Abstract
Adiponectin is one of the most clinically relevant cytokines associated with obesity. However, circadian rhythmicity of adiponectin in human adipose tissue (AT) has not been analyzed. To assess whether the mRNA levels of adiponectin and its receptors (ADIPOR1 and ADIPOR2) might show daily circadian rhythms in visceral and sc fat explants obtained from morbid obese women, visceral and sc abdominal AT biopsies (n = 6) were obtained from morbidly obese women (body mass index ≥40 kg/m2). Anthropometric variables were measured and fasting plasma glucose, lipid, and lipoprotein concentrations were analyzed. To investigate rhythmic expression pattern, AT explants were cultured during 24 h, and gene expression was analyzed at the following times: 0800, 1400, 2000, and 0200 h, using quantitative real-time PCR. All genes investigated showed a circadian rhythmicity and oscillated accurately and independently of the suprachiasmatic nucleus in both AT explants (P < 0.05). Adiponectin gene expression fluctuated in the same phase as its receptors. Correlation analyses between the genetic circadian oscillation and components of the metabolic syndrome revealed that adiposity and abdominal obesity correlated with a decrease in adiponectin and adiponectin receptors ADIPOR1 and ADIPOR2 amplitude (P < 0.05). Visceral fat showed a trend toward a phase delay and dampening of the mRNA amplitude of adiponectin as compared with sc fat. The mRNA expression of adiponectin and its receptors showed 24-h rhythmicity in human AT from morbidly obese patients.
Rhythm amplitude of circadian rhythmicity of adiponectin in human adipose tissue is related to metabolic syndrome features.
Metabolic syndrome (MetS) is becoming a major health challenge, with a quarter of the world adult population displaying one or more of its individual components. However, despite its widespread presence, the identification of its underlying causes continues to be a challenge. Recently some studies suggested that the disruption of the circadian system (chronodisruption) may be associated with manifestations of MetS (1). Thus, shift work, sleep deprivation, and exposure to bright light at night increase the prevalence of adiposity and MetS (2). These discoveries have resulted in new paradigms regarding the involvement of the circadian system in the regulation of multiple pathophysiological disturbances (2).
One of the most exciting recent findings in the area of chronobiology is that circadian clock genes exist in not only the brain but also peripheral tissues relevant to energy metabolism and cardiovascular functions, including the heart, liver, pancreas, and adipose tissue (3,4,5).
Adipose tissue (AT), present in several peripheral depots, acquires special relevance in the study of obesity and MetS. It is now known that AT is not a mere inert storage for triglycerides. In fact, adipocytes are currently seen as a dynamic endocrine cell type, releasing free-fatty acids, and secreting a number of factors or adipokines (6). The presence of active circadian clock mechanisms in this tissue has been recently published, and its periodic nature has been confirmed (5,7). The persistency of gene expression oscillations in vitro strongly suggests the existence of an intracellular circadian clock system directly regulating local cell functions.
In this context, adiponectin is one adipokine that has recently attracted much attention because it functions as a key modulator of insulin sensitivity (8,9) and a protective factor against MetS alterations (10,11). The beneficial effects of this hormone are predominantly mediated by binding to two cell membrane receptors, adiponectin receptor (ADIPOR)-1 and ADIPOR2 (12). ADIPOR1, a high-affinity receptor for globular adiponectin but a low-affinity receptor for full-length adiponectin, is most abundantly expressed in muscle, whereas ADIPOR2, an intermediate-affinity receptor for both full-length and globular adiponectin, is predominantly expressed in liver (12). Both receptors are also present in adipose tissue, suggesting that adiponectin may have biological effects in AT in an autocrine/paracrine manner (13).
The diurnal and ultradiurnal dynamics of circulating adiponectin concentrations have been demonstrated in humans (14) and also in AT from experimental animals (15). However, it remains to be characterized whether this cytokine exerts a circadian oscillation in human adipose tissue and whether this oscillation differs between visceral and subcutaneous fat depots.
