Abstract
Melatonin has been reported to protect neurons from a variety of neurotoxicity. However, the underlying mechanism by which melatonin exerts its neuroprotective property has not yet been clearly understood. We previously demonstrated that melatonin protected kainic acid-induced neuronal cell death in mouse hippocampus, accompanied by sustained activation of Akt, a critical mediator of neuronal survival. To further elucidate the neuroprotective action of melatonin, we examined in the present study the causal mechanism how Akt signaling pathway is regulated by melatonin in a rat primary astrocyte culture model. Melatonin resulted in increased astrocytic Akt phosphorylation, which was significantly decreased with wortmannin, a specific inhibitor of PI3K, suggesting that activation of Akt by melatonin is mediated through the PI3K-Akt signaling pathway. Furthermore, increased Akt activation was also significantly decreased with luzindole, a non-selective melatonin receptor antagonist. As downstream signaling pathway of Akt activation, increased levels of CREB phoshorylation and GDNF expression were observed, which were also attenuated with wortmannin and luzindole. These results strongly suggest that melatonin exerts its neuroprotective property in astrocytes through the activation of plasma membrane receptors and then PI3K-Akt signaling pathway.
Keywords: Melatonin, Akt phosphorylation, Melatonin receptor, luzindole, PI3K, Primary astrotyctes
INTRODUCTION
Melatonin (5-methoxy-N-acetyltryptamine), the major secretory product of the pineal gland, has a variety of important physiological functions in the neuroimmunoendocrine system (Conti and Maestroni, 1995). It plays a role in the regulation of the circadian rhythm of several biological functions and also has been reported to protect neurons in vivo and in vitro (Maestroni et al., 1986).
In our previous study, we found that melatonin induced the phosphorylation of serine/threonine kinase Akt in astrocytes of mouse hippocampus (Lee et al., 2006). It has also been also reported that melatonin causes the activation of Akt in rat hippocampus by acting on melatonin receptors (Anhe et al., 2004), which is a critical step for neuronal survival in pathological neuronal cell death such as excitotoxic injury (Henshall et al., 2002; Kim et al., 2002). However, the exact mechanism of astrocytic Akt phosphorylation by melatonin, downstream signaling, and phenotypic changes have not yet been studied in astrocytes. In the present study, therefore, to understand the exact role of melatonin in astrocytes, we investigated the extent and mechanism of astrocytic Akt activation by melatonin and its downstream effects in rat primary astrocytes.
METHODS
Reagents
Melatonin was purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Wortmannin and luzindole were purchased from Calbiochem (San Diego, CA, U.S.A.). Melatonin was dissolved in 99% ethanol and stored at -20℃, covered with aluminum foil. Wortmannin and luzindole were prepared as 10 mM and 100 mM stock solution in dimethylsulfoxide, respectively, and aliquots were stored at -20℃ until use.
Primary astrocyte culture
Primary astrocyte culture was derived from 1 to 3 day postnatal SD rat (Daehan Biolink, Ltd., Seoul, Korea). Briefly, cerebral cortices were dissected out. After removal of the meninges and blood vessels, the cerebral cortices were collected and minced with scalpel in a solution containing 20µg/ml DNase and 0.3% BSA in DPBS (8.0 g/l NaCl, 0.4 g/l KCl, 0.06 g/l NaH2PO4, 0.06 g/l K2HPO4, 1.0 g/l glucose, 0.1 g/l MgSO4, 0.14 g/l CaCl2, 0.35 g/l NaHCO3). The tissues were centrifuged and incubated in 0.25% trypsin/EDTA solution for 10 min at 37℃. The suspension was filtered through a 70µm nylon filter, pelleted by centrifugation to remove trypsin, and then suspended in 10% (v/v) fetal bovine serum in Dulbecco's modified Eagle's medium containing penicillin and streptomycin antibiotic mixture (PS; GIBCO, Grand Island, NY, USA), and transferred to culture flasks and maintained at 37℃, 5% CO2 and 90% relative humidity. Medium was changed after 24 hr and every third day thereafter. When cells reached confluence, flasks were gently shaken to remove microglia and oligodendrocytes. After shaking, cells were rinsed three times with phosphate-buffered saline (PBS), suspended in trypsin-containing solution as above, and subcultured at 5×105 cells/60 mm flask. Using this method, cultures containing over 95% astrocytes, as determined by immunostaining for glial fibrillary acidic protein, were obtained (Xu et al., 2000). The cells at 80~85% confluence were treated with medium containing 1% lipoprotein deficient serum, replacing the 10% fetal bovine serum, and incubated for 16 hr prior to experimentation. All experiments were done using confluent astrocytes.
