Abstract
Although growth associated protein-43 (GAP-43) is known to play a significant role in the regulation of axonal growth and the formation of new neuronal connections in the hippocampus, there is only a few studies on the effects of acute stress on GAP-43 mRNA expression in the hippocampus. Moreover, the effects of repeated citalopram treatment on chronic mild stress (CMS)-induced changes in GAP-43 mRNA expression in the hippocampus have not been explored before. To explore this question, male rats were exposed to acute immobilization stress or CMS. Also, citalopram was given prior to stress everyday during CMS procedures. Acute immobilization stress significantly increased GAP-43 mRNA expression in all subfields of the hippocampus, while CMS significantly decreased GAP-43 mRNA expression in the dentate granule cell layer (GCL). Repeated citalopram treatment decreased GAP-43 mRNA expression in the GCL compared with unstressed controls, but this decrease was not further potentiated by CMS exposure. Similar decreases in GAP-43 mRNA expression were observed in CA1, CA3 and CA4 areas of the hippocampus only after repeated citalopram treatment in CMS-exposed rats. This result indicates that GAP-43 mRNA expression in the hippocampus may differently respond to acute and chronic stress, and that repeated citalopram treatment does not change CMS-induced decreases in GAP-43 mRNA expression in the GCL.
Keywords: Stress, Antidepressant, GAP-43, Hippocampus
INTRODUCTION
Growth associated protein-43 (GAP-43) is a nervous tissue specific protein which is highly expressed in neurons during development and nerve regeneration (Snipes et al, 1987; McGuire et al, 1988). GAP-43 is localized in growth cones (Skene et al, 1986) and plays an important role in guiding the axonal growth and modulating the formation of new neuronal connections (Benowitz & Routtenberg, 1997). Accordingly, GAP-43 has been used as a marker for axonal growth and presynaptic axonal plasticity. In hippocampus, GAP-43 is increased by epileptic insult such as kainate (Cantallops & Routtenberg, 1996; Bendotti et al, 1997) or pilocarpine (Naffah-Mazzacoratti et al, 1999) as well as by hypoxia-ischemia (Miyake et al, 2002). In addition to the regulation by external insult, GAP-43 is regulated by various endogenous compounds such as hormones, neurotransmitters and drugs. For example, norepinephrine, estradiol and mood stabilizer valproic acid promote an increase of GAP-43 mRNA expression in the hippocampus (Ferrini et al, 2002; Watterson et al, 2002; Laifenfeld et al, 2005).
Recent preclinical and clinical studies suggest that stress-induced impairment in neuroplasticity of the brain, in particular hippocampus, occurs in depression (Duman, 2002; Pittenger & Duman, 2008), and that chronic antidepressant treatments can reverse these impairments (Manji et al, 2000). Therefore, chronic stress seems to decrease GAP-43 mRNA expression in the hippocampus (Kuroda & McEwen, 1998), however, with some inconsistency (Rosenbrock et al, 2005). Moreover, chronic imipramine and desipramine treatment increase GAP-43 mRNA expression in the hippocampus (Chen et al, 2003; Sairanen et al, 2007), whereas chronic fluoxetine or fluvoxamine treatment tend to decrease GAP-43 mRNA expression (Iwata et al, 2006; Larsen et al, 2008). However, the changes of GAP-43 in the hippocampus by chronic antidepressant treatment in normal laboratory animals may not necessarily reflect the mechanism of antidepressant effect in the depressed animals, since antidepressant treatment in human without depression induces typical side effects rather than antidepressant effect (Nestler et al, 2002). In this regard, it is interesting to note that chronic restraint stress decreases GAP-43 mRNA expression in CA3 region of the hippocampus, however, tianeptine pretreatment does not prevent chronic restraint stress-induced decrease in GAP-43 mRNA expression in the hippocampus (Kuroda & McEwen, 1998). Nevertheless, growing evidence suggests that chronic mild stress (CMS), rather than chronic restraint stress, is more suitable for animal depression model to study the antidepressant effect, because a similar state of anhedonia, which is one of the two core symptoms of depression, can be induced in rats by CMS procedures (Moreau et al, 1992; Stout et al, 2000). Moreover, tianeptine promotes serotonin reuptake (Mennini et al, 1987), unlike typical antidepressants that block serotonin reuptake in presynaptic terminal.
Thus, it is not clear at present whether repeated treatment with selective serotonin reuptake inhibitor prior to CMS may affect CMS-induced alteration of GAP-43 mRNA expression in the hippocampus. Moreover, although there are only a few studies that examined the effects of chronic stress on the GAP-43 mRNA expression in the hippocampus, there are several studies that observed the effects of acute stress on GAP-43 mRNA levels in the hippocampus. Therefore, we observed the effects of acute stress and CMS on the GAP-43 mRNA levels in the hippocampus, and then, the effects of chronic citalopram pretreatment on the CMS-induced changes in GAP-43 mRNA levels in the hippocampus. In the present study, we used citalopram as a selective serotonin reuptake inhibitor, since it has negligible effects on norepinephrine and dopamine reuptake (Goodnick & Goldstein, 1998; Stahl, 1998). Furtheremore, the effects of CMS and repeated citalopram treatment on GAP-43 mRNA levels in the hippocampus were also examined.
METHODS
Animals and drug/stress treatment
Sprague Dawley adult male rats (initial weight, 150~180 g; Orient, Korea) were used. Rats were brought into the laboratory one week before the experiment. Three rats were housed per cage under a 12 h light-dark cycle (light on at 6 A.M). Food and water were available ad libitum except for water and food deprivation periods of CMS schedule. At first, to observe the effects of acute stress and CMS on the GAP-43 mRNA levels in the hippocampus, rats were exposed to either acute stress or CMS. For acute immobilization stress, rats were placed in plastic restraint cone bags (Harvard Apparatus, South Natick, MA, USA) for 2 h (n=4). Unstressed animals were used as a control (n=4). As for CMS, the stress regimen consisted of each week of a variety of unpredictable, mild stressors such as repeated 1-h periods of confinement to small (24×10×9 cm) cages as previously described (Moreau et al, 1992; Stout et al, 2000) with some modification (Table 1). CMS procedure was applied for 19 days (n=7). Control animals were briefly handled each day, similarly to the experimental group, but without restraint, and were kept under standard housing condition (n=7). To observed the effects of citalopram treatment on CMS-exposed animals, animals were i.p. injected with citalopram (10 mg/kg) every morning before the stress procedure, and control animals received an equivalent volume of saline (2 ml/kg, i.p.): This dose of citalopram (10 mg/kg, i.p.) has previously been shown to have antidepressant effects in CMS model (Papp et al, 2002). Thus, animals were divided into 4 groups; saline treated unstressed group (SAL+CON, n=9), citalopram treated unstressed group (CIT+CON, n=9), saline treated CMS-exposed group (SAL+CMS, n=9) and citalopram treated CMS-exposed group (CIT+CMS, n=9). All procedures used in this study were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Table 1.
Schedule of chronic mild stress
Perfusion and brain section
Rats exposed to acute immobilization stress were deeply anesthesized with sodium pentobarbital (100 mg/kg, i.p.) 2 h after an immobilization stress. CMS-exposed rats were deeply anestheized with sodium pentobarbital 6~8 h after the last CMS procedure, and control animals were perfused midway through the sacrificing of the CMS-exposed rats to reduce the time of difference due to sacrifice. Rats were perfused intracardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PPB, pH 7.2). The brains were fixed in situ for 1 h, removed, post-fixed in PPB for 2 h, and finally placed in 20% sucrose/PPB overnight at 4℃. Serial coronal sections (30 µm) of whole brain were prepared using a freezing microtome and were stored in cryoprotectant solution [30% RNase free sucrose, 30% ethylene glycol, and 1% polyvinyl pyrrolidone (PVP-40) in 100 mM sodium phosphate buffer, pH 7.4] at -20℃.
Probe construction
PCR fragments encompassing nucleotides 124-950 of rat GAP-43 (Genebank accession number NM_017195) were subcloned into pBluescript II. The PCR primer sequence was as follows; sense 5'-CCCAAGCTTAGCAAGCGAGCAGAAAAGAG-3' and antisense 5'-CGCGGATCCGCAGGAGAGACAGGGTTCAG-3'. For in situ hybridization, the GAP-43 plasmid was linearized with Hind III and transcribed with T3 RNA polymerase to obtain antisense riboprobes. Linearized DNA was labeled with 35S-UTP (Amersham, Cardiff, UK) by in vitro transcription. A 1 µl aliquot of labeled mRNA was suspended in 4 ml of scintillation fluid, and activity in cpm was determined by a scintillation counter.