In view of the above mentioned, the objective of the present research was to analyze in human adipose tissue the putative circadian rhythmicity of the expression of adiponectin and its two receptors, ADIPOR1 and ADIPOR2, as well as to assess the potential differences between two adipose depots, sc and visceral AT, in morbid obese female subjects.
Materials and Methods
Subjects
Visceral and sc abdominal AT biopsies were obtained from morbidly obese women (n = 6), aged 51 ± 9 yr with body mass index (BMI) 44.1 ± 5.5 kg/m2, undergoing laparoscopic gastric bypass surgery due to obesity at the General Surgery Service of Virgen de la Arrixaca Hospital (Murcia, Spain). The women studied were postmenopausal and were not under hormone replacement therapy. The day before surgery, all patients were synchronized having lunch at 1430 and dinner at 2100 h. The AT biopsies were taken as paired samples from the two AT depots (visceral and sc) at the beginning of the surgical procedure (estimated time of biopsies sampling at 1300 h).
The protocols were approved by the Ethics Committee of the Virgen de la Arrixaca University Hospital, and the subjects signed a written informed consent before the biopsies were obtained.
Clinical characteristics
Arterial pressure, BMI, and waist and hip circumference were assessed by standard procedures, whereas skinfolds (biceps, triceps, suprailiac, and subscapular) were measured with a Harpenden caliper (Holtain Ltd., Bryberian, Crymmych, Pembrokeshire, UK). Total body fat (percent) was evaluated by bioimpedance with a TBF-300 (Tanita Corp. of America, Arlington Heights, IL). Sagittal diameter and coronal diameter were measured at the level of the iliac crest (L4-5) using a Holtain Kahn abdominal caliper. Women were classified in visceral and sc obesity calculating the index visceral area (VA)/sc area (SA) after applying the following equation (16): VA/SApredicted = 0.868 + 0.064 × sagittal diameter − 0.036 × coronal diameter − 0.022 × triceps skinfold. Those patients with VA/SA greater than 0.42 were classified as having visceral obesity. Fasting plasma concentrations of glucose, triacylglycerols, total cholesterol, and high-density lipoprotein cholesterol were determined with commercial kits (Roche Diagnostics GmbH, Mannheim, Germany).
Adipose tissue culture
Immediately after the surgery, a part of AT biopsies were immediately frozen at −80 C and used for analyzing the basal gene expression. The rest of AT was used for culture; thus, 800-1000 mg AT explants (minimal pieces of 1–2 mm3 to allow the maximal contact of adipose tissue with the medium) were transferred to cell culture bottles with membrane filter screw cap to safeguard the viability of the culture and placed in 5 ml of DMEM supplemented with 10% fetal bovine serum and kept at 37 C for 24 h in a humidified atmosphere containing 7% CO2.
On the next day, the adipose explants were collected to perform gene expression analysis at the following times (T): 0, 6, 12, and 18, T0 being arbitrarily defined as 0800 h because this was the usual waking time for patients, T6 as 1400 h, T12 as 2000 h, and T18 as 0200 h. Gene expression was measured for only one circadian cycle (24 h) but in the second day of cultured. All cultures were performed in duplicates.
Analysis of gene expression
Total RNA was extracted from AT explants using RNeasy kit (QIAGEN, Courtabeouf, France). Reverse transcription was performed using random hexamers as primers and Thermoscript reverse transcriptase (Invitrogen, Cergy Pontoise, France) with 1 μg total RNA for each sample. Quantitative real-time PCR was performed using an ABI PRISM 7000 HT sequence detection system (Applied Biosystems, Foster City, CA), using TaqMan universal PCR master mix and specific TaqMan probes (Applied Biosystems). The primers used in the study are the following references from Applied Biosystems: human AdipoQ (Hs00605917_m1), ADIPOR1 (Hs01114951_m1) and ADIPOR2 (Hs00226105_m1). We used 18S rRNA (reference Hs99999901_s1; Applied Biosystems) as internal control. We selected 18S because this gene showed a lower variance along time compared with the glyceraldehyde-3-phosphate dehydrogenase gene. In addition, we carried out a one-way (Zeitgeber time) ANOVA for 18S and observed no significant difference in any of the adipose depots studied (P > 0.05). In addition, there was no significant difference in 18S cycle threshold (Ct) between sc (mean Ct = 23.3 ± 3.3) and visceral depots (mean Ct = 23.2 ± 3.3). In conclusion, we selected 18S as the better housekeeping gene for this experiment. Gene mRNA levels were normalized to 18s using the 2−ΔΔCt method (17).
Rhythm calculation and statistical analysis
Clinical and anthropometric data are presented as means ± sd. The results for gene expression, expressed in arbitrary units, are presented as means ± sem. Wilcoxon nonparametric paired test was used for comparing data from the samples derived from the two adipose depots in each individual subject. Pearson’s correlations coefficients were used for analyzing associations between the relative expression of adiponectin and ADIPOR1 and ADIPOR2 in both adipose depots.
The single cosinor method was used to analyze for circadian rhythm individually (18). This inferential method involves fitting a curve of a predefined period by the least squares method. The rhythm characteristics and their 95% confidence intervals estimated by this method include the mesor (middle value of the fitted cosine representing a rhythm adjusted mean), the amplitude (half the difference between the minimum and maximum of the fitted cosine function), and the temporal location of maximum value or acrophase (the time at which the peak of a rhythm occurs, expressed in hours) and the percent rhythm (PR; percentage of variability accounted for by cosine curve). Relative amplitude was expressed as a percentage of mesor values [relative amplitude = (amplitude/ mesor) × 100]. The significance of the rhythms was determined by rejection of the zero amplitude hypotheses with a threshold of 60%.
Mean circadian gene expression of the total population into each fat depot was analyzed by using repeated-measures ANOVA test, with a post hoc test of least significant difference (LSD) correction. Differences in acrophase between sc and visceral depots were analyzed by Mann-Whitney nonparametric test for the three genes studied. Pearson’s correlation analyses were used for finding associations between the genetic circadian oscillation (amplitude) and components of the MetS. All statistical analyses were carried out using SPSS for Windows (release 15.0; SPSS Inc., Chicago, IL). The level of significance for all statistical tests and hypotheses was set at P < 0.05.
Results
Characteristics of the population
Table 1 contains basal characteristics of the women studied. BMI was higher than 40 kg/m2 in these patients and VA/SA higher than 0.4, indicating that women suffered from visceral and morbid obesity. All subjects were defined as MetS patients, according to the International Diabetes Federation (IDF) criteria (19). The individual and average values for waist circumference, glucose, and systolic pressure exceeded the cutoff points proposed by the IDF (Table 2).
Table 1.
Anthropometric characteristics of the population studied
| Patients (n = 6) | |
|---|---|
| Age (yr) | 51 ± 9 |
| Weight (kg) | 107.9 ± 11.2 |
| Height (cm) | 156 ± 5.0 |
| BMI (kg/m2) | 44.1 ± 5.5 |
| Body fat (%) | 43 ± 6 |
| WC (cm) | 126 ± 8 |
| HC (cm) | 139 ± 9 |
| WHR | 0.91 ± 0.03 |
| Sagittal diameter (cm) | 22 ± 4 |
| Coronal diameter (cm) | 31 ± 4 |
| Bicipital sinkfold (mm) | 28 ± 3 |
| Tricipital sinkfold (mm) | 28 ± 4 |
| Subscapular sinkfold (mm) | 33 ± 4 |
| Suprailliac sinkfold (mm) | 31 ± 3 |
| VA/SApredicted | 0.54 ± 0.28 |
Data are presented as means ± sd. WC, Waist circumference; HC, hip circumference.
Table 2.
MetS characteristics of the subjects studied
| Patients (n = 6) | IDF MeS worldwide definition | |
|---|---|---|
| WC (cm) | 126 ± 8 | ≥80 |
| Plus any two of the following | ||
| Triglycerides (mmol/liter) | 1.02 ± 0.42 | ≥1.70 |
| HDL cholesterol (mmol/liter) | 1.34 ± 0.22 | <1.29 |
| Glucose (mmol/liter) | 6.13 ± 0.82 | ≥5.60 |
| Systolic pressure (mm Hg) | 148 ± 23 | ≥130 |
| Diastolic pressure (mm Hg) | 73 ± 14 | ≥85 |
Data are presented as means ± sd. Bold characters indicate values higher than cutoff points proposed by the IDF (19). WC, Waist circumference; HDL, high-density lipoprotein.
Basal gene expression
Paired adipose tissue biopsies from the sc and visceral depots were obtained from each patient. Attending to basal gene expression, significant differences in adiponectin were observed between AT depots (Fig. 1), with a higher expression in sc than visceral fat, whereas no significant differences were found in the relative expression of both adiponectin receptors, ADIPOR1 and ADIPOR2, within each fat compartment (Fig. 1). Pearson’s correlation analysis showed significant positive correlations among adiponectin and ADIPOR1 and ADIPOR2 in sc (ADIPOQ vs. ADIPOR1: P = 0.039, r = 0.834; ADIPOQ vs. ADIPOR2: P = 0.036, r = 0.841; ADIPOR1 vs. ADIPOR2: P = 0.033, r = 0.848) and visceral AT (ADIPOQ vs. ADIPOR1: P = 0.010, r = 0.915; ADIPOQ vs. ADIPOR2: P = 0.000, r = 0.989; ADIPOR1 vs. ADIPOR2: P = 0.008, r = 0.927).
Figure 1.
Basal relative expression of genes studied (ADIPOQ, ADIPOR1, and ADIPOR2) in sc and visceral adipose tissue. mRNA levels were measured with the real-time PCR and normalized to 18S using the ΔΔCt method of relative quantitation. Data are reported as means ± sem, and results are presented as percent of ADIPOR2 relative expression (ADIPOR2 value = 1). Asterisk represents significant differences (Wilcoxon nonparametric paired test). NS, Not significant.
Circadian gene expression
Once we examined the basal expression of these genes, we focused on their circadian expression pattern in cultured adipose tissue explants. Parameters imputed from each subject obtained by cosinor analysis, defining the circadian rhythms as mesor, amplitude, relative amplitude, acrophase, and percent rhythm are reported in Table 3. According to these results, circadian expression patterns were found for all the genes investigated (represented in bold).
Table 3.
Parameters imputed from cosinor analysis
| SC AT
|
Visceral AT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Mesor (AU) | Amplitude (AU) | Relative amplitude (%) | Acrophase (h) | PR (%) | Mesor (AU) | Amplitude (AU) | Relative amplitude (%) | Acrophase (h) | PR (%) |
| Adiponectin | ||||||||||
| P2 | 1.703 | 0.587 | 34.47 | 2.11 | 87.41 | 4.592 | 4.655 | 101.37 | 1.8 | 83.22 |
| P3 | 2.856 | 2.59 | 90.69 | 1.72 | 93.71 | 2.923 | 1.25 | 42.76 | 8.8 | 43.21 |
| P4 | 14.463 | 20.047 | 138.61 | 23.8 | 82.37 | 1.852 | 2.332 | 125.92 | 0.67 | 93.23 |
| P5 | 7.098 | 10.754 | 151.51 | 0.1 | 63.05 | 3.301 | 3.323 | 100.67 | 11.76 | 55.86 |
| P6 | 33.976 | 57.466 | 169.14 | 0.17 | 64.02 | 5.11 | 4.782 | 93.58 | 6.16 | 96.25 |
| ADIPOR1 | ||||||||||
| P1 | 3.637 | 3.517 | 96.70 | 9.5 | 99.61 | 1.76 | 1.122 | 63.75 | 10.27 | 91.1 |
| P2 | 3.335 | 1.767 | 52.98 | 6.65 | 27.39 | 2.16 | 1.487 | 68.84 | 2.49 | 94.34 |
| P3 | 1.935 | 1.384 | 71.52 | 7.1 | 86.19 | 1.38 | 0.53 | 38.41 | 10.77 | 75.63 |
| P4 | 50.332 | 35.102 | 69.74 | 21.23 | 53.61 | 1.778 | 2.279 | 128.18 | 0.5 | 91.8 |
| P5 | 14.171 | 2.467 | 17.41 | 22.04 | 2.08 | 3.242 | 3.225 | 99.48 | 10.69 | 79.6 |
| P6 | 7.821 | 10.895 | 139.30 | 10.21 | 94.27 | 3.859 | 1.495 | 38.74 | 15.42 | 23.18 |
| ADIPOR2 | ||||||||||
| P1 | 3.021 | 2.749 | 91.00 | 8.16 | 97.69 | 2.862 | 2.205 | 77.04 | 11.39 | 51.52 |
| P2 | 1.785 | 0.736 | 41.23 | 20.37 | 67.92 | 2.997 | 2.289 | 76.38 | 3.02 | 95.01 |
| P3 | 2.668 | 1.607 | 60.23 | 8.53 | 88.9 | 1.645 | 0.615 | 37.39 | 10.66 | 50.15 |
| P4 | 22.318 | 24.478 | 109.68 | 20.98 | 94.74 | 2.083 | 2.602 | 124.92 | 0.96 | 94.63 |
| P5 | 7.414 | 2.933 | 39.56 | 0.02 | 9.5 | 3.718 | 4.548 | 122.32 | 11.24 | 75.69 |
| P6 | 26.679 | 31.139 | 116.72 | 0.52 | 51.24 | 1.544 | 0.652 | 42.23 | 5.91 | 43.25 |
Parameters imputed from each subject obtained by cosinor analysis defining the circadian rhythms as mesor, amplitude, relative amplitude, acrophase, and PR are represented in this table. Subjects in which expression of the gene was considered to have circadian rhythmicity are shown in bold. AU, Arbitrary units.
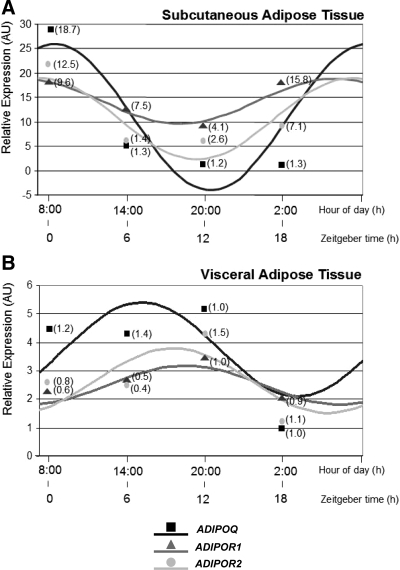
Figure 2 shows mean circadian rhythms for the examined group concerning the three genes studied (ADIPOQ, ADIPOR1, and ADIPOR2) in sc (Fig. 2A) and visceral AT (Fig. 2B). When relative gene expression among different times was analyzed by using repeated-measures ANOVA test, statistical differences were found for ADIPOQ in sc (P = 0.010) and visceral (P = 0.045) ATs, evidencing the circadian rhythmicity of adiponectin expression.
Figure 2.
Rhythmic expression of genes studied in human sc (A) and visceral AT (B). Adipose depots were isolated at 6-h intervals over the course of the day from AT cultures (time at 0, 6, 12, and 18 h). Results are presented relative to the lowest basal relative expression for each gene. Data of relative expression are represented as arbitrary units (AU). Data are reported as means ± sem (sem of ΔCt are represented in parentheses).
When comparing both AT depots, different circadian patterns were observed. Thus, whereas the sc tissue displayed a strong oscillatory trend, adiponectin circadian expression in visceral fat was dampened in all patients, with only one exception, as observed when comparing relative amplitude from both AT locations (Table 3). Moreover, the three genes cycled in synchrony in both depots, but while in sc adipose tissue, they reached their zenith (highest levels) around Zeitgeber time 0 (0800 h) and their nadir (lowest levels) around time 12 (2000 h); in omental AT both zenith and nadir were significantly delayed 6 h with respect to sc AT for the three genes studied (P < 0.05).
Correlation analyses between the genetic circadian oscillation (amplitude) and components of the MetS revealed that higher adiposity and abdominal obesity (BMI, body fat percent, waist, sagittal diameter, and conicity index) correlated with a decrease in ADIPOQ, ADIPOR1, and ADIPOR2 amplitude (Table 4).
Table 4.
Correlations between the gene expression, circadian oscillation (amplitude), and components of the metabolic syndrome in visceral AT
| Amplitude | BMI (kg/m2)
|
Weight (kg)
|
Body fat (%)
|
Waist (cm)
|
Sagittal diameter (cm)
|
Conicity index
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| ADIPOQ | −0.871 | 0.024 | −0.796 | 0.058 | NS | NS | NS | NS | −0.826 | 0.043 | NS | NS |
| ADIPOR1 | −0.864 | 0.027 | −0.965 | 0.002 | −0.844 | 0.035 | −0.906 | 0.013 | NS | NS | −0.847 | 0.034 |
| ADIPOR2 | −0.889 | 0.018 | −0.912 | 0.011 | −0.817 | 0.047 | −0.850 | 0.032 | NS | NS | NS | NS |
NS, Not significant.
A simple statistical analysis was performed correlating the phase difference between both AT depots (differences in achrophase from sc and visceral fat) in adiponectin expression and obesity parameters such as BMI, waist circumference, waist to hip ratio (WHR), and sagittal diameter, and no significant associations were found for those parameters (BMI: r = 0.235, P = 0.70; waist circumference: r = −0.62; P = 0.26; WHR: r = 0.09; P = 0.89; sagittal diameter r = 0.08, P = 0.89).
Discussion
Adiponectin is one of most intensively studied cytokines. The interest on this novel adipocyte hormone is mainly derived from its protective role in the different alterations associated with obesity, such as insulin resistance and inflammation (20). Interestingly, in humans, circulating adiponectin levels exhibit diurnal variations (14). However, as far as we know, specific information concerning circadian rhythmic expression of this adipokine in human AT has not been reported. In the present study, we show in AT from obese women that adiponectin mRNA levels fluctuate during the day in the same phase as its receptors and that this circadian rhythmicity is attenuated with MetS features.
Women from the present work displayed significant differences in basal adiponectin expression between AT depots, being higher in the sc expression. These findings are in agreement with a previous study performed in a similar population (21) and could be associated with the cardioprotective feature of this adipose area, termed by some authors as healthy adipose tissue (22). With respect to adiponectin receptors, no significant differences were found between both AT location, as previously shown in experimental animals (23).
The main objective of the present work was to assess whether the mRNA levels of adiponectin and its receptors showed daily circadian rhythms in visceral and sc fat explants obtained from morbidly obese women. Results indicate that the three genes examined (ADIPOQ, ADIPOR1, and ADIPOR2) exhibited 24-h rhythmicity, suggesting that the expression of adiponectin is regulated in a time-dependent manner, at least at the mRNA level.
Thus, our data suggest that these genes can oscillate accurately and independently of the suprachiasmatic nucleus in human AT during 24 h. This situation has been previously demonstrated for other genes in human AT cultures, such as clock genes (PER2, CRY1, and BMAL1) (7), cortisol-related genes, such as glucocorticoid receptor (GR) and 11β-hydroxysteroid dehydrogenase 1 (11βHSD1), and PPARγ (24). However, this is the first study to show the circadian expression of adiponectin and its receptors in human AT. From a physiological point of view, these new data are not surprising if we consider that, besides glucose and lipid homeostasis, there are many hormones (insulin, glucagon, GH, and cortisol) and adipose-specific molecules implicated in energy metabolism and MetS (leptin, adipsin, resistin, and visfatin), which display circadian oscillation with different daily pattern (2).
Only a limited number of studies previously examined ADIPOR expression in AT (13,16,25). Here we first explore their circadian rhythmicity, finding that they fluctuate in the same phase as adiponectin. This synergy could magnify the physiological effect of adiponectin and facilitate its autocrine/paracrine action. Indeed, the simultaneous circadian expression of adiponectin and its receptors may lead to an increase in adiponectin binding and thus result in increased adiponectin effects. These results emphasize the importance of circadian rhythmicity in the physiological role of adiponectin.
A previous study performed in experimental animals (26) have shown that adiponectin gene expression follows a circadian rhythm in adipose tissue depots with a nadir (minimum expression) during the morning (1000–1100 h). In the present study, performed in humans, the expression of adiponectin achieved its zenith (maximum) at the same hours. As expected, circadian expression of human adiponectin occurs in antiphase as compared with mice, a rodent characterized by its nocturnal behavior.
In humans, there are no studies showing circadian adiponectin gene expression in other tissues and none performed in women or obese women. When comparing AT adiponectin circadian expression in the present work with serum diurnal variability from a previous work (14), it can be observed that the nadir in AT in the present paper anticipated to that found in plasma, thus implying a 7-h phase delay between the transcription and the secretion of the protein to blood. These data must be discussed with caution because the study by Gavrila et al. (14) was performed in healthy males, whereas the present work is followed up in morbidly obese women. However, these results could be explained by the kinetics of expression, synthesis, and secretion of adiponectin.
Recent findings suggest that the circadian system regulates the metabolism in all tissues and organs implicated in the pathological changes leading the MetS. Moreover, glucose and lipid homeostasis are also known to exhibit circadian variation (27,28,29,30). In this sense, adiponectin is highly implicated in glucose metabolism (31). An additional relevant finding in our results was that adiponectin expression was decreased in the late evening (2000 h), which coincides with the hours of maximal insulin resistance in humans (32). Additionally, adiponectin has been called the fat-burning molecule because it is able to redirect fatty acids to the muscle for their oxidation (31). This special capability is of great interest because the influx of fatty acids to the liver decreases and so does total triglyceride content, leading to a higher insulin-sensitivity state (31). The fact that adiponectin displays its higher expression during the morning hours (1000 h) could be implicated in the maximal withdrawal of fatty acids at these times and the improvement in glucose tolerance, which characterized the subjects in these particular daily hours (32).
Circadian disruption or chronodisruption is characterized by an attenuation of the circadian rhythms (33). Recently some studies suggested that chronodisruption may lead to obesity (1). Disrupted biological rhythms lead to attenuated circadian feeding rhythms, hyperphagia, obesity, cancer proneness, and reduced life expectancy (1). In this regard, our correlation analyses performed between the genetic circadian oscillation (amplitude) and components of the MetS revealed that the rhythmicity of adiponectin expression in the women studied was attenuated with obesity. Indeed, our results show that an increase of adiposity markers and mainly abdominal obesity (BMI, body fat percent, weight, waist, sagittal diameter, and conicity index) significantly correlated with a decrease in adiponectin and ADIPOR1 and ADIPOR2 amplitude, which characterized a dampened rhythm. It is important to highlight that this situation was present only in the visceral fat. Another interesting result was that when comparing AT locations, visceral fat showed a trend toward a dampening of the mRNA amplitude of components of adiponectin function, compared with sc fat. This consideration perhaps may help to explain why MetS features are highly related to visceral fat. Previously Ando et al. (15) showed that the rhythmic expression of clock genes (Bmal1, Per1, Per2, Cry1, Cry2, and Dbp) and adipocytokines (adiponectin, resistin, and visfatin) was attenuated in visceral adipose tissue from obese KK mice. Other animal models revealed that mice with Clock gene disruption are prone to develop obesity and a phenotype resembling MetS (34).
Another interesting characteristic that provides information about rhythmicity in AT is the potential phase delay between the different AT locations. In this line, in visceral AT from the women studied, both nadir and zenith were significantly delayed 6 h with respect to sc. A similar situation was present for two of the clock genes studied (hPer2 and hCry1) but not for hBmal1 (data not shown). It is noteworthy that this phase delay between both AT location was not related to obesity. Indeed, no significant correlations were obtained between obesity parameters and the differences in achrophase from both AT locations. Interestingly, in humans, alterations in the dynamics of circulating adiponectin with obesity have also been reported (35). Together with previous findings, we could then speculate that circadian rhythm disruption is clearly important in determining the severity of the metabolic impairