Abstract
In order to reproduce chronic cerebral hypoperfusion as it occurs in human aging and Alzheimer's disease, we introduced permanent, bilateral occlusion of the common carotid arteries (BCCAO) in rats (Farkas et al, 2007). Here, we induced BCCAO in two different rat strains in order to determine whether there was a strain difference in the pathogenic response to BCCAO. Male Wistar and Sprague-Dawley (SD) rats (250-270 g) were subjected to BCCAO for three weeks. Klüver-Barrera and cresyl violet staining were used to evaluate white matter and gray matter damage, respectively. Wistar rats had a considerably higher mortality rate (four of 14 rats) as compared to SD rats (one of 15 rats) following BCCAO. Complete loss of pupillary light reflex occurred in all Wistar rats that survived, but loss of pupillary light reflex did not occur at all in SD rats. Moreover, BCCAO induced marked vacuolation in the optic tract of Wistar rats as compared to SD rats. In contrast, SD rats showed fewer CA1 hippocampal neurons than Wistar rats following BCCAO. These results suggest that the neuropathological process induced by BCCAO takes place in a region-specific pattern that varies according to the strain of rat involved.
Keywords: Chronic cerebral hypoperfusion, Wistar strain, Sprague-Dawley strain, White matter damage, Hippocampal neurodegeneration, Rat
INTRODUCTION
Vascular dementia (VaD) is the second most common type of dementia following Alzheimer's disease-related dementia (Rockwood et al, 2000). VaD occurs when the blood supply to the brain is reduced by a blocked or diseased vascular system (Roman et al, 2002) and leads to a progressive decline in memory and cognitive function (Jellinger et al, 2007). Pathological features of VaD are diffuse myelin pallor, astrocytic gliosis, and the loss of oligodendrocytes leading to rarefaction, vacuolization, and the loss of myelin and axons without definite necrosis, ultimately culminating in white matter lesions and lacunes (Roman et al, 2002).
Chronic cerebral hypoperfusion can be induced by permanent, bilateral occlusion of common carotid arteries (BCCAO) in rats, resulting in significant white matter lesions, learning and memory impairment, and hippocampal neuronal damage (Tsuchiya et al, 1993; Sarti et al, 2002). Thus, BCCAO in rats provides a model useful for understanding the pathophysiology of chronic cerebrovascular hypoperfusion and for screening drugs with potential therapeutic value for VaD (Wakita et al, 1994). Rats are suitable species for this purpose because their complete circle of Willis affords constant blood flow after the onset of BCCAO (Farkas et al, 2007). However, to date, the reason why different strains of rats are used to study various aspects of chronic cerebral hypoperfusion has remained obscure. Wistar rats have been used to investigate BCCAO-induced white matter lesions (Nakaji et al, 2006; Watanabe et al, 2006), whereas Sprague-Dawley (SD) rats have been adopted to evaluate the hippocampal neuronal damage and cognitive impairment after BCCAO (Pappas et al, 1996; Farkas et al, 2006; Liu et al, 2007).
Therefore, we studied Wistar and SD rats to determine whether there was a strain difference in the response to BCCAO in terms of mortality, pupillary light reflex (PLR), and neurodegeneration.
METHODS
Animals and surgery
All animal procedures were approved by the Ethics Committee of the Catholic University of Korea and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). Chronic cerebral hypoperfusion was induced in male Wistar-ST rats (Joongang Lab, Seoul, Korea) and male SD rats (Koatech, Kyungki-do, Korea) weighing 250~270 g, as previously described with minor modifications (Cho et al, 2006). Briefly, after induction with 4% halothane, rats were anesthetized with 1.5% halothane in a 70% nitrous oxide and 30% oxygen mixture using a face mask; anesthesia continued throughout the surgical procedure. A midline incision was made and both common carotid arteries were exposed; care was taken to avoid the vagus nerves. The carotid arteries were double ligated using silk sutures. With the exception of occlusion of the carotid arteries, surgical procedures in sham-operated animals were the same as those in the BCCAO-operated animals. During surgery, rectal temperature was maintained at 37.0~37.9℃ with a heating pad. After the operation, all animals were returned to their cages with free access to food and water. The mortality rate within 48 h of BCCAO was recorded (Ni et al, 1994). On the 21st day after surgery (BCCAO induction or sham) the animals were euthanized with 15% chloral hydrate. Body weight was measured before the surgery and on the day of euthanasia.
Pupillary light reflex
Direct and indirect PLR was examined before the surgery and once per day for seven days after surgery. Each animal was allowed to adapt to darkness for 1 min prior to the initiation of an examination. Then a light was directed to the right eye for evaluating the direct PLR. Subsequently, the beam was moved quickly to the left eye to assess the indirect reflex. Then, the rat was allowed to readapt to the dark for 1 min and the procedure was repeated for the left eye. PLR loss was defined as the failure of a pupil to constrict within a 10 sec exposure to light (Lavinsky et al, 2006).
Tissue preparation
To obtain tissue specimens for histological analysis, animals were transcardially perfused with normal saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffered solution (pH 7.4). After decapitation, the whole brain was post-fixed with 4% paraformaldehyde for 4 h. Then, the brain samples were dehydrated with 30% sucrose in 0.1 M phosphate buffer, embedded in Tissue-Tek (Sakura Fine-technical, Tokyo, Japan), and rapidly frozen with liquid nitrogen. The brain was sectioned on a cryotome, and 20-µm sections were used for assessing neuronal injury and white matter damage.
Klüver-Barrera staining
The white matter region investigated in this study was located at -2.64 to -3.12 mm from the bregma, according to the atlas of Paxinos and Watson (2007). The tissue sections were incubated with 0.1% Luxol fast blue (Sigma, St. Louis, MO, USA) at 56℃ overnight. Then, the slides were soaked in 0.05% lithium carbonate solution, distilled water, and 70% ethanol. Finally, the slides were dehydrated in absolute ethanol, cleared in xylene, and mounted. Lesion severity was evaluated by an examiner who was blinded to the experimental conditions and was graded as normal (grade 0), disarrangement of nerve fibers (grade 1), formation of marked vacuoles (grade 2), and disappearance of myelinated fibers (grade 3; Wakita et al, 1998)
Assessment of neuronal damage
Neuronal damage was visualized by cresyl violet staining. Slides were hydrated in serial concentrations from absolute alcohol to tap water and incubated in 0.1% cresyl violet solution for 20 min. Excessive stain was removed by briefly immersing the slides in 95% alcohol solution and followed by dehydration. After clearing in two serial concentrations of xylene solution, the sections were mounted with Canada balsam and observed under light microscopy (BX51, Olympus, Tokyo, Japan). Every fifth section (i.e., a total of three) was processed for cresyl violet staining. In each CA1 subfield of the left hippocampus, we counted the number of pyramidal cells within a 1.35-mm length from the beginning of the CA1 pyramidal cells that showed a distinct nucleus and nucleolus (×400 magnification; Kuang et al, 2007).
Statistical analysis
Data are expressed as the mean ± the standard deviation of the mean (SD). Statistical analysis was performed by one-way analysis of the variance (ANOVA) followed by Tukey's post-hoc test. Differences were designated as significant when p<0.05.
RESULTS
Mortality rate due to BCCAO
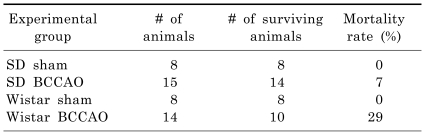
As shown in Table 1, four of 14 Wistar rats subjected to BCCAO died due to ischemic seizure within 48 h of BCCAO induction. Meanwhile, 14 of 15 SD rats survived for up to three weeks post BCCAO induction. Most of the surviving rats recovered within seven days of the operation and displayed no evidence of ingestion difficulty or marked motor impairment.
Table 1.
Mortality following permanent bilateral occlusion of common carotid arteries (BCCAO) in Sprague-Dawley (SD) and Wistar rats
The mortality rate within 48 h of BCCAO induction was recorded. SD Sham, SD rats subjected to sham operation; SD BCCAO, SD rats subjected to BCCAO for three weeks; Wistar sham, Wistar rats subjected to sham operation; Wistar BCCAO, Wistar rats subjected to BCCAO for three weeks.
Pupillary light reflex
Before BCCAO, all Wistar and SD rats exhibited intact PLRs. However, all Wistar rats that survived BCCAO eventually lost the PLR in both eyes, whereas all surviving SD rats showed intact PLRs in both eyes. The PLR loss was detectable by one-day post BCCAO induction and remained unchanged throughout the experiment (Table 2).
Table 2.
The loss of pupillary light reflex (PLR) after bilateral occlusion of common carotid arteries (BCCAO) in Sprague-Dawley (SD) and Wistar rats
Direct and indirect PLR were examined before the surgery and once per day for seven days after the procedure. SD Sham, SD rats subjected to sham operation; SD BCCAO, SD rats subjected to BCCAO for three weeks; Wistar sham, Wistar rats subjected to sham operation; Wistar BCCAO, Wistar rats subjected to BCCAO for three weeks.
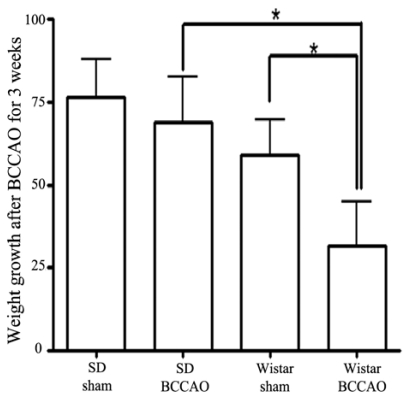
Weight gain
Fig. 1 shows the mean weight gain during the three weeks of BCCAO based on the pre-operative weight. The weights of SD rats subjected to three weeks of BCCAO caught up with the weights of sham-operated SD animals by the end of the experiment (weight gained over three weeks: SD sham, 76.63±11.38 g; SD BCCAO, 63.86±13.81 g). However, Wistar rats subjected to BCCAO failed to attain the sham-operated animal weight gain within three weeks (Wistar sham: 58.88±10.62 g; Wistar BCCAO: 31.70±13.00; p<0.05). Thus, weight gain in Wistar rats subjected to BCCAO is protracted as compared to that of SD rats subjected to BCCAO.
Fig. 1.
The difference in weight gain between Wistar and Sprague-Dawley (SD) rats after bilateral common carotid artery occlusion (BCCAO). Wistar rats failed to catch up to the weights of sham-operated animals during 21 days of BCCAO. Data reported are means ± SD. *p<0.05 by one-way ANOVA.
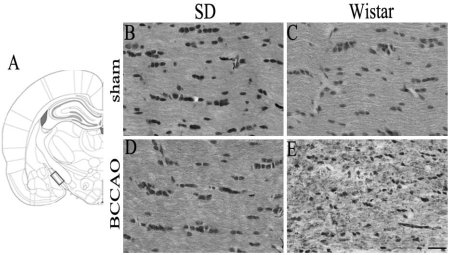
Klüver-Barrera staining for white matter damage after BCCAO
No white matter damage was detected in sham-operated Wistar and SD rats (Fig. 2B and C). However, when examined at three weeks post BCCAO induction, Wistar rats showed an increase in vacuolation and disarrangement of myelin fibers in the optic tract, indicating severe rarefaction, whereas SD rats had an almost intact optic tract (Fig. 2D and E).
Fig. 2.
Klüver-Barrera staining for white matter damage after 21 days of bilateral common carotid artery occlusion (BCCAO). Schematic representation of the rat optic tract (A). The rectangle identifies the section of the optic tract evaluated in this study (B-E). Sections were taken from (2.92 mm relative to bregma in the anteroposterior plane (adapted from Paxinos and Watson, 2007). Optic tract of Sprague-Dawley (SD) rats subjected to sham operation (B) or BCCAO (D). Optic tract of Wistar rats subjected to sham operation (C) or BCCAO (E). Note that BCCAO caused more serious white matter damage in the optic tract of Wistar rats than in that of SD rats. Scale bar=50 µm.
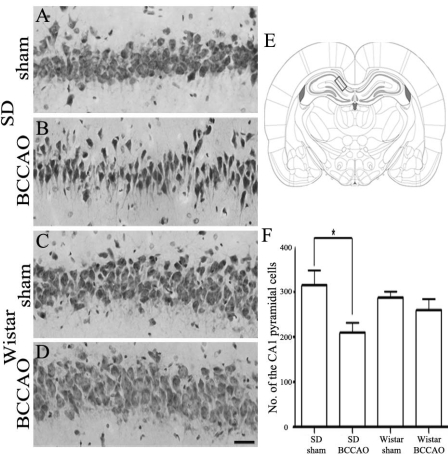
Cresyl violet staining for hippocampal neuronal damage after BCCAO
Fig. 3 shows representative photomicrographs of cresyl violet staining in the hippocampal CA1 at day-21 of BCCAO. There were no infarctions or hemorrhages in any of the brain areas examined in either Wistar or SD rats. No neuronal death was seen in the hippocampus of either sham-operated rat strains (Fig. 3A, C, and F). However, three weeks post BCCAO induction, SD rats showed significant neuronal loss, neuronal shrinkage, and marked vacuolar changes in CA1 areas of the hippocampus (Fig. 3B). SD rats displayed a greater decrease in the number of pyramidal cells in the hippocampal CA1 subfield (SD sham: 314.4±34.23 vs. SD BCCAO: 210.1±21.79 cells) as compared to Wistar rats after BCCAO (Wistar sham: 287.9±13.43 vs. Wistar BCCAO: 259.7±25.01; Fig. 3F).
Fig. 3.
Cresyl violet staining of the hippocampal CA1 damage after 21 days of bilateral common carotid artery occlusion (BCCAO). Hippocampal CA1 lesion of Sprague-Dawley (SD) rats subjected to sham operation (A) or BCCAO (B). Hippocampal CA1 region of Wistar rats subjected to sham operation (C) or BCCAO (D). Schematic representation of the rat hippocampal CA1 region (E, adapted from Paxinos and Watson, 2007). The rectangle indicates the area of the brain that was examined in this study. The number of cells in the hippocampal CA1 region in SD and Wistar rats subjected to sham operation or BCCAO (F). Note that BCCAO induced significantly more pyramidal neuronal damage in CA1 subfield of the hippocampus of SD rats than in that of Wistar rats. There was no difference in the number of CA1 pyramidal neurons between sham-operated and BCCAO-induced Wistar rats. The data shown are the mean number of intact pyramidal neurons (±SD). Scale bar in A-D = 50 µm. *p<0.05 by one-way ANOVA.
DISCUSSION
VaD can be studied using a BCCAO model that induces cerebral hypoperfusion, learning and memory impairments, white matter damage, and neuropathological changes in the hippocampus (Wakita et al, 1994; Farkas et al, 2007). In this study, two different strains (Wistar and SD) of rats were used to investigate the pathophysiology of VaD. By convention, Wistar rats are used to study the white matter lesions that can result from chronic cerebral hypoperfusion associated with BCCAO (Nakaji et al, 2006; Watanabe et al, 2006), and SD rats are used as a model for BCCAO-associated hippocampal neuronal damage and cognitive impairment (Pappas et al, 1996; Farkas et al, 2006; Liu et al, 2007). However, to date, the reason why these different strains of rats exhibit different histological pathology due to chronic cerebral hypoperfusion has been unclear. In order to investigate this strain-specific discrepancy, we compared the effects of inducing BCCAO in these two rat strains.
Chronic cerebral hypoperfusion causes hippocampal neurodegeneration (Pappas et al, 1996; Bennett et al, 1998). The most obvious signs of neurodegeneration are the loss of neuronal cell bodies and synaptic contacts (Farkas et al, 2007). In this study, we found that the pyramidal neurons in the CA1 subfield of the hippocampus were degenerated only in SD rats when examined three weeks post BCCAO induction. Similarly, other authors have reported that two weeks of BCCAO induced damage to the CA1 hippocampal neurons in SD rats (Pappas et al, 1996; Bennett et al, 1998). On the other hand, the majority of the studies using Wistar rats have detected no hippocampal damage following BCCAO (Sarti et al, 2002; Schmidt et al, 2005). Moreover, the few studies claiming hippocampal damage due to BCCAO in Wistar rats reported that histological changes occurred at least two months after the onset of injury (Ni et al, 1995; Liu et al, 2005). Thus, for investigating the neuronal damage caused by chronic cerebral hypoperfusion within one month of injury onset, SD rats appear to be more useful than Wistar rats.
By tradition, Wistar rats have been used to study white matter lesions resulting from BCCAO. Studies have shown that one of the white matter regions most vulnerable to chronic cerebral hypoperfusion is the optic tract, which exhibits the most severe rarefaction. The medial corpus callosum and the internal capsule are similarly sensitive, but show less intense fiber derangement (Wakita et al, 1998; 2002). That the optic tract exhibits the most severe histopathology in response to BCCAO can be attributed to the dependence of the optic tract on the direct blood supply from the internal carotid artery (Pantoni et al, 1996; Ohta et al, 1997; Butte et al, 2002; Wakita et al, 2002), which fits nicely with our observations of perfect PLR loss in Wistar rats following BCCAO. BCCAO can result in visual impairment, retinal degeneration, and atrophy of the optic nerve in rats (Stevens et al, 2002). In addition, damage to the optic nerve has been reported to be an early event and is known to be directly caused by ischemia (Davidson et al, 2000), which coincides with our observation of PLR loss within 48 h of BCCAO onset. This observation might be rather discouraging for researchers who investigate BCCAO-induced cognitive dysfunction using Wistar rats because spatial memory tests, such as the Morris water maze or the radial arm maze, are based on visual cues. Therefore, maze tests with visual cues cannot differentiate between cognitive and visual impairments after BCCAO. However, behavioral tests with non-visual cues, such as the elevated T-maze, object recognition test, or radial arm maze test with auditory or tactile stimuli, would allow researchers to examine BCCAO-induced cognitive impairment. Taken together, these data suggest that investigators should use caution when evaluating cognitive deficits caused by BCCAO in Wistar rats.
The mortality rate after BCCAO varies according to the strain or the age of the rats. In the case of Wistar rats, researchers have reported mortality rates ranging between 19.2% and 25%, comparable to the 29% mortality observed in this study (Hirabayashi et al, 2004; Liu et al, 2005). For SD rats, Sopala et al (2001) reported a 7% (2/27) mortality rate following BCCAO, which agrees with our observed rate of 7% (1/15) at three weeks post BCCAO induction. Intriguingly, we found a difference between SD and Wistar rats with regard to body weight recovery after BCCAO induction. Wistar rats failed to attain the sham-operated animals' weight gain after three weeks of BCCAO, whereas SD rats did. Similarly, Hirabayashi et al (2004) reported that the weight gain in Wistar rats during four weeks of BCCAO was significantly lower than that of the sham group, which implies perturbed nutritional intake behavior in rats with BCCAO. However, further studies are required to address the mechanism underlying the observed difference between the two strains.
In summary, we demonstrated higher mortality rate and lower weight gain in Wistar rats as compared to SD rats following the induction of BCCAO. In addition, we showed that there were differences between the strains in the neuropatholgical responses to three weeks of BCCAO. White matter damage occurred in the Wistar strain, whereas hippocampal neuronal damage occurred in the SD strain. Further, BCCAO-induced impairment of PLR was observed only in Wistar rats and was related to damage to their optic tracts. In conclusion, our results suggest that Wistar and SD strain rats are appropriate BCCAO rat models for investigating white matter damage and hippocampal neurodegeneration related to chronic cerebral hypoperfusion, respectively.
ACKNOWLEDGEMENT
This study was supported by a grant of the Biomedical Brain Research Center of Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (A04-0042-A21004-07A4-00090B).
ABBREVIATIONS
- BCCAO
bilateral occlusionof the common carotid artery
- PLR
pupillarylight reflex
- SD rat
Sprague-Dawley rat
- VaD
vascular dementia
References
- 1.Butte DM, Fortin T, Pappas BA. Pinealectomy: behavioral and neuropathological consequences in a chronic cerebral hypoperfusion model. Neurobiol Aging. 2002;23:309–317. doi: 10.1016/s0197-4580(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 2.Bennett SA, Tenniswood M, Chen JH, Davidson CM, Keyes MT, Fortin T, Pappas A. Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. Neuroreport. 1998;9:164–166. doi: 10.1097/00001756-199801050-00033. [DOI] [PubMed] [Google Scholar]
- 3.Cho KO, La HO, Cho YJ, Sung KW, Kim SY. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J Neurosci Res. 2006;83:285–291. doi: 10.1002/jnr.20727. [DOI] [PubMed] [Google Scholar]
- 4.Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SAL. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 2000;859:96–103. doi: 10.1016/s0006-8993(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 5.Farkas E, Institoris A, Domoki F, Mihaly A, Bari F. The effect of pre- and post-treatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res. 2006;1087:168–174. doi: 10.1016/j.brainres.2006.02.134. [DOI] [PubMed] [Google Scholar]
- 6.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Hirabayashi H, Kurita D, Takizawa S, Shinohara Y. Phosphate-related energy compounds are not exhausted in chronically hypoperfused rat brain cortex after cortical spreading depression. J Stroke Cerebrovasc Dis. 2004;3:271–279. doi: 10.1016/j.jstrokecerebrovasdis.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007;113:349–388. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 9.Kuang X, Du JR, Liu YX, Zhang GY, Peng HY. Postischemic administration of Z-Ligustilide ameliorates cognitive dysfunction and brain damage induced by permanent forebrain ischemia in rats. Pharmacol Biochem Behav. 2008;88:213–221. doi: 10.1016/j.pbb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Lavinsky D, Arterni NS, Achaval M, Netto CA. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefes Arch Clin Exp Ophthalmol. 2006;244:199–204. doi: 10.1007/s00417-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Wu J, Gu J, Xiong Z, Wang F, Wang J, Wang W, Chen J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2007;86:423–430. doi: 10.1016/j.pbb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Liu HX, Zhang JJ, Zheng P, Zhang Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res. 2005;139:169–177. doi: 10.1016/j.molbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Nakaji K, Ihara M, Takahashi C, Itohara S, Noda M, Takahashi R, Tomomoto H. Matrix metalloproteinase-2 plays a critical role in the pathogenesis of white matter lesions after chronic cerebral hypoperfusion in rodents. Stroke. 2006;37:2816–2823. doi: 10.1161/01.STR.0000244808.17972.55. [DOI] [PubMed] [Google Scholar]
- 14.Ni JW, Matsumoto K, Li HB, Murakami Y, Watanabe H. Neuronal damage and decrease of centeral acetylcholine level following permanent occlusion of bilateral common carotid arteries in rat. Brain Res. 1995;673:290–296. doi: 10.1016/0006-8993(94)01436-l. [DOI] [PubMed] [Google Scholar]
- 15.Ni JW, Ohta H, Matsumoto K, Watanabe H. Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res. 1994;653:231–236. doi: 10.1016/0006-8993(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 16.Ohta H, Nishikawa H, Kimura H, Anayama H, Miyamoto M. Chronic cerebral hypoperfusion by permanent internal carotid ligation produces learning impairment without brain damage in rat. Neuroscience. 1997;79:1039–1050. doi: 10.1016/s0306-4522(97)00037-7. [DOI] [PubMed] [Google Scholar]
- 17.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 18.Pappas BA, Torre JC, Davidson CM, Keyes MT, Fortin T. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res. 1996;708:50–58. doi: 10.1016/0006-8993(95)01267-2. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Burlington: Academic Press; 2007. [Google Scholar]
- 20.Rockwood K, Wentzel C, Hachinski V, Hogan DB, MacKnight C, McDowell I. Prevalence and outcomes of vascular cognitive impairment. Neurology. 2000;54:447–451. doi: 10.1212/wnl.54.2.447. [DOI] [PubMed] [Google Scholar]
- 21.Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 22.Sarti C, Pantoni L, Bartolini L, Inzitari D. Cognitive impairment and chronic cerebral hypoperfusion: what can be learned from experimental models. J Neurol Sci. 2002;203-204:263–266. doi: 10.1016/s0022-510x(02)00302-7. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Kastner R, Aguirri-Chen C, Saul I, Yick L, Hamasaki D, Busto R, Ginsberg MD. Astrocytes react to oligemia in the forebrain induced by chronic bilateral common carotid artery occlusion in rat. Brain Res. 2005;1052:28–39. doi: 10.1016/j.brainres.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Sopala M, Danysz W. Chronic cerebral hypoperfusion in the rat enhances age-related deficits in spatial memory. J Neural Transm. 2001;108:1445–1456. doi: 10.1007/s007020100019. [DOI] [PubMed] [Google Scholar]
- 25.Stevens WD, Fortin T, Pappas BA. Retinal and optic nerve degeneration after chronic carotid ligation: time course and role of light exposure. Stroke. 2002;33:1107–1112. doi: 10.1161/01.str.0000014204.05597.0c. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya M, Sako K, Yura S, Yonemasu Y. Local cerebral glucose utilization following acute and chronic bilateral carotid artery ligation in Wistar rat: relation to change in local cerebral blood flow. Exp Brain Res. 1993;95:1–7. doi: 10.1007/BF00229648. [DOI] [PubMed] [Google Scholar]
- 27.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994;87:484–492. doi: 10.1007/BF00294175. [DOI] [PubMed] [Google Scholar]
- 28.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res. 1998;792:105–113. doi: 10.1016/s0006-8993(98)00126-7. [DOI] [PubMed] [Google Scholar]
- 29.Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, McGeer PL. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924:63–70. doi: 10.1016/s0006-8993(01)03223-1. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 2006;37:1539–1545. doi: 10.1161/01.STR.0000221783.08037.a9. [DOI] [PubMed] [Google Scholar]