In situ hybridization
Brain sections were permeabilized with proteinase K (1 µg/ml, 37℃, 30 min), treated with acetic anhydride in 0.1 M triethanolamine (pH 8.0), washed in 2 × SSC (pH 7.0), and were then transferred into 500 µl of hybridization solution in 24-well culture plates. The hybridization solution comprised 50% formamide, 0.01% polyvinyl pyrrolidone, 0.01% Ficoll, 0.01% bovine serum albumin, 50 µg/ml denatured salmon sperm DNA, 250 µg/ml yeast tRNA, 40 mM dithiothreitol (DTT), 10% dextran sulfate, and 35S-labeled GAP-43 cRNA probes at 1×107 cpm/ml. Sections were hybridized with the GAP-43 riboprobe for 18 h at 55 ℃ in the hybridization solution. After overnight incubation in a humidified chamber, sections were washed twice for 30 min at 55℃ in 4×SSC with 5 mM DTT, treated with RNase A (20 µg/ml, 30 min at 45℃), and washed four times (15 min/wash) in 2×SSC containing 5 mM DTT at room temperature. The sections were then washed twice (30 min/wash) in 0.5×SSC containing 5 mM DTT, once for 30 min in 0.1×SSC containing 5 mM DTT at 50~55℃, and once in 0.1×SSC with 5 mM DTT at room temperature. Sections were then mounted on gelatin-coated microscope slides and air-dried overnight. Tissue sections were exposed to Hyperfilm β-max (Amersham, Arlington Heights, IL, USA) for 3 days. In situ hybridization of brain section which was conducted on free-floating sections in the present study (Gold et al, 1997) yielded the result equivalent to that conducted on slide-mounted sections (Ni et al, 1999).
Data analysis and statistics
The sections for analysis were selected through hippocampus (-3.60 to -3.80 mm from bregma) according to Paxinos and Watson (1998). The level of GAP-43 mRNA was analyzed by outlining the regions of interest on in situ hybridization sections and then densitometrically quantifying the regions using Scion Image beta 4.2 software (Scion Corporation, Frederick, MD, USA). The relative values of optical density measured with known [14C]-labeled radioactive standards (Amersham, Arlington Heights, IL, USA) were used to construct a third degree polynomial calibration curve. For each animal, the relative radioactivity values from three to five individual sections were analyzed, yielding three to five determinations, and they were then used to calculate mean values. The results are expressed as the mean percentage of the respective control values, and data are expressed as mean ± standard error of mean (S.E.M.). Statistical analysis was performed by Student's t-test or one-way analysis of variance (ANOVA) followed by the Fisher's LSD post-hoc test. Significance was accepted for p-values less than 0.05.
RESULTS
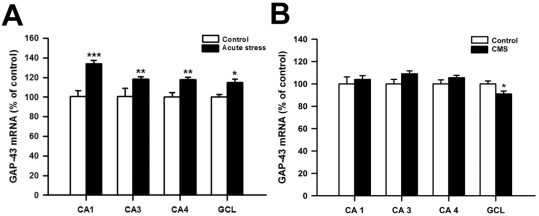
Acute stress significantly increased GAP-43 mRNA levels of the CA1 compared with the levels of CA1 in control rats (Student's t-test, p<0.001). Similar trend of increases was found in CA3, CA4 and dentate granule cell layer (GCL) of the hippocampus, but the magnitude of increases was lower than that of CA1 (Fig. 1, 2). CMS induced a small but significant decrease of GAP-43 mRNA levels in the GCL, compared to control rats (Fig. 1; Student's t-test, p<0.05).
Fig. 1.
(A) The effects of acute stress on GAP-43 mRNA levels in CA1, CA3, CA4 and dentate granular cell layer (GCL) of the hippocampus (n=4 per group) (B) The effects of chronic mild stress (CMS) on GAP-43 mRNA levels in the CA1, CA3, CA4 and GCL of the hippocampus (n=7 per group). The results are expressed as percent of mean value from control group and are mean standard error of mean (S.E.M.). Data were compared by Student's t-test. *p<0.05, **p<0.01 and ***p<0.001 vs. control group.
Fig. 2.
Representative autoradiographs of 35S-labeled GAP-43 mRNA expression in the hippocampus from each treatment group are shown. (A) control (B) acute immobilization stress.
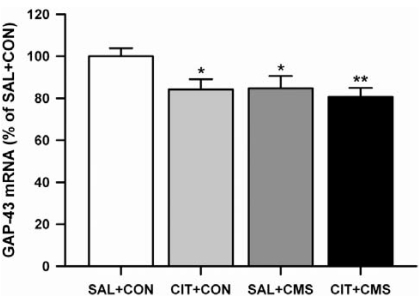
A two-way ANOVA revealed a significant main effect of repeated citalopram treatment (F1,32=4.39, p<0.05) on GAP-43 mRNA levels in the GCL. Although there was no significant interaction between repeated citalopram treatment and CMS (F1,32=1.55, p=0.223), a two-way ANOVA test for CMS was close to statistical significance F1,32=3.89, p=0.057), indicating that CMS tended to decrease GAP-43 mRNA levels in the GCL compared to unstressed controls. A one-way ANOVA showed that there was a significant difference among these groups (Fig. 3, 4; F3,32=3.27, p<0.05). Post hoc analysis using Fisher's LSD test demonstrated that both repeated citalopram treatment and CMS significantly decreased GAP-43 mRNA levels in the GCL, compared to saline-treated unstressed controls (p<0.05). However, repeated citalopram treatment prior to CMS did not further enhance CMS-induced decrease of GAP-43 mRNA level in the GCL.
Fig. 3.
The effects of repeated citalopram treatment and CMS on GAP-43 mRNA levels in the GCL. Repeated citalopram treatment and CMS significantly decreased GAP-43 mRNA levels in the GCL, but there was no significant interaction between repeated citalopram treatment and CMS. The results are expressed as percent of mean value from SAL+CON group and are mean±S.E.M (n=9 per group). Data were compared using a one-way ANOVA with Fisher's LSD post hoc test. *p<0.05 and **p<0.01 vs. saline-treated unstressed control (SAL+CON). Abbreviations used: citalopram treated unstressed group (CON+CIT), saline treated CMS-exposed group (CMS+SAL), citalopram treated CMS-exposed group (CMS+CIT).
Fig. 4.
Representative autoradiographs of 35S-labeled GAP-43 mRNA in the hippocampus from each treatment group are shown. (A) saline treated unstressed group (B) citalopram treated unstressed group (C) saline treated CMS-exposed group (D) citalopram treated CMS-exposed group.
A two-way ANOVA revealed significant main effects of repeated citalopram treatment (F1,32=10.16, p<0.01) as well as interaction between repeated citalopram treatment and CMS (F1,32=5.97, p<0.05) on GAP-43 mRNA levels in the CA3. A one-way ANOVA showed that there was a significant difference among these groups (F3,32=5.54, p<0.01). Post hoc analysis using Fisher's LSD test demonstrated that GAP-43 mRNA levels of CA3 in rats, which were given repeated citalopram treatment prior to CMS, were significantly lower than those in other groups. A similar pattern of decreases was found in CA1 and CA4 of the hippocampus in rats which were given repeated citalopram treatment before CMS (Table 2).
Table 2.
Effect of chronic mild stress and repeated citalopram treatment on the GAP-43 mRNAs levels in rat hippocampus
The results are expressed as percent of mean value from SAL+CON group and are mean±S.E.M (n=9 per group). Data were compared using a one-way ANOVA with Fisher's LSD post hoc test. *p<0.05 and **p<0.01 vs. saline-treated unstressed control (SAL+CON). Abbreviations used are the same as those in Fig. 3.
DISCUSSION
In the present study, acute stress significantly increased GAP-43 mRNA levels in all subfields of the hippocampus (CA1, CA3, CA4 and GCL). Similar increase of GAP-43 mRNA expression in CA1, CA3 and GCL of the hippocampus was observed following learned helplessness paradigm (Iwata et al, 2006). Learned helplessness paradigm is one of animal models of depression. However, learned helplessness is composed of electric shock for 2 days, and therefore, is rather close to an acute stress paradigm. Thus, learned helplessness seems likely to increase GAP-43 mRNA expression in hippocampus, similar to acute stress. Although the region of interest is different, acute restraint stress in mice has been shown to increase GAP-43 mRNA expression similary in the amygdala (Pawlak et al, 2003). However, compared to acute stress, CMS in the present study induced a small but significant decrease of GAP-43 mRNA levels in the GCL. Interestingly, chronic restraint stress for 3 weeks induces a decrease in GAP-43 mRNA expression in the CA3 region of the hippocampus (Kuroda & McEwen, 1998). Moreover, chronic intermittent stress in mice for 3 weeks significantly decreases GAP-43 levles in the hippocampus (Pawlak et al, 2005), while a chronic intermittent stress in rats does not induce any change in GAP-43 mRNA expression in the hippocampus (Rosenbrock et al, 2005). The reason of such discrepancy is not clear at present, nevertheless differences in stress intensity may be responsible for this discrepancy: The stress intensity of CMS model may be greater than that of chronic restraint or chronic intermittent stress, since animals in chronic restraint or chronic intermittent stress are repeatedly exposed to the same procedures of stress (i.e. homotype stress). This type of stress model tends to develop habituation in contrast to CMS in which rats are exposed to daily stresses of different nature (i.e., heterotype stress) (Herman & Cullinan, 1997).
In the present study, when the effects of repeated citalopram treatment on GAP-43 mRNA levels in either CMS-exposed group or unstressed controls were compared, repeated citalopram treatment significantly decreased GAP-43 mRNA levels in all subfields of the hippocampus in CMS-exposed group. However, there was a difference in changes of GAP-43 mRNA expression following repeated citalopram treatment in CMS-exposed group, depending on the subfields of hippocampus: repeated citalopram treatment and CMS significantly decreased GAP-43 mRNA levels in the GCL, however, there were no significant changes in CA1, CA3 and CA4 areas of the hippocampus. It is not clear at present how GAP-43 mRNA expression in the hippocampus changes in response to CMS or repeated citalopram treatment. One possibility is that increased serotonergic transmission in the hippocampus decreases GAP-43 mRNA expression. In the present study, although the magnitude of decrease in unstressed control group by repeated citalopram treatment was small, a two-way ANOVA test revealed a significant main effect of repeated citalopram treatment on the decrease of GAP-43 mRNA levels in all subfields of the hippocampus. A similar trend of decrease in GAP-43 mRNA expression in CA1 and CA3 has also been observed after chronic treatment with fluvoxamine and fluoxetine (Iwata et al, 2006; Larsen et al, 2008). Considering the facts that chronic treatment with citalopram (10 mg/kg) significantly increases serotonin release in the hippocampus (Invernizzi et al, 1995) and that repeated treatments with citalopram, fluoxetine and fluvoxamine are required to observe a decrease of GAP-43 mRNA expression, increased serotonergic neurotransmission in the hippocampus for a certain period of time would result in a decrease of GAP-43 mRNA levels in the hippocampus. On the contrary, however, decreased serotonergic transmission in the hippocampus may lead to an increase of GAP-43 mRNA expression. For example, adrenalectomy, which is known to decrease serotonin turnover in the hippocampus, increased GAP-43 mRNA expression in the hippocampus (De Kloet et al, 1982; Korte-Bouws et al, 1996). In addition, repeated daily exposure to the stressor increased basal extracellular serotonin release in the hippocampus, suggesting that repeated stress elicits a sustained increase of serotonin release and turnover in the hippocampus (Torres et al, 2002; Storey et al, 2006). In accordance with this result, there was a trend toward an increase of serotonin release in the hippocampus of CMS-exposed rats (Bekris et al, 2005) with some inconsistency (Kang et al, 2005). Although there are some controversies, with regard to acute stress, accumulating evidence has demonstrated that acute stress does not change or decrease serotonin release in the hippocampus (Kirby et al, 1995; Kirby et al, 1997; Mokler et al, 2007), further reinforcing the view that decreased serotonergic transmission in the hippocampus may lead to increased GAP-43 mRNA expression.
Alternatively, GAP-43 mRNA expression is also regulated by glutamate and its receptors. For example, MK801 treatment, NMDA receptor antagonist, significantly decreased GAP-43 mRNA expression in hippocampal neurons in vitro (Hirai et al, 2005), strengthening the notion that GAP-43 mRNA expression is influenced by neuronal activity. Interestingly, chronic unpredictable stress for 3 weeks significantly reduced NR2B subunit of NMDA receptor in the GCL without altering other subunits of NMDA and AMPA receptors (Qin et al, 2004). Similar decreases in NR1, NR2A and NR2B subunits of NMDA receptor in the hippocampus were observed also in chronic restraint stress-exposed mice (Pawlak et al, 2005). Since changes in GAP-43 mRNA expression closely follow those of NMDA receptors (Pawlak et al, 2005), it is quite possible that decrease of GAP-43 mRNA expression in GCL is likely to be a consequence of the decrease of NMDA receptors. Conversely, acute immobilization stress increased NR1 subunit of NMDA receptor in CA1 and CA3 areas of the hippocampus, however, it is not clear whether acute stress changes glutamate receptors in the GCL, because Bartanusz et al (1995) did not examine the changes in glutamate receptors in the GCL. Similar to chronic stress, chronic treatment of mice with citalopram significantly reduced NR2 subunits in the hippocampus (Boyer et al, 1998). Consistent with the above result, chronic imipramine treatment of rats induced a significant reduction in the density of NMDA receptor binding sites without altering NMDA receptor binding affinity in the hippocampus (Harvey et al, 2002). These results implicate complex interaction between serotonergic transmission and NMDA receptor in the regulation of GAP-43 mRNA expression in the hippocampus.
As accumulating evidence indicates that GAP-43 plays a key role in guiding axonal growth and modulating the formation of new neuronal connections (Benowitz & Routtenberg, 1997), decreased GAP-43 mRNA expression in the GCL may be accompanied by reduced mossy fiber axonal growth. Indeed, chronic unpredictable stress induces an atrophy of mossy fiber terminal, as evidenced by the marked reduction in the total number of mossy fiber terminal-CA3 synapses and a reduction in the volume and surface area of the mossy fiber terminal (Sousa et al, 2000). The changes of mossy fiber terminal following chronic unpredictable stress are prominent at stratum lucidum, where the synaptic contacts between mossy fiber terminals and CA3 pyramidal cells are observed (Stewart et al, 2005). By contrast, repeated fluoxetine treatment of rats did not alter mossy fiber sprouting, which was evaluated by Timm staining (Lamont et al, 2001). As there are only a few earlier studies on the effect of repeated citalopram treatment on the ultrastructual changes in mossy fiber terminal in the hippocampus, it is possible that repeated citalopram treatment may have some subtle effects on mossy fiber terminal, therefore, ultrastructual analysis has as yet failed to detect. Further studies are needed to elucidate the ultrastructual changes in the hippocampus following the chronic treatment with antidepressants.
Taken together, the present study demonstrates that acute stress significantly increased GAP-43 mRNA levels in all subfields of the hippocampus. However, CMS significantly decreased GAP-43 mRNA levels in the GCL, suggesting that GAP-43 mRNA expression responds differently to acute and chronic stress. Interestingly, repeated citalopram treatment decreased GAP-43 mRNA levels in the GCL in unstressed controls, but this decrease was not further potentiated by CMS exposure. Similar decreases of GAP-43 mRNA expression were observed also in CA1, CA3 and CA4 areas of the hippocampus only after repeated treatment of CMS-exposed rats with citalopram. Together with previous morphologic studies, this result suggests parallel changes of GAP-43 mRNA expression and axonal remodeling following stress, and that repeated citalopram treatment did not change CMS-induced GAP-43 mRNA expression in the hippocampus.
ACKNOWLEDGEMENTS
This work was supported by the Korean Ministry of Health and Welfare [Korea Health 21 R&D Project (KPGRN-R-04-04)] and Korea Science and Engineering Foundation [KOSEF(R13-2003-016-02004-0)] grants funded by the Korea government (MOST).
ABBREVIATIONS
- GAP-43
growth associated protein-43
- CMS
chronic mild stress
- GCL
dentate granule cell layer
- PPB
paraformaldehyde in 0.1 M sodium phosphate buffer
- DTT
dithiothreitol
- ANOVA
one-way analysis of variance
References
- 1.Bartanusz V, Aubry JM, Pagliusi S, Jezova D, Baffi J, Kiss JZ. Stress-induced changes in messenger RNA levels of N-methyl-D-aspartate and AMPA receptor subunits in selected regions ofthe rat hippocampus and hypothalamus. Neuroscience. 1995;66:247–252. doi: 10.1016/0306-4522(95)00084-v. [DOI] [PubMed] [Google Scholar]
- 2.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stressapplied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Bendotti C, Baldessari S, Pende M, Southgate T, Guglielmetti F, Samanin R. Relationship between GAP-43 expression in the dentate gyrus and synaptic reorganization of hippocampal mossy fibres in rats treated with kainic acid. Eur J Neurosci. 1997;9:93–101. doi: 10.1111/j.1460-9568.1997.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant ofneuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 5.Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 1998;10:219–233. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- 6.Cantallops I, Routtenberg A. Rapid induction by kainic acid of both axonal growth and F1/GAP-43 protein in the adult rat hippocampal granule cells. J Comp Neurol. 1996;366:303–319. doi: 10.1002/(SICI)1096-9861(19960304)366:2<303::AID-CNE9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Wang JF, Sun X, Young LT. Regulation of GAP-43 expression by chronic desipramine treatment in rat cultured hippocampal cells. Biol Psychiatry. 2003;53:530–537. doi: 10.1016/s0006-3223(02)01551-2. [DOI] [PubMed] [Google Scholar]
- 8.De Kloet ER, Kovacs GL, Szabo G, Telegdy G, Bohus B, Versteeg DH. Decreased serotonin turnover in the dorsal hippocampus of rat brain shortly after adrenalectomy: selective normalization after corticosterone substitution. Brain Res. 1982;239:659–663. doi: 10.1016/0006-8993(82)90546-7. [DOI] [PubMed] [Google Scholar]
- 9.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17(Suppl 3):306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- 10.Ferrini M, Bisagno V, Piroli G, Grillo C, Deniselle MC, De Nicola AF. Effects of estrogens on choline-acetyltransferase immunoreactivity and GAP-43 mRNA in the forebrain of young and aging male rats. Cell Mol Neurobiol. 2002;22:289–301. doi: 10.1023/A:1020767917795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--I. Basic pharmacology. J Psychopharmacol. 1998;12:S5–S20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- 13.Gronli J, Fiske E, Murison R, Bjorvatn B, Sorensen E, Ursin R, Portas CM. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav Brain Res. 2007;181:42–51. doi: 10.1016/j.bbr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Harvey BH, Jonker LP, Brand L, Heenop M, Stein DJ. NMDA receptor involvement in imipramine withdrawal-associated effects on swim stress, GABA levels and NMDA receptor binding in rat hippocampus. Life Sci. 2002;71:43–54. doi: 10.1016/s0024-3205(02)01561-8. [DOI] [PubMed] [Google Scholar]
- 15.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 16.Hirai T, Taniura H, Goto Y, Tamaki K, Oikawa H, Kambe Y, Ogura M, Ohno Y, Takarada T, Yoneda Y. Counteraction by repetitive daily exposure to static magnetism against sustained blockade of N-methyl-D-aspartate receptor channels in cultured rat hippocampal neurons. J Neurosci Res. 2005;80:491–500. doi: 10.1002/jnr.20497. [DOI] [PubMed] [Google Scholar]
- 17.Invernizzi R, Bramante M, Samanin R. Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 1995;696:62–66. doi: 10.1016/0006-8993(95)00730-e. [DOI] [PubMed] [Google Scholar]
- 18.Iwata M, Shirayama Y, Ishida H, Kawahara R. Hippocampal synapsin I, growth-associated protein-43, and microtubule-associated protein-2 immunoreactivity in learned helplessness rats and antidepressant-treated rats. Neuroscience. 2006;141:1301–1313. doi: 10.1016/j.neuroscience.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 19.Kang M, Pyun KH, Jang CG, Kim H, Bae H, Shim I. Nelumbinis Semen reverses a decrease in hippocampal 5-HT release induced by chronic mild stress in rats. J Pharm Pharmacol. 2005;57:651–656. doi: 10.1211/0022357056055. [DOI] [PubMed] [Google Scholar]
- 20.Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- 21.Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- 22.Korte-Bouws GA, Korte SM, De Kloet ER, Bohus B. Blockade of corticosterone synthesis reduces serotonin turnover in the dorsal hippocampus of the rat as measured by microdialysis. J Neuroendocrinol. 1996;8:877–881. doi: 10.1046/j.1365-2826.1996.05389.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda Y, McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res. 1998;59:35–39. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- 24.Laifenfeld D, Karry R, Grauer E, Klein E, Ben-Shachar D. Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol Dis. 2005;20:432–441. doi: 10.1016/j.nbd.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Lamont SR, Paulls A, Stewart CA. Repeated electroconvulsive stimulation, but not antidepressant drugs, induces mossy fibre sprouting in the rat hippocampus. Brain Res. 2001;893:53–58. doi: 10.1016/s0006-8993(00)03287-x. [DOI] [PubMed] [Google Scholar]
- 26.Larsen MH, Hay-Schmidt A, Ronn LC, Mikkelsen JD. Temporal expression of brain-derived neurotrophic factor (BDNF) mRNA in the rat hippocampus after treatment with selective and mixed monoaminergic antidepressants. Eur J Pharmacol. 2008;578:114–122. doi: 10.1016/j.ejphar.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 27.Manji HK, Moore GJ, Rajkowska G, Chen G. Neuroplasticity andcellular resilience in mood disorders. Mol Psychiatry. 2000;5:578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 28.McGuire CB, Snipes GJ, Norden JJ. Light-microscopic immunolocalization of the growth- and plasticity-associated protein GAP-43 in the developing rat brain. Brain Res. 1988;469:277–291. doi: 10.1016/0165-3806(88)90189-7. [DOI] [PubMed] [Google Scholar]
- 29.Mennini T, Mocaer E, Garattini S. Tianeptine, a selective enhancer of serotonin uptake in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:478–482. doi: 10.1007/BF00169302. [DOI] [PubMed] [Google Scholar]
- 30.Miyake K, Yamamoto W, Tadokoro M, Takagi N, Sasakawa K, Nitta A, Furukawa S, Takeo S. Alterations in hippocampal GAP-43, BDNF, and L1 following sustained cerebral ischemia. Brain Res. 2002;935:24–31. doi: 10.1016/s0006-8993(02)02420-4. [DOI] [PubMed] [Google Scholar]
- 31.Mokler DJ, Torres OI, Galler JR, Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res. 2007;1148:226–233. doi: 10.1016/j.brainres.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulationbehavior in rats. Eur Neuropsychopharmacol. 1992;2:43–49. doi: 10.1016/0924-977x(92)90035-7. [DOI] [PubMed] [Google Scholar]
- 33.Naffah-Mazzacoratti MG, Funke MG, Sanabria ER, Cavalheiro EA. Growth-associated phosphoprotein expression is increased in thesupragranular regions of the dentate gyrus following pilocarpine-induced seizures in rats. Neuroscience. 1999;91:485–492. doi: 10.1016/s0306-4522(98)00631-9. [DOI] [PubMed] [Google Scholar]
- 34.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 35.Ni YG, Gold SJ, Iredale PA, Terwilliger RZ, Duman RS, Nestler EJ. Region-specific regulation of RGS4 (Regulator of G-protein-signaling protein type 4) in brain by stress and glucocorticoids: in vivo and in vitro studies. J Neurosci. 1999;19:3674–3680. doi: 10.1523/JNEUROSCI.19-10-03674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papp M, Nalepa I, Antkiewicz-Michaluk L, Sanchez C. Behavioural and biochemical studies of citalopram and WAY 100635 in ratchronic mild stress model. Pharmacol Biochem Behav. 2002;72:465–474. doi: 10.1016/s0091-3057(01)00778-x. [DOI] [PubMed] [Google Scholar]
- 37.Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 38.Pawlak R, Rao BS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in themouse hippocampus. Proc Natl Acad Sci U S A. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- 40.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 41.Qin Y, Karst H, Joels M. Chronic unpredictable stress alters gene expression in rat single dentate granule cells. J Neurochem. 2004;89:364–374. doi: 10.1111/j.1471-4159.2003.02332.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 43.Sairanen M, O'Leary OF, Knuuttila JE, Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 44.Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 45.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233:783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- 47.Snipes GJ, Chan SY, McGuire CB, Costello BR, Norden JJ, Freeman JA, Routtenberg A. Evidence for the coidentification of GAP-43, a growth-associated protein, and F1, a plasticity-associated protein. J Neurosci. 1987;7:4066–4075. doi: 10.1523/JNEUROSCI.07-12-04066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 49.Stahl SM. Using secondary binding properties to select a not so selective serotonin reuptake inhibitor. J Clin Psychiatry. 1998;59:642–643. doi: 10.4088/jcp.v59n1201. [DOI] [PubMed] [Google Scholar]
- 50.Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL, Popov VI. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 51.Storey JD, Robertson DA, Beattie JE, Reid IC, Mitchell SN, Balfour DJ. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res. 2006;166:220–229. doi: 10.1016/j.bbr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- 53.Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002;27:519–525. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- 54.Watterson JM, Watson DG, Meyer EM, Lenox RH. A role for protein kinase C and its substrates in the action of valproic acid in the brain: implications for neural plasticity. Brain Res. 2002;934:69–80. doi: 10.1016/s0006-8993(02)02362-4. [DOI] [PubMed] [Google Scholar]