Abstract
We report a nonhydrolytic bimolecular aminolysis reaction catalyzed by an antibody. The stereospecific formation of an amide from racemic lactone plus an aromatic amine is described by a random equilibrium bireactant kinetic sequence. The observed turnover rate may be approximated from the measured difference between the binding of reactants and the transition state analog.
Full text
PDF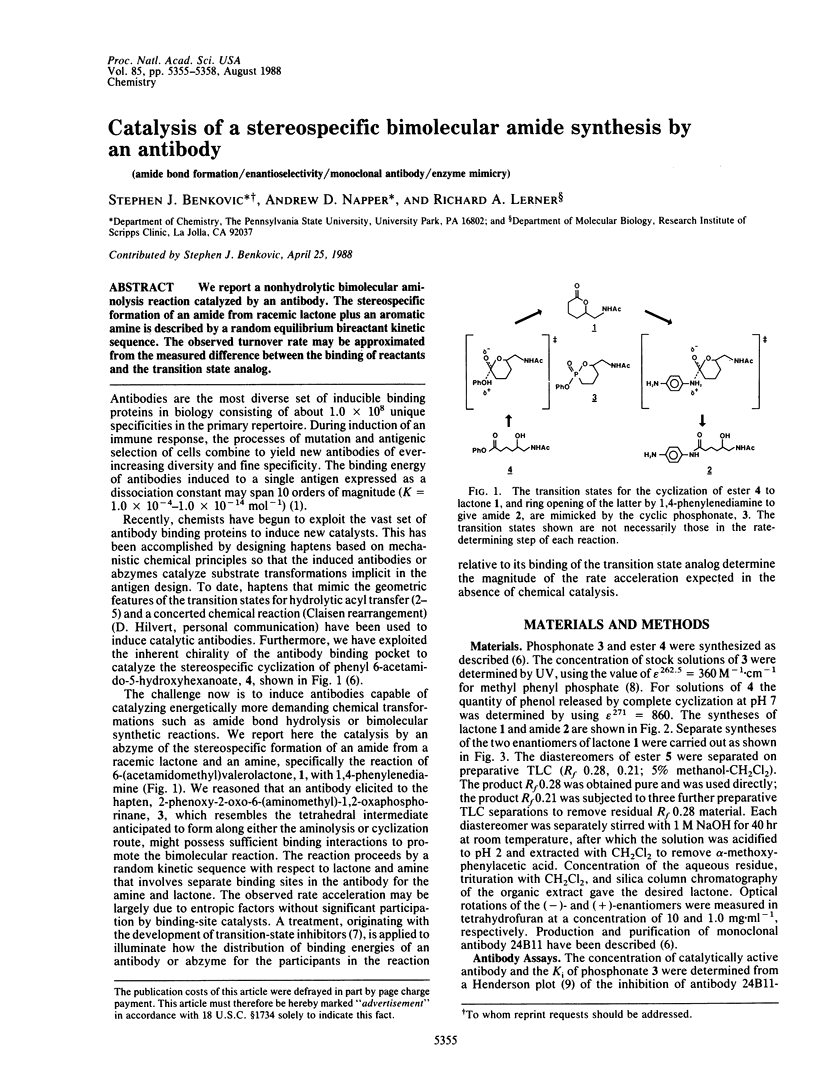

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jencks W. P. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- Napper A. D., Benkovic S. J., Tramontano A., Lerner R. A. A stereospecific cyclization catalyzed by an antibody. Science. 1987 Aug 28;237(4818):1041–1043. doi: 10.1126/science.3616626. [DOI] [PubMed] [Google Scholar]
- Tramontano A., Janda K. D., Lerner R. A. Catalytic antibodies. Science. 1986 Dec 19;234(4783):1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- Tramontano A., Janda K. D., Lerner R. A. Chemical reactivity at an antibody binding site elicited by mechanistic design of a synthetic antigen. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6736–6740. doi: 10.1073/pnas.83.18.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]