Immunoblotting
Cells were harvested, washed twice with ice-cold PBS, resuspended in lysis buffer, and sonicated on ice. Protein concentrations of the homogenates were determined using the bicinochoninic acid (BCA) method (Pierce, Rockford, IL, USA) and diluted to a final concentration of 1 mg/ml with 2X reducing stop buffer (0.25 M Tris-HCl, pH 6.8, 5 mM EDTA, 5 mM EGTA, 25 mM dithiothreitol, 2% SDS, and 10% glycerol with bromophenol blue as the tracking dye). Samples were resolved on 8% and 15% SDS-polyacrylamide gels and transferred to nitrocellulose membrances. Blots were blocked in 5% nonfat dry milk in TBST (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.05% Tween 20) for 1 hr at room temperature. The blots were then incubated with the anti-phospho-Akt, anti-phospho-CREB antibody, anti-Akt antibody, anti-CREB (1:1,000, Cell Signalling Technology), anti-GDNF antibody (1:1,000, Santa Cruz), and anti-β-actin antibody (1:2000, Sigma), respectively, in the same buffer overnight at 4℃. The membranes were then washed three times with TBST and incubated with HRP-conjugated goat anti-rabbit IgG (1:2,000, Jackson Immuno Research) or HRP-conjugated goat anti-mouse IgG (1:2,000, Jackson Immuno Research) for 2 hr at room temperature. The membranes were rinsed three times for 30 min with TBST. The blots were visualized with enhanced chemiluminescence (ECL; Amersham) and exposed to ECL film (Kodak). Gel images in ECL films were scanned and the digital images were quantified using Image Quant software (Molecular Dynamics, Sunnyvale, California, USA). For fair comparison, total protein levels in each samples loaded were equalized. In addition, actin, Akt, and CREB levels were used as denominators for calculating the relative expression levels of target proteins.
Statistical analysis
The data were analyzed using the unpaired t-test, and values were considered significantly different when the two-tailed p value was <0.05. The results are expressed as mean±SEM values.
RESULTS
Effect of melatonin on Akt phosphorylation in primary astrocytes
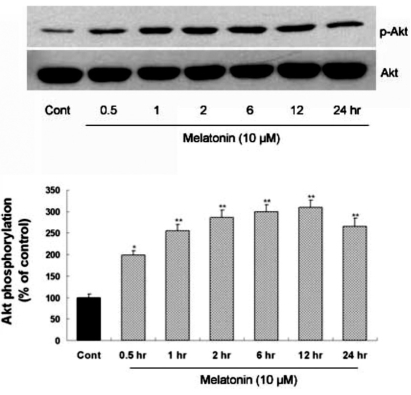
To clarify whether melatonin can directly induce Akt phosphorylation in astrocytes, time-course of Akt phosphorylation level was evaluated in primary cultures of cortical astrocytes after melatonin treatment (Fig. 1). Akt phosphorylation was induced by melatonin treatment in primary cultures of rat cortical astrocytes and reached the peak level 12 hr after melatonin treatment.
Fig. 1.
Time-course of melatonin-stimulated Akt phosphorylation at Ser473 in primary cultures of rat cortical astrocytes. Quiescent astrocytes were treated with melatonin (10µM) for indicated time periods. Data are representative of four independent experiments. The phospho-Akt and total Akt bands of the samples were densitometrically evaluated and phospho-Akt/total Akt ratio was expressed as mean±SEM. *p<0.05 and **p<0.01 indicate statistically significant difference from the control group.
Melatonin-mediated activation of Akt is PI3K dependent
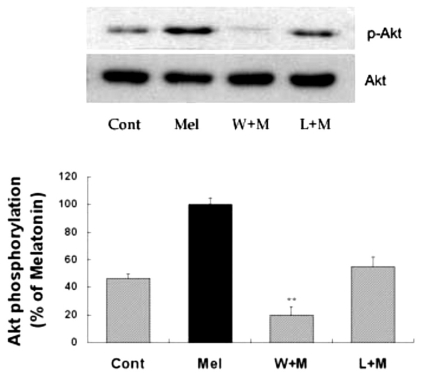
To evaluate the upstream pathway of Akt phosphorylation activated by melatonin, specific inhibitors were used prior to the administration of melatonin. Wortmannin, a specific inhibitor of PI3K, was effective in reducing melatonin-induced phosphorylation of Akt (Fig. 2). Luzindole, a non-selective melatonin receptor antagonist, was also effective in reducing melatonin-induced phosphorylation of Akt (Fig. 2). Similar phenomenon was also observed in vivo (data not shown). Wortmannin completely blocked the melatonin-induced phosphorylation of Akt, wherease luzindole partially blocked Akt phosphorylation. These results suggest that PI3K activation, which is mediated through melatonin receptor, leads to the Akt activation, thereby mediating biological action of melatonin in astrocytes.
Fig. 2.
Effects of PI3K inhibition and melatonin receptor blockade on melatonin-induced Akt phosphorylation. Melatonin (10µM) was applied to primary cultures for 12 hr in the absence or presence of wortmannin or luzindole. Concentrations and preincubation times of these compounds were as follows: wortmannin (W), 10µM, 1 hr; luzindole (L), 100µM, 1 hr. Right panel represents quantitative analysis. The phospho-Akt/Akt ratio was determined and expressed as mean±SEM. Akt phosphorylation by melatonin was inhibited by pretreatment of the cells with wortmannin or luzindole. *p<0.05 and **p<0.01 indicate statistically significant difference from the melatonin treated group.
Melatonin increases CREB phosphorylation via Akt phosphorylation
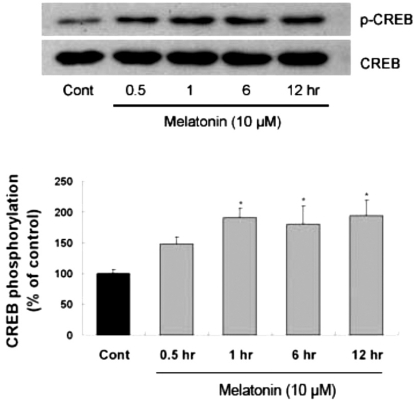
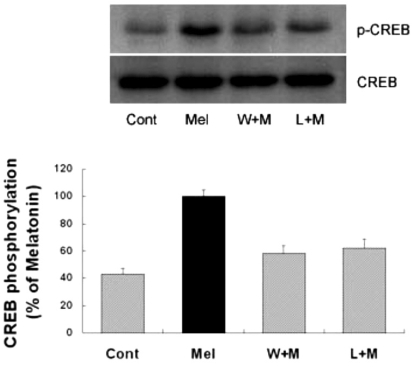
Several transcription factors, such as CREB and NF-κB (Du and Montminy, 1998) are known to play a role in the anti-apoptotic effect of the PI3K/Akt pathway. To determine whether melatonin can regulate CREB phosphorylation, immunoblotting on brains which were collected 2, 4, 6, and 12 hr after melatonin treatment was carried out. Increased levels of astrocytic CREB phosphorylation in hippocampus was observed as early as 2 hr after melatonin treatment and maintained up to 12 hr (data not shown). Similarly, CREB phosphorylation in primary cultures of rat cortical astrocytes was significantly increased up to 12 hr after melatonin treatment (Fig. 3). Next, to determine whether CREB phosphorylation is influenced by Akt activation, primary astrocytes were treated with wortmannin and luzindole 1 hr before the melatonin treatment. Western blot analysis showed that CREB phosphorylation by melatonin was inhibited by pretreatment of the cells with wortmannin or luzindole (Fig. 4). These results indicate that astrocytic Akt phosphorylation can regulate CREB phosphorylation.
Fig. 3.
Time-course of melatonin-stimulated CREB phosphorylation in primary cultures of rat cortical astrocytes. Quiescent cultured astrocytes were treated with melatonin (10µM) for indicated time periods. CREB phosphorylation in mouse hippocampus was increased by melatonin treatment. Left panel is the representative blot, and right panel shows quantitative verification. The phospho-CREB/CREB ratio was expressed as mean±SEM. *p<0.05 indicates statistically significant difference from the control group.
Fig. 4.
Effects of PI3K inhibition and melatonin receptor blockade on melatonin-induced CREB phosphorylation. Melatonin (10µM) was applied to primary cultures for 12 hr in the absence or presence of the inhibitor indicated. Left panel is the representative blot, and right panel shows quantitative verification. The phospho-CREB/CREB ratio was expressed as mean±SEM. CREB phosphorylation by melatonin was partially inhibited by pretreatment of the cells with wortmannin or luzindole. *p<0.05 indicates statistically significant difference from the melatonin treated group.
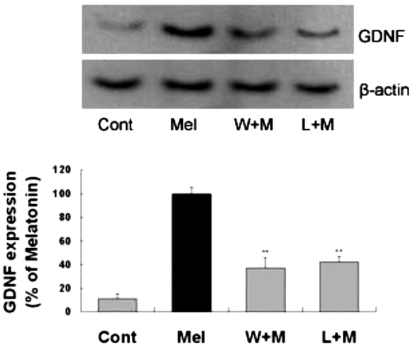
Melatonin-mediated Akt activation can increase GDNF expression
In our previous study (Lee et al., 2006), melatonin administration resulted in increased expression of GDNF in astrocytes. To investigate the role of Akt activation in upregulation of the GDNF by melatonin, GDNF expression level was measured after melatonin treatment for 24 hr in the presence of wortmannin or luzindole. Western blot analysis showed that GDNF expression by melatonin was inhibited by pretreatment of cells with wortmannin and luzindole. These results indicate that melatonin can regulate GDNF expression in astrocytes via Akt phosphorylation (Fig. 5).
Fig. 5.
Effects of PI3K inhibition or melatonin receptor blockade on melatonin-induced neurotrophic factors expression. Melatonin (10µM) was applied to primary cultures for 24 hr in the absence or presence of the agents indicated. Left panel is a representative blot, and right panel shows quantitative verification. The GDNF/β-actin ratio was expressed as mean±SEM. GDNF expression by melatonin was inhibited by pretreatment of the cells with wortmannin and luzindole. **p<0.01 indicates statistically significant difference from the melatonin treated group.
DISCUSSION
In our previous study, we found that melatonin can induce Akt phosphorylation in astrocytes in vivo (Lee et al., 2006). Recently, it has been shown that melatonin activated Akt in the ischemic rat brain via extracellular-regulated kinase-1/2 and/or Jun kinase-1/2 pathway to provide protection against ischemia/reperfusion injury (Kilic et al., 2005). However, mechanism and intracellular signaling of astrocytic Akt activation have not been studied. To determine whether melatonin can directly induce astrocytic Akt phosphorylation, the level of Akt phosphorylation was examined in cultured astrocytes. The results showed that melatonin induced astrocytic Akt phosphorylation in primary astrocytes: Akt phosphorylation started to increase as early as 30 minutes after melatonin treatment and reached the peak level 12 hr after treatment (Fig. 1).
It has been known that Akt phosphorylation can be induced by PI3K-dependent or independent pathway (Yano et al., 1998). It has also been reported that stimulation of Akt by melatonin is achieved through the activation of insulin tyrosine receptor kinase, which in turn activates PI3K, an upstream kinase of Akt (Anhe et al., 2004). Melatonin exerts its effects through its receptor or its antioxidant activity or its inhibition of QR2 enzymatic activity (Boutin et al., 2005). Some of the biological effects of melatonin are mediated by two distinct G protein-coupled receptors, which are high-affinity membrane-bound receptors; MT1 and MT2 receptors (Reppert et al., 1996). Both melatonin receptors have been reported to be present in hippocampal areas such as dentate gyrus, CA1, and CA3 (Musshoff et al., 2002). In order to further elucidate the Akt phosphorylation in astrocytes by melatonin, wortmannin, a PI3K inhibitor, and luzindole, a melatonin receptor antagonist, were used. Both inhibitors inhibited Akt phosphorylation in vitro (Fig. 2) and also in vivo (data not shown). The above results are in support of the fact that melatonin receptors, MT1 and MT2, are expressed in astrocytes and C6 glioma cells (Peters et al., 2005). In the present study, luzindole was found to inhibit Akt phosphorylation to a lesser extent than wortmannin, and this is most likely due to the possibility that melatonin might exert its action through not only its membrane-bound receptors, but also other pathways. Furthermore, it is also possible that, although activation of melatonin receptors results in Akt phosphorylation, other receptors such as growth factor receptors are also strongly involved in PI3K-mediated Akt phosphorylation.
Several transcription factors are known to play a role in the anti-apoptotic effects of the PI3K/Akt pathway. These include phosphorylated CREB (Du and Montminy, 1998), and activated NF-κB (Romashkova and Makarov, 1999). Furthermore, CREB activation is associated with BDNF and GDNF expression (Young et al., 1999). Melatonin up-regulates glial cell line-derived neurotrophic factor (GDNF) (Tang et al., 1998). It has also been shown that melatonin treatment results in the enhanced expression of GDNF mRNA in rat brain (Tang et al., 1998). Recent studies have suggested complex regulatory mechanisms of GDNF expression. GDNF expression can be induced by diverse extracellular stimuli (Castro et al., 2005), and multiple transcription factor binding sites have been identified in the promoter sequence of the GDNF gene (Woodbury et al., 1998), such as cAMP response element (CRE) binding protein (CREB) (Baecker et al., 1999). Previous studies showed that CREB activation is associated with GDNF expression (Lenhard et al., 2002), implying that CREB may participate in regulating GDNF expression as a transcriptional factor. We, therefore, hypothesized that the activation of transcription factor, such as CREB, and the increase of neurotrophic factors expression such as GDNF in astrocytes can be mediated by Akt phosphorylation-dependent pathway. As expected, CREB was phosphorylated by melatonin in astrocytes (Fig. 3). The changes of CREB phosphorylation has similar pattern with that of Akt phosphorylation in primary astrocytes. Similarly to Akt phosphorylation, CREB phosphorylation was inhibited by wortmannin and luzindole (Fig. 4). In accordance with our previous in vivo study (Lee et al., 2006), GDNF expression was induced by melatonin treatment and was inhibited by blockade of melatonin receptor and PI3K pathway in primary astrocytes.
These results described above indicate that Akt phosphorylation in astrocytes induced by melatonin is mediated through melatonin receptors and PI3K/Akt pathway, suggesting that the PI3K/Akt signaling pathway may be crucial for neuroprotective action of melatonin.
ACKNOWLEDGEMENT
This work was supported by the Korea Science and Engineering Foundation (R05-2004-000-11023-0).
ABBREVIATIONS
- PI3K
Phosphoinositide-3 kinase
- CREB
cAMP-responsive element-binding protein
- GDNF
glial cell line-derived neurotrophic factor
- NF-kB
nuclear factor-kB
References
- 1.Anhe GF, Caperuto LC, Pereira-Da-Silva M, Souza LC, Hirata AE, Velloso LA, Cipolla-Neto J, Carvalho CR. In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J Neurochem. 2004;90:559–566. doi: 10.1111/j.1471-4159.2004.02514.x. [DOI] [PubMed] [Google Scholar]
- 2.Baecker PA, Lee WH, Verity AN, Eglen RM, Johnson RM. Characterization of a promoter for the human glial cell line-derived neurotrophic factor gene. Brain Res Mol Brain Res. 1999;69:209–222. doi: 10.1016/s0169-328x(99)00106-0. [DOI] [PubMed] [Google Scholar]
- 3.Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–419. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Castro LM, Gallant M, Niles LP. Novel targets for valproic acid: up-regulation of melatonin receptors and neurotrophic factors in C6 glioma cells. J Neurochem. 2005;95:1227–1236. doi: 10.1111/j.1471-4159.2005.03457.x. [DOI] [PubMed] [Google Scholar]
- 5.Conti AG, Maestroni J. The clinical neuroimmunotherapeutic role of melatonin in oncology. J Pineal Res. 1995;19:103–110. doi: 10.1111/j.1600-079x.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 6.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 7.Henshall DC, Araki T, Schindler CK, Lan JQ, Tiekoter KL, Taki W, Simon RP. Activation of Bcl-2-associated death protein and counter-response of Akt within cell populations during seizureinduced neuronal death. J Neurosci. 2002;22:8458–8465. doi: 10.1523/JNEUROSCI.22-19-08458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilic U, Kilic E, Reiter RJ, Bassetti CL, Hermann DM. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J Pineal Res. 2005;38:67–71. doi: 10.1111/j.1600-079X.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Chun W, Kong PJ, Han JA, Cho BP, Kwon OY, Lee HJ, Kim SS. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal Res. 2006;40:79–85. doi: 10.1111/j.1600-079X.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 11.Lenhard T, Schober A, Suter-Crazzolara C, Unsicker K. Fibroblast growth factor-2 requires glial-cell-line-derived neurotrophic factor for exerting its neuroprotective actions on glutamate-lesioned hippocampal neurons. Mol Cell Neurosci. 2002;20:181–197. doi: 10.1006/mcne.2002.1134. [DOI] [PubMed] [Google Scholar]
- 12.Maestroni GJ, Conti A, Pierpaoli W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986;13:19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- 13.Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002;12:165–173. doi: 10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- 14.Peters JL, Earnest BJ, Tjalkens RB, Cassone VM, Zoran MJ. Modulation of intercellular calcium signaling by melatonin in avian and mammalian astrocytes is brain region-specific. J Comp Neurol. 2005;493:370–380. doi: 10.1002/cne.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci. 1996;17:100–102. doi: 10.1016/0165-6147(96)10005-5. [DOI] [PubMed] [Google Scholar]
- 16.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 17.Tang YP, Ma YL, Chao CC, Chen KY, Lee EH. Enhanced glial cell line-derived neurotrophic factor mRNA expression upon (-)-deprenyl and melatonin treatments. J Neurosci Res. 1998;53:593–604. doi: 10.1002/(SICI)1097-4547(19980901)53:5<593::AID-JNR9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Woodbury D, Schaar DG, Ramakrishnan L, Black IB. Novel structure of the human GDNF gene. Brain Res. 1998;803:95–104. doi: 10.1016/s0006-8993(98)00627-1. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Li Y, Cyras C, Sanan DA, Cordell B. Isolation and characterization of apolipoproteins from murine microglia. Identification of a low density lipoprotein-like apolipoprotein J-rich but E-poor spherical particle. J Biol Chem. 2000;275:31770–31777. doi: 10.1074/jbc.M002796200. [DOI] [PubMed] [Google Scholar]
- 20.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 21.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]