical to maintain the amplitude of ICa(L) under our recording conditions. Application of chelerythrine (3 µM), a more specific inhibitor of PKC, showed similar effect on basal ICa(L) (47.4±5.5% of control). After the effect of PKC inhibitors reached to steady state, LPC-induced enhancement of ICa(L) was completely abolished (Fig. 3). On the other hand, pretreatment of the cells with 100 nM PMA, a PKC activator, enhanced the basal ICa(L) activity (123.5±3.1% of control) and significantly suppressed the LPC-induced enhancement of ICa (% of ICa change in PMA treated group vs. PMA+LPC treated group; 123.5±3.1% vs 121.4±4.0%, n=3).
Fig. 3.
Effect of PKC on LPC-induced change of ICa(L). Plot of Ca2+ currents evoked by repetitive step depolarization from -80 mV to 0 mV versus time. Temporal course of change of ICa(L) during treatment of LPC (1 µM) alone and LPC with 3 µM chelerythrine (A), or with 100 nM PMA (B). Horizontal bars indicate the time of drugs application. (C) Summary of the effect of pretreatment of staurosporine (100 nM), chelerythrine (3 µM), and PMA (100 nM) on LPC-induced change of ICa(L). Data are expressed as the % of control. Number of cells in each experimental condition is indicated in parenthesis.
DISCUSSION
We report herein a novel property of LPC to activate a L-type Ca2+ current (ICa(L)) in the rabbit portal vein smooth muscle cells. To the best of our knowledge, this is the first study to show that LPC can increase ICa(L) in vascular smooth muscle cells and its effect is mediated by activation of PKC. The enhancement of ICa(L) by LPC may be involved in augmented vascular contractility which is seen in atherosclerotic artery, because the activity of Ca2+ channel determines the amount of Ca2+ influx and intracellular Ca2+ concentration.
It has earlier been reported that LPC affects several kinds of ionic current, including Na+ current in cardiac myocytes and nonselective cation current in canine renal arterial cells (Magishi et al, 1996; Watson & Gold, 1997; Jabr et al, 2000). In the present study, nonselective cation current evoked by a prolonged exposure to LPC might contaminate to the recording of ICa(L) and involve in LPC-induced increase of ICa(L). However, this does not seem to be the case because 1) we used very low concentration of LPC (1 µM) compared with that used for activation of nonselective cation current (10 µM LPC; Magishi et al, 1996), and 2) application of nifedipine, which selectively blocks ICa(L), completely inhibited LPC-induced ICa(L) increase (Fig. 1A). These results suggest that LPC-induced enhancement of inward current in our experimental condition was due to mainly activation of Ca2+ current, but not a modification of other current, such as nonselective cation currents.
There are several possible mechanisms by which ICa(L) could be increased by LPC. One possibility is that LPC indirectly increased ICa(L) activity via destabilizing sarcolemma. At relatively high concentrations (>10 µM), LPC may induce a change of phospholipid packing when it is inserted into the lipid bilayer and this alone can modulate the activity of membrane proteins (Epand & Lester, 1990; McHowat et al, 1993). In the present experiment, the activity of ICa(L) was significantly enhanced by LPC at a concentration of 1 µM. Furthermore, pretreatment of the muscle cells with PKC inhibitors effectively inhibited LPC-induced change of ICa(L). These results suggest that LPC-induced increase of ICa(L) may not be due to alteration of membrane properties.
Another possibility is that LPC can increase ICa(L) through activation of PKC. In vitro as well as in vivo studies demonstrated that LPC activates PKC in some cell types, including vascular tissues (Sugiyama et al, 1994; Murohara et al, 1996; Watson & Gold, 1997). Activation of PKC has been implicated in regulation of various ion channel activities, such as Ca2+ channels, in vascular smooth muscle cells (Cole et al, 1996; Keef et al, 2001). Theses reports suggest the possibility that LPC increased the ICa(L) in portal vein smooth muscle cells through activation of PKC. As shown in Fig 3, the LPC-induced activation of ICa(L) was effectively antagonized by pretreatment with 100 nM staurosporine, indicating the involvement of PKC in the LPC-induced activation of ICa(L). It has been reported that staurosporine lacks specificity against PKC action, and staurosporine-induced inhibition of LPC activity might be due to inhibition of protein kinases other than PKC (Ruegg & Burgess, 1989). However, this was not the case in the present study because 1) pretreatment of chelerythrine, which is more specific for inhibition of PKC other than protein kinases (Zheng et al, 2001), showed results similar to those of staurosporine pretreatment, and 2) LPC-induced increase of ICa(L) was also effectively antagonized by pretreatment of PMA, an activator of PKC. Theses results suggest that LPC increased the ICa(L) through activation of PKC in rabbit portal vein smooth muscle cells.
In vascular smooth muscle, 11 isoforms of PKC have been reported, and they are divided to 3 groups: 1) classic or conventional PKCs that are activated by diacylglycerol or phorbol ester and are Ca2+ sensitive (including α, βI, βII, γ), 2) novel or new PKCs that are activated by diacylglycerol or phorbol ester but are not Ca2+ dependent (δ, ε, θ, η, Λ, µ), and 3) atypical PKCs that are not activated by either diacylglycerol or Ca2+ (λ/ι, ζ) (Hoffman, 1997). In portal vein smooth muscle cells, 3 groups of PKCs (α, ε, ζ) have been reported (Clement-Chomienne et al, 1996). In our present experiment, we did not directly measure which type of PKC isoforms was involved in LPC-induced increase in ICa(L). However, in our experimental condition, novel PKC in portal vein smooth muscle cells was most likely activated by LPC, because 1) we used 10 mM BAPTA-containing internal solution (see Method), and the involvement of conventional PKCs, which needs a [Ca2+]i for its activation, could be excluded, and 2) enhancement of basal ICa(L) by PMA also ruled out the involvement of atypical PKCs in LPC-induced increase of ICa(L). Nevertheless, the activation of other type of PKC isoforms by LPC can not still be excluded.
Thus, it may be concluded that LPC may increase the activity of ICa(L) via the activation of PKC in portal vein smooth muscle cells. And these LPC-induced increases in ICa(L) may be responsible for the increase of intracellular Ca2+ concentration and for the LPC-induced abnormal vascular reactivity in atherosclerotic artery.
ACKNOWLEDEGMENT
This work was supported by a grant 2005 from Myungsun Kim Foundation.
ABBREVIATIONS
- LPC
Lysophosphatidylcholine
- ICa(L)
L-type Ca2+ current
- PKC
protein kinase C
- PMA
phorbol myristic acid
References
- 1.Auge N, Fitoussi G, Bascand JL, Pieraggi M, Junquero D, Valet P. Mildly oxidized LDL evokes a sustained Ca2+-dependentretraction of vascular smooth muscle cells. Circ Res. 1996;79:871–880. doi: 10.1161/01.res.79.4.871. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Morimoto S, Kitano S, Koh E, Fukuo K, Jiang B, Chen S, Yasuda O, Hirotani A, Ogihara T. Lysophosphatidylcholine causes Ca2+ influx, enhanced DNA synthesis and cytotoxicity incultured vascular smooth muscle cells. Atherosclerosis. 1995;112:69–76. doi: 10.1016/0021-9150(94)05400-d. [DOI] [PubMed] [Google Scholar]
- 3.Clement-Chomienne O, Walsh MP, Cole WC. Antiotensin II activation of protein kinase C decreases delayed rectifier K+ current in rabbit vascular myocytes. J Physiol. 1996;495:689–700. doi: 10.1113/jphysiol.1996.sp021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol. 1996;74:439–447. doi: 10.1139/o96-048. [DOI] [PubMed] [Google Scholar]
- 5.Cox DA, Cohen ML. Effects of oxidized low-density lipoprotein on vascular contraction and relaxation: clinical and pharmacological implications in atherosclerosis. Pharmacol Rev. 1996a;48:3–19. [PubMed] [Google Scholar]
- 6.Cox DA, Cohen ML. Selective enhancement of 5-hyroxytryptamine-induced contraction of porcine coronary artery by oxidized low density lipoprotein. J Pharmacol Exp Ther. 1996b;276:1095–1103. [PubMed] [Google Scholar]
- 7.Cox RH, Tulenko TN. Altered contractile and ion channel function in rabbit protal vein with dietary atherosclerosis. Am J Physiol. 1995;268:H2522–H2530. doi: 10.1152/ajpheart.1995.268.6.H2522. [DOI] [PubMed] [Google Scholar]
- 8.Eizawa H, Yui Y, Inoue R, Kosuga K, Hattori R, Aoyama T. Lysophosphatidylcholine inhibits endothelium-dependent hyperpolarization and N-omega-nitro-L-arginine/indomethacin-resistant endothelium-dependent relaxation in the porcine coronary artery. Circulation. 1995;92:3520–3526. doi: 10.1161/01.cir.92.12.3520. [DOI] [PubMed] [Google Scholar]
- 9.Epand RM, Lester DS. The role of membrane biophysical properties in the regulation of protein kinase C activity. Trends Pharmacol Sci. 1990;11:317–320. doi: 10.1016/0165-6147(90)90234-y. [DOI] [PubMed] [Google Scholar]
- 10.Galle J, Bassenge E, Busse R. Oxidized low density lipoproteins potentiate vasoconstriction to various agonists by direct interaction with vascular smooth muscle. Circ Res. 1990;66:1287–1293. doi: 10.1161/01.res.66.5.1287. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J. 1997;11:649–669. doi: 10.1096/fasebj.11.8.9240967. [DOI] [PubMed] [Google Scholar]
- 12.Jabr RI, Yamazaki J, Hume JR. Lysophosphatidylcholine triggers intracellular calcium release and activation of non-selective cation channels in renal arterial smooth muscle cells. Pflugers Arch. 2000;439:495–500. doi: 10.1007/s004249900206. [DOI] [PubMed] [Google Scholar]
- 13.Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca2+ channels by protein kinases. Am J Physiol. 2001;281:C1743–C1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- 14.Lepretre N, Mironneau J. Alpha 2-adrenoreceptors activate dihydropyridine-sensitive calcium channels via Gi-proteins and protein kinase C in rat portal vein myocytes. Pflugers Arch. 1994;429:253–261. doi: 10.1007/BF00374320. [DOI] [PubMed] [Google Scholar]
- 15.Magishi K, Kimura J, Kubo Y, Abiko Y. Exogenous lysophosphatidylcholine increases non-selective cation current in guinea-pig ventricular myocytes. Pflugers Arch. 1996;432:345–350. doi: 10.1007/s004240050142. [DOI] [PubMed] [Google Scholar]
- 16.McHowat J, Yamada KA, Wu J, Yan GX, Corr BB. Recent insights pertaining to sarcolemmal phospholipid alterations underlying arrhythmogenesis in the ischemic heart. J Cardiovasc Electrophysiol. 1993;4:288–310. doi: 10.1111/j.1540-8167.1993.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 17.Murohara T, Kugiyama K, Ohgushi M, Sugiyama S, Ohta Y, Yasue H. LPC in oxidized LDL elicits vasocontraction and inhibits endothelium-dependent relaxation. Am J Physiol. 1994;267:H2441–H2449. doi: 10.1152/ajpheart.1994.267.6.H2441. [DOI] [PubMed] [Google Scholar]
- 18.Murohara T, Scalia R, Lefer AM. Lysophosphatidylcholine promotes P-selectin expression in platelets and endothelial cells: possible involvement of protein kinase C activation and its inhibition by nitric oxide donors. Circ Res. 1996;78:780–789. doi: 10.1161/01.res.78.5.780. [DOI] [PubMed] [Google Scholar]
- 19.Obejero-Paz CA, Auslender M, Scarpa A. PKC activity modulates availability and long openings of L-type Ca2+ channels in A7r5 cells. Am J Physiol. 1998;275:C535–C543. doi: 10.1152/ajpcell.1998.275.2.C535. [DOI] [PubMed] [Google Scholar]
- 20.Ruegg UT, Burgess GN. Staurosporine, K-252 and NCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 21.Shimamura K, Kusaka M, Sperelakis N. Protein kinase C stimulates Ca2+ current in pregnant rat myometrial cells. Can J Physiol Pharmacol. 1994;72:1304–1307. doi: 10.1139/y94-187. [DOI] [PubMed] [Google Scholar]
- 22.Stoll LL, Spector AA. Lysophosphatidylcholine causes cGMP-dependent verapamil-sensitive Ca2+ influx in vascular smooth muscle cells. Am J Physiol. 1993;264:C885–C893. doi: 10.1152/ajpcell.1993.264.4.C885. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama S, Kugiyama K, Ohgushi M, Fujimoto K, Yasue H. Lysophosphatidylcholine in oxidized low-density lipoprotein increases endothelial susceptibility to polymorphonuclear leukocyte-induced endothelial dysfunction in porcine coronary arteries: Role of protein kinase C. Circ Res. 1994;74:565–575. doi: 10.1161/01.res.74.4.565. [DOI] [PubMed] [Google Scholar]
- 24.Watson CL, Gold MR. Lysophosphatidylcholine modulates cardiac INa via multiple protein kinase pathways. Circ Res. 1997;81:387–395. doi: 10.1161/01.res.81.3.387. [DOI] [PubMed] [Google Scholar]
- 25.Yeon DS, Kwon SC, Nam TS, Ahn DS. Lysophosphatidylcholine decreases delayed rectifier K current in rabbit coronary smooth muscle cells. J Vet Med Sci. 2001;63:395–399. doi: 10.1292/jvms.63.395. [DOI] [PubMed] [Google Scholar]
- 26.Zheng T, Li W, Altura BT, Altura BM. Use of protein kinase C inhibitors in rapid [Mg2+]i mobilization in primary cultured rat aortic smooth muscle cells: are certain protein kinase C isoforms natural homeostatic regulators of cytosolic free Mg2+? Eur J Pharmacol. 2001;413:R1–R3. doi: 10.1016/s0014-2999(01)00729-4. [DOI] [PubMed] [Google Scholar]