Abstract
The present study examined the inhibitory effect of licorice compounds glycyrrhizin and a metabolite 18β-glycyrrhetinic acid on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse and on the 1-methyl-4-phenylpyridinium (MPP+)-induced cell death in differentiated PC12 cells. MPTP treatment increased the activities of total superoxide dismutase, catalase and glutathione peroxidase and the levels of malondialdehyde and carbonyls in the brain compared to control mouse brain. Co-administration of glycyrrhizin (16.8 mg/kg) attenuated the MPTP effect on the enzyme activities and formation of tissue peroxidation products. In vitro assay, licorice compounds attenuated the MPP+-induced cell death and caspase-3 activation in PC12 cells. Glycyrrhizin up to 100µM significantly attenuated the toxicity of MPP+. Meanwhile, 18β-glycyrrhetinic acid showed a maximum inhibitory effect at 10µM; beyond this concentration the inhibitory effect declined. Glycyrrhizin and 18β-glycyrrhetinic acid attenuated the hydrogen peroxide- or nitrogen species-induced cell death. Results from this study indicate that glycyrrhizin may attenuate brain tissue damage in mice treated with MPTP through inhibitory effect on oxidative tissue damage. Glycyrrhizin and 18β-glycyrrhetinic acid may reduce the MPP+ toxicity in PC12 cells by suppressing caspase-3 activation. The effect seems to be ascribed to the antioxidant effect.
Keywords: Glycyrrhizin, MPTP, MPP+, Brain tissue damage, Cell death, Inhibitory effect
INTRODUCTION
Mitochondrial dysfunction and increased oxidative stress have been shown to be implicated in dopaminergic cell degeneration in Parkinson's disease (Jenner, 2003). The major mitochondrial defect in Parkinson's disease appears to be associated with complex I at the electron transport chain. Impairment of complex I activity leads to excess formation of ROS, which is involved in mitochondrial dysfunction and cell death (Fleury et al, 2002; Jenner, 2003). Implication of oxidative stress in the pathogenesis and progression of Parkinson's disease is supported by the decrease in GSH contents, increase in levels of lipid peroxidation products, increased production of ROS, and increase in iron content in substantia nigra (Olanow & Tatton, 1999).
MPTP produces an irreversible and severe parkinsonian-like syndrome in human and nonhuman primates (Przedborski & Jackson-Lewis, 1998). The inhibition of complex I in the mitochondrial electron transport chain induced by MPP+, the active metabolite of MPTP, results in impaired ATP production, loss of mitochondrial membrane potential and formation of ROS (Cassarino et al, 1997; Schulz et al, 1997). The membrane permeability transition of mitochondria is known as a central event in the course of a variety of toxic and oxidative forms of cell injury as well as apoptosis (Mignotte & Vayssiére, 1998). Along with respiratory chain inhibition, neuronal cell death by MPP+ is suggested to be mediated by formation of the mitochondrial permeability transition that leads to the release of cytochrome c and activation of caspases, and by disturbance of intracellular Ca2+ homeostasis (Cassarino et al, 1999; Lee et al, 2006, 2007).
Licorice root is a traditional herbal remedy, which has been used for the treatment of various pathologic conditions, including chronic hepatitis and gastric ulcer (Shibata, 2000). Increasing evidence indicates that glycyrrhizin (GL), a triterpenoid saponin found in Glycyrrhiza glabrata, and its hydrolyzed metabolite 18β-glycyrrhetinic acid (GA) have anti-inflammatory, anti-cancer and anti-hepatotoxic effects (Jeong et al, 2002; Matsui et al, 2004; Agarwal et al, 2005). GL and GA have an anti-oxidant ability and reduce oxidative damage due to carbon tetrachloride, tert-butyl hydroperoxide or ischemia-reperfusion injury (Nagai et al, 1991; Jeong et al, 2002; Kinjo et al, 2003). GA attenuates tumor necrosis factor-α-, lipopolysaccharide-induced cell death in human hepatoblastoma cell line and cultured liver cells (Yoshikawa et al, 1999; Zheng & Lou, 2003).
Mitochondrial dysfunction, increased oxidative stress and inflammation are involved in neurodegenerative process in Parkinson's disease (Olanow & Tatton, 1999; Jenner, 2003). Therefore, the compounds that have properties such as mitochondrial function enhancement, anti-inflammatory activity and anti-oxidant ability may provide the neuroprotective effect on the degeneration of striatal dopaminergic neurons (Bonuccelli & Del Dotto, 2006). GA and GL are known to exhibit an anti-oxidant and anti-inflammatory effect. However, the effect of licorice compounds against the neurotoxicity of MPTP and MPP+ remains uncertain. The aim of the present study was therefore to elucidate the protective effect of GL on the MPTP neurotoxicity in the mouse and assess the inhibitory effect of GL and GA on the toxicity of MPP+ in differentiated PC12 cells.
METHODS
Materials
MPTP, MPP+, glycyrrhizin, 18β-glycyrrhetinic acid, superoxide dismutase (from bovine erythrocytes; 2,500~7,000 units/mg protein), catalase (from bovine liver; 10,000 ~25,000 units/mg protein), carboxy-PTIO, MTT and RPMI 1640 medium were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). ApoAlert™ CPP32/caspase-3 assay kit was purchased from CLONTECH Laboratories Inc. (Palo Alto, CA, USA), and trolox was from OXIS International Inc. (Portland, OR, USA).
MPTP treatment
Adult male ICR mice (weighing 25~30 g) were treated with 30 mg/kg MPTP intraperitoneally for 5 days at 24-h intervals and killed 24 h after the last MPTP injection and ether anesthesia (Cassarino et al, 1997; Hung & Lee, 1998). Animals (32 mice) were divided into four groups, and saline and compounds were administered intraperitoneally: One group received saline (0.9% NaCl) injection for 5 days, the second group received MPTP injection alone, the third group received both MPTP and 16.8 mg/kg GL injection, and the fourth group received GL injection alone. The GL injection started one day before the MPTP injection and continued for 5 days. Animal care was in accordance with the NIH guidelines for animal experiments of USA and the guide for animal experiments of Korean Academy of Medical Sciences (2000 yr). Mice were maintained under a 12-h light and dark cycle in a temperature-regulated (23±1℃) animal room with water and food continuously available.
Preparation of brain homogenates
Mouse brain [basal ganglia, diencephalon plus midbrain (BDM), cortex and cerebellum] homogenates were prepared in 150 mM KCl and centrifuged at 800 × g at 10 min. The supernatants were used in the experiments. Protein concentration was determined by the method of Bradford according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA, USA).
Measurement of SOD activity
The total SOD activity in brain homogenates was assayed by using the SOD-inhibitable ferricytochrome c reduction (McCord & Fridovich, 1969). Brain homogenates (0.1 mg of protein/ml) were suspended in one ml of reaction mixtures containing 10 mM ferricytochrome c, 0.03% sodium deoxycholate, 100 mM EDTA, 50 mM xanthine and 50 mM potassium phosphate, pH 7.4. The reaction was started by addition of xanthine oxidase and absorbance was measured at 550 nm spectrophotometrically (model DU-70; Beckman Instruments, Fullerton, CA, USA). The absorbance change of cytochrome c induced by xanthine oxidase without tissue homogenates was adjusted to show 0.021~0.025 at the same wavelength. Fifty percent inhibition of cytochrome c reduction is defined as 1 unit of enzyme activity.
Measurement of catalase activity
Catalase activity in brain homogenates was measured by the decomposition of H2O2 at 240 nm (Aebi, 1984). Tissue homogenates (0.1 mg of protein/ml) were added to one ml of reaction mixtures containing 15 mM H2O2, 0.1% Triton X-100, and 50 mM potassium phosphate, pH 7.4. Units of enzyme activity were determined using the extinction coefficient of 0.0394 mM-1cm-1 for H2O2. One unit of catalase activity is defined as 1 mmol H2O2 consumed/ min/mg of protein.
Measurement of glutathione peroxidase activity
Glutathione peroxidase activity was assayed by the method of Flohe & Gunzler (1984). Brain homogenates (0.1 mg of protein/ml) were suspended in 30℃ reaction mixtures (one ml) containing 1 mM glutathione, 0.96 units/ml glutathione reductase, 100 mM EDTA, and 50 mM potassium phosphate (pH 7.4). The reaction was started by sequential addition of 150 mM NADPH and 1.2 mM tert-butyl hydroperoxide, and then absorbance was measured at 340 nm. Units of enzyme activity were determined using the extinction coefficient of 6.22 mM-1cm-1. One unit of the enzyme is defined as 1 nmol NADPH consumed/min/mg of protein.
Measurement of lipid peroxidation
The lipid peroxidation product in brain homogenates was assayed by measurement of the MDA concentration using the thiobarbituric acid method. Brain homogenates (0.4 mg of protein/ml) of mice exposed to MPTP were mixed with 1 ml of 1% thiobarbituric acid in 50 mM NaOH and 1 ml of 2.8% trichloroacetic acid, and the concentration of MDA was measured (Gutteridge et al, 1982). The MDA concentration is expressed as nanomoles per milligram of protein using the molar extinction coefficient of 1.56×105 M-1cm-1.
Quantification of carbonyl groups
The protein oxidation product of brain homogenates was quantified by carbonyl assay using 2,4-dinitrophenylhydrazine (Levine et al, 1990). Brain homogenates (1 mg of protein/ml) of mice exposed to MPTP were reacted with carbonyl assay mixture. Four milliliters of 10 mM 2,4-dinitrophenylhydrazine in 2.5 M HCl was added to mixtures, and tubes were left for 1 h at room temperature in the dark. The mixtures were sequentially treated with 20 and 10% trichloroacetic acid. After centrifugation, pellets were washed three times with 4 ml of ethanol/ethyl acetate mixture (1:1 vol/vol). The final pellets were dissolved in 2 ml of 6 M guanidine HCl and left for 15 min at 30℃ with mixing. Absorbance of the supernatants was measured at 370 nm and protein carbonyls were quantified using the molar extinction coefficient of 2.2×104 M-1cm-1.
Cell culture
Rat PC12 cells (adrenal gland; pheochromocytoma) were obtained from Korean cell line bank (Seoul, Korea). PC12 cells were cultured in RPMI medium supplemented with 10% heat-inactivated horse serum, 5% heat-inactivated fetal bovine serum (FBS), 100 units/ml of penicillin and 100µg/ml of streptomycin according to the manual of the cell line bank. Cells were differentiated by treating with 100 ng/ml 7S nerve growth factor for 9 days (Tatton et al, 2002). Cells were washed with RPMI medium containing 1% FBS 24 h before experiments and replated onto the 96- and 24-well plates.
Cell viability assay
Cell viability was measured by using the MTT assay, which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenases (Mosmann, 1983). PC12 cells (4×104) were treated with MPP+ in the presence of licorice compounds for 24 h at 37℃. The medium (200µl) was incubated with 10µl of 10 mg/ml MTT solution for 2 h at 37℃. After centrifugation at 412 × g for 10 min, culture medium was removed and 100µl dimethyl sulfoxide added to each well to dissolve the formazan. Absorbance was measured at 570 nm using a microplate reader (Spectra MAX 340, Molecular Devices Co., Sunnyvale, CA, USA). Cell viability was expressed as a percentage of the value in control cultures.
Measurement of caspase-3 activity
The activation of caspase-3 occurred during the apoptotic process in cells was assessed (Mignotte & Vayssière, 1998). PC12 cells (2×106 cells/ml) were treated with MPP+ for 24 h at 37℃ and caspase-3 activity was determined according to the user's manual for the ApoAlert™ CPP32/Caspase-3 assay kit. The supernatant obtained by a centrifugation of cells dissolved was added to the reaction mixture containing dithiothreitol and caspase-3 substrate (N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide) and incubated for 1 h at 37℃. Absorbance of the chromophore p-nitroanilide produced was measured at 405 nm. The standard curves were obtained from the absorbance of p-nitroanilide standard reagent diluted with cell lysis buffer (up to 20 nM). One unit of the enzyme was defined as the activity producing 1 nmol of p-nitroanilide.
Statistical analysis
Data are expressed as means±SEM. Statistical analysis was performed by one-way analysis of variance. When significance was detected, post hoc comparisons between the different groups were made using the Duncan's test for multiple comparisons. A probability less than 0.05 was considered to be statistically significant.
RESULTS
Inhibitory effect of GL on elevated antioxidant enzyme activities in MPTP-treated mouse brain
Increased ROS formation and alteration of endogenous antioxidant enzyme activities, which seems to reflect enhanced oxidative stress, are found in the brains of MPTP-treated mice (Cassarino et al, 1997; Hung & Lee, 1998). In agreement with these reports, the present study showed an increase in the antioxidant enzyme activities in various brain areas [basal ganglia, diencephalon plus midbrain (BDM), cortex and cerebellum] of mice treated with 30 mg/kg MPTP intraperitoneal injection for 5 days. The MPTP treatment exhibited a significant increase in the activities of total SOD, catalase and glutathione peroxidase in the brains of mice compared to saline-injected mice. Increase in three antioxidant enzyme activities in the brain BDM of MPTP-treated mice (increase of 1.54~1.70 fold compared to control) was greater than those of cortex (1,25~1,35 fold increase) and cerebellum (1.31~1.46 fold increase). Co-administration of GL (16.8 mg/kg=20µM) significantly attenuated the activity increase of the antioxidant enzymes in mice treated with MPTP (Table 1). The enzyme activities in the brain of mice treated with GL alone was similar to that of saline-injected mice.
Table 1.
Effect of GL on increased antioxidant enzyme activities in the brains of mice treated with MPTP
Mice were treated with MPTP (30 mg/kg) and GL (16.8 mg/kg) for 5 days. Enzyme activities in tissue homogenates of BDM, cortex and cerebellum were measured as described in Methods. Data represent units/mg protein and means ± SEM (n=8). †p<0.05 compared to control; and *p<0.05 compared to MPTP alone.
Inhibitory effect of GL on lipid peroxidation and carbonyl formation in MPTP-treated mouse brain
Oxidative tissue damage in the brains of mice treated with MPTP was assessed by measuring the formation of lipid oxidation product MDA and protein oxidation product carbonyls. In the various brain areas of mice treated with MPTP, the formations of MDA and carbonyls were significantly increased compared to saline-injected mice. As observed in the endogenous antioxidant enzymes, increases in the amounts of MDA and carbonyls (increase of 1.59~1.72 fold compared to control) were greater than those of cortex (1.32~1.33 fold increase) and cerebellum (1.34~1.37 fold increase). Co-administration of GL reduced the formation of MDA and carbonyls induced by MPTP treatment (Table 2). The amounts of MDA and carbonyls in the brains of mice treated with GL alone were similar to those of control mice.
Table 2.
Effect of GL on formation of MDA and carbonyls in the brains of mice treated with MPTP
Mice were treated with MPTP (30 mg/kg) and GL (16.8 mg/kg) for 5 days. Amounts of MDA and carbonyls in tissue homogenates of BDM, cortex and cerebellum were quantified as described in Methods. Data represent nmol/mg protein and means ± SEM (n=8). †p<0.05 compared to control; and *p<0.05 compared to MPTP alone.
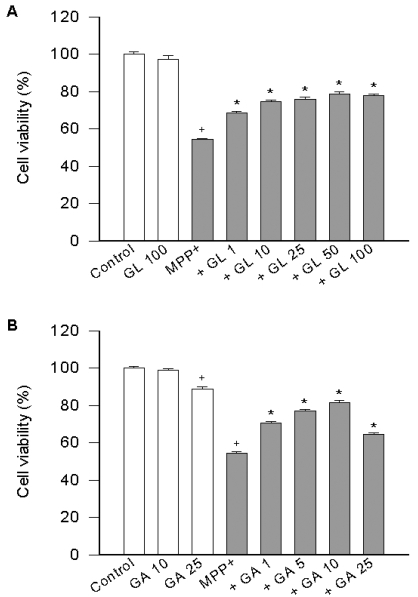
Inhibition of MPP+-induced cell death by licorice compounds
To assess the toxicity of MPTP at the level of cells, we examined the toxic effect of MPP+, an active metabolite of MPTP, against PC12 cells that are differentiated by nerve growth factor. The incidence of cell death after exposure to 500µM MPP+ for 24 h was about 46%. GL (1~100µM) significantly reduced the 500µM MPP+-induced cell death with a maximal inhibitory at 50µM; beyond this concentration the inhibitory effect declined (Fig. 1). The present study also examined the effect of GA, a metabolite of GL, against the MPP+-induced cell viability loss in PC12 cells. The present data shows that GA showed a maximal inhibitory effect at 10µM; beyond this concentration the inhibitory effect declined (Fig. 1). Although GL at 100 µM and GA at 25µM caused about 3 and 11% cell death, respectively, they reduced the MPP+-induced cell death.
Fig. 1.
Effect of licorice compounds on MPP+-induced cell death. PC12 cells were pre-treated with licorice compounds (1~100µM GL in A or 1~25µM GA in B) for 20 min, exposed to 500µM MPP+ for 24 h and cell viability was determined. Data represent means ± SEM (n=6). +p<0.05 compared to control (percentage of control); and *p<0.05 compared to MPP+ alone.
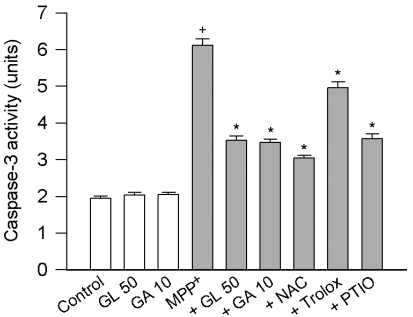
Inhibitory effect of licorice compounds on MPP+-induced activation of caspase-3
Opening of the mitochondrial permeability transition pore causes the release of cytochrome c from mitochondria into the cytosol and subsequent activation of caspases as one of the mitochondria-mediated cell death signaling events (Kim et al, 2006). We examined the inhibitory effect of licorice compounds on the MPP+-induced cell death by performing the ELISA-based quantitative analysis for caspase-3 activity. Cells treated with MPP+ exhibited an increase in caspase-3 activity, whose response was significantly attenuated by the addition of 1 mM N-acetylcysteine, 30µM trolox (a scavenger of hydroxyl radicals and peroxynitrite), or 30µM carboxy-PTIO (a scavenger of nitric oxide). Licorice compounds (50µM GL and 10µM GA) attenuated the MPP+-induced activation of caspase-3, while treatment of 50µM GL or 10µM GA alone did not cause significant changes in caspase-3 activity (Fig. 2).
Fig. 2.
Effect of licorice compounds on MPP+-induced activation of caspase-3. PC12 cells were treated with 500µM MPP+ in the presence of licorice compounds (10~50µM) or scavengers [1 mM N-acetylcysteine (NAC), 30µM trolox or 30 µM carboxy-PTIO (PTIO)] for 24 h. Data are expressed as units for caspase-3 activity and represent means ± SEM (n=6). +p<0.05 compared to control; and *p<0.05 compared to MPP+ alone.
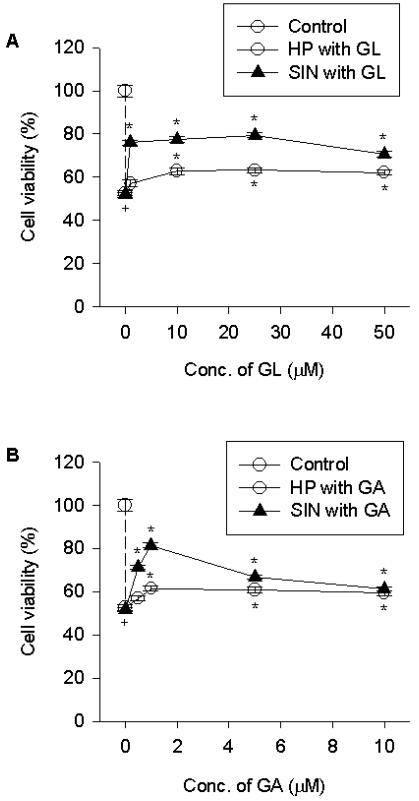
Inhibition of hydrogen peroxide- and reactive nitrogen species-induced cell death by licorice compounds
The present study examined that GL and GA protect PC12 cells against damaging action of ROS or nitrogen species. As observed in the data, GL and GA significantly attenuated the hydrogen peroxide (200µM)-induced cell death in PC12 cells and showed a maximal inhibitory effect at 25µM and 1µM, respectively; beyond these concentrations the inhibitory effect declined (Fig. 3). We examined the effect of licorice compounds against the cytotoxicity of reactive nitrogen species. 3-Morpholinosydnonime liberates a strong oxidant peroxynitrite that is formed by reaction of nitric oxide with superoxide (Muijsers et al, 1997). Treatment of GL and GA reduced cell death due to exposure of 750µM 3-morpholinosydnonime and showed a maximal inhibitory effect at 10µM and 1µM, respectively (Fig. 3). The inhibitory potency of licorice compounds on the toxicity of 3-morpholinosydnonime was greater than on that of hydrogen peroxide.
Fig. 3.
Effect of licorice compounds on cell death due to hydrogen peroxide or 3-morpholinosydnonime. PC12 cells were pre-treated with licorice compounds [1~50µM GL (A) or 1~10µM GA (B)] for 15 min, exposed to 200µM hydrogen peroxide for 4 h or 750µM 3-morpholinosydnonime for 24 h, and cell viability was determined. Data represent means ± SEM (n=6). +p<0.05 compared to control; and *p<0.05 compared to hydrogen peroxide (HP) or 3-morpholinosydnonime (SIN).
DISCUSSION
In substantia nigra from patients with Parkinson's disease, diminished activity of mitochondrial complex I, and increased activities of SOD and glutathione peroxidase are detected (Olanow & Tatton, 1999). The continuous production of free radicals in dopaminergic neurons is compensated by the induction of endogenous antioxidant enzymes. MPTP neurotoxicity has been shown to be ascribed to the inhibition of complex I at the mitochondrial electron transport chain (Cassarino et al, 1997; Schulz et al, 1997). The MPP+-induced mitochondrial dysfunction appears to induce a pathologic cascade involving both excitotoxicity and free radical production. The brains of mice treated with MPTP exhibited the decreased complex I activity, the increased hydroxyl radical production and the elevated activities of SOD, catalase and glutathione peroxidase (Cassarino et al, 1997; Hung & Lee, 1998). In the present study, the brains of mice treated with MPTP exhibited increase in the activities of antioxidant enzymes (total SOD, catalase and glutathione peroxidase). Compared to cortex and cerebellum, the marked increase in antioxidant enzyme activities in the BDM area suggests that after exposure to MPTP, the brain BDM seems to be chiefly affected. And such finding appears to be mostly involved in the elicitation of motor control disturbance.
Licorice compounds GA and GL are known to exhibit an anti-oxidant and anti-inflammatory effect. GL and GA reduce oxidative tissue damage due to tert-butyl hydroperoxide or ischemia-reperfusion injury (Nagai et al, 1991; Kinjo et al, 2003). Nevertheless, the effect of licorice compounds against the MPTP/MPP+ toxicity remains uncertain. The present study examined whether GL added intraperitoneally exhibited an inhibitory effect against oxidative tissue damage in the brains of mice treated with MPTP. The results suggest that GL seems to prevent oxidative tissue damage in the brain (BDM, cortex and cerebellum) of mice treated with MPTP through the inhibitory action on the activation of the antioxidant enzymes (total SOD, catalase, and glutathione peroxidase) and the formation of MDA and carbonyls. GL administration alone did not cause significant changes of antioxidant enzyme activities and formation of MDA and carbonyls in the brain. This finding shows that GL at the stated concentration has no toxic effect against the brain tissue and may have a therapeutic safety. Therefore, licorice compounds seem to exert an effective inhibitory effect against oxidative tissue damage in the brains of patients with Parkinson's disease.
To assess the toxicity of MPTP on cell viability, we examined the toxic effect of MPP+ instead of MPTP against differentiated PC12 cells. PC12 cells upon the nerve growth factor stimulation not only display abundant neuritic growth, but also adopt a neurochemical dopaminergic phenotype (Kadota et al, 1996). The MPP+-induced apoptosis in neuronal cells is mediated by formation of the mitochondrial permeability transition, which results in the release of mitochondrial cytochrome c and subsequent activation of caspase-3 (Cassarino et al, 1999; Lee et al, 2006). In agreement with these reports, the MPP+ induced cell death seems to be mediated by activation of caspase-3.
GA attenuates the tumor necrosis factor-induced apoptotic cell death without cell surface Fas activation (Yoshikawa et al, 1999). In contrast, GL could enhance Fas-mediated apoptosis without alteration of caspase-3-like activity (Ishiwata et al, 1999). Therefore, along with variable effects, it is uncertain by which mechanism licorice compounds exert a protective effect. In this study, GL up to 100µM and its metabolite GA up to 25µM significantly reduced the MPP+-induced cell viability loss in PC12 cells. The preventive effect of licorice compounds on MPP+-induced cell death seems to be ascribed to the inhibitory effect on caspase-3 activation. Although there is a difference of inhibitory potency on the basis of concentration, a metabolite GA as well as GL exhibits a protective effect. GA at low concentration seems to exert an effective protective effect the MPP+-induced cell damage. Meanwhile, the toxicity at high concentrations appears to diminish the protective of licorice compounds against the MPP+-induced neuronal cell death.
The MPP+ treatment causes the respiratory chain inhibition, leading to the formation of ROS and nitrogen species (Jenner, 2003). ROS act upon mitochondria, causing a disruption of mitochondrial membrane potential and the release of cytochrome c (Fleury et al, 2002). It has been shown that MPP+ induces the formation of ROS and nitrogen species, which is involved in mitochondrial dysfunction and cell death (Lee et al, 2006, 2007). Inhibitory effect of antioxidants such as N-acetylcysteine and carboxy-PTIO on the MPP+-induced activation of caspase-3 may support the results of the previous reports. GL and GA are demonstrated to attenuate either renal injury due to ischemia-reperfusion or FeCl2 plus ascorbate-induced lipid peroxidation in liver homogenates by scavenging free radicals (Yokozawa et al, 2000; Jeong et al, 2002). In contrast, GA induces apoptosis of HL60 cells by increasing production of ROS (Makino et al, 2006). These reports indicate on uncertainty whether licorice compounds have antioxidant ability and reveal a cyto-protective effect. In this study, the inhibitory effect on the enhanced antioxidant enzyme activity and the formation of tissue oxidation products in the brain of mice treated with MPTP clearly indicates that GL exhibit a neuroprotective effect by suppressing oxidative tissue damage. In addition, licorice compounds may exert an inhibitory effect against cell death due to exposure of ROS and nitrogen species. Therefore, GL and GA seem to prevent the MPP+-induced cell death and caspase-3 activation by suppressing oxidative stress.
Overall, the in vivo results show that licorice compound GL may prevent brain tissue damage in mice treated with MPTP through inhibitory effect on oxidative tissue damage. At the cellular levels, GL and GA seem to reduce the MPP+-induced cell viability loss in PC12 cells by suppressing caspase-3 activation. The effect seems to be ascribed to the antioxidant effect. GL in combination with drugs such as levodopa may provide a beneficial effect in the treatment of Parkinson's disease.
ACKNOWLEDGEMENT
This research was supported by the Chung-Ang University Research Grants in 2007.
ABBREVIATIONS
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium iodide
- GL
glycyrrhizin
- GA
18β-glycyrrhetinic acid
- carboxy-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- SOD
superoxide dismutase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MDA
malonedialdehyde
- ROS
reactive oxygen species
References
- 1.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal MK, Iqbal M, Athar M. Inhibitory effect of 18β-glycyrrhetinic acid on 12-O-tetradecanoyl phorbol-13-acetate- induced cutaneous oxidative stress and tumor promotion in mice. Redox Rep. 2005;10:151–157. doi: 10.1179/135100005X57346. [DOI] [PubMed] [Google Scholar]
- 3.Bonuccelli U, Del Dotto P. New pharmacological horizons in the treatment of Parkinson disease. Neurology. 2006;67(7 Suppl 2):S30–S38. doi: 10.1212/wnl.67.7_suppl_2.s30. [DOI] [PubMed] [Google Scholar]
- 4.Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD, Jr, Bennet JP., Jr Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim Biophys Acta. 1997;1362:77–80. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 5.Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The Parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 6.Fleury C, Mignotte B, Vayssière J-L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–141. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 7.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 8.Gutteridge JMC, Rowley DA, Halliwell B. Superoxide dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of 'catalytic' iron and antioxidant activity in extracellular fluids. Biochem J. 1982;206:605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung HC, Lee EHY. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med. 1998;24:76–84. doi: 10.1016/s0891-5849(97)00206-2. [DOI] [PubMed] [Google Scholar]
- 10.Ishiwata S, Nakashita K, Ozawa Y, Niizeki M, Nozaki S, Tomioka Y, Mizugaki M. Fas-mediated apoptosis is enhanced by glycyrrhizin without alteration of caspase-3-like activity. Biol Pharm Bull. 1999;22:1163–1166. doi: 10.1248/bpb.22.1163. [DOI] [PubMed] [Google Scholar]
- 11.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(suppl 3):S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 12.Jeong HG, You HJ, Park SJ, Moon AR, Chung YC, Kang SK, Chun HK. Hepatoprotective effects of 18β-glycyrrhetinic acid on carbon tetrachloride-induced liver injury:inhibition of cytochrome P450 2E1 expression. Pharmacol Res. 2002;46:221–227. doi: 10.1016/s1043-6618(02)00121-4. [DOI] [PubMed] [Google Scholar]
- 13.Kadota T, Yamaai T, Saito Y, Akita Y, Kawashima S, Moroi K, Inagaki N, Kadota K. Expression of dopamine transporter at the tips of growing neurites of PC12 cells. J Histochem Cytochem. 1996;44:989–996. doi: 10.1177/44.9.8773564. [DOI] [PubMed] [Google Scholar]
- 14.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57:545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 15.Kinjo J, Hirakawa T, Tsuchihashi R, Nagao T, Okawa M, Nohara T, Okabe H. Hepatoprotective constituents in plants. 14. Effects of soyasapogenol B, sophoradiol, and their glucuronides on the cytotoxicity of tert-butyl hydroperoxide to HepG2 cells. Biol Pharm Bull. 2003;26:1357–1360. doi: 10.1248/bpb.26.1357. [DOI] [PubMed] [Google Scholar]
- 16.Lee CS, Kim YJ, Ko HH, Han ES. Modulation of 1-methyl-4-phenylpyridinium-induced mitochondrial dysfunction and cell death by KATP channel block. J Neural Transm. 2007;114:297–305. doi: 10.1007/s00702-006-0594-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Han YS, Han ES, Bang H, Lee CS. Differential involvement of intracellular Ca2+ in 1-methyl-4-phenylpyridiniumor 6-hydroxydopamine-induced cell viability loss in PC12 cells. Neurochem Res. 2006;31:851–860. doi: 10.1007/s11064-006-9088-9. [DOI] [PubMed] [Google Scholar]
- 18.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, Ahn B-W, Shaltiel S, Stadman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 19.Makino T, Tsubouchi R, Murakami K, Haneda M, Yoshino M. Generation of reactive oxygen species and induction of apoptosis of HL60 cells by ingredients of traditional herbal medicine, Sho-saiko-to. Basic Clin Pharmacol Toxicol. 2006;98:401–415. doi: 10.1111/j.1742-7843.2006.pto_328.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsui S, Matsumoto H, Sonoda Y, Ando K, Aizu-Yokota E, Sato T, Kasahara T. Glycyrrhizin and related compounds downregulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 2004;15:1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCord JM, Fridovich I. Superoxide dismutase. Enzymatic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 22.Mignotte B, Vayssière JL. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival:application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Muijsers RBR, Folkerts G, Henricks PA, Sadeghi-Hashjin G, Nijkamp FP. Peroxynitrite:a two faced metabolite of nitric oxide. Life Sci. 1997;60:1833–1845. doi: 10.1016/s0024-3205(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 25.Nagai T, Egashira T, Yamanaka Y, Kohno M. The protective effect of glycyrrhizin against injury of the liver caused by ischemia-reperfusion. Arch Environ Contam Toxicol. 1991;20:432–436. doi: 10.1007/BF01064416. [DOI] [PubMed] [Google Scholar]
- 26.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 27.Przedborski S, Jackson-Lewis V. Mechanism of MPTP toxicity. Mov Disord. 1998;13:35–38. [PubMed] [Google Scholar]
- 28.Schulz JB, Matthews RT, Klockgether T, Dichgans J, Beal MF. The role of mitochondrial dysfunction and neuronal nitric oxide in animal models of neurodegenerative diseases. Mol Cell Biochem. 1997;174:193–197. [PubMed] [Google Scholar]
- 29.Shibata S. A drug over the millennia:pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 30.Tatton WG, Chalmers-Redman RME, Ju WJH, Mammen M, Carlile GW, Pong AW, Tatton NA. Propargylamines induce antiapoptotic new protein synthesis in serum-and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther. 2002;301:753–764. doi: 10.1124/jpet.301.2.753. [DOI] [PubMed] [Google Scholar]
- 31.Yokozawa T, Liu ZW, Chen CP. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine. 2000;6:439–445. doi: 10.1016/S0944-7113(00)80072-0. [DOI] [PubMed] [Google Scholar]
- 32.Yoshikawa M, Toyohara M, Ueda S, Shirol A, Takeuchi H, Nishiyama T, Yamada T, Fukui H, Ishizaka S. Glycyrrhizin inhibits TNF-induced, but not Fas-mediated, apoptosis in the human hepatoblastoma line HepG2. Biol Pharm Bull. 1999;22:951–955. doi: 10.1248/bpb.22.951. [DOI] [PubMed] [Google Scholar]
- 33.Zheng QZ, Lou YJ. Pathologic characteristics of immunologic injury in primary cultured rat hepatocytes and protective effect of glycyrrhizin in vitro. Acta Pharmacol Sin. 2003;24:771–777. [PubMed] [Google Scholar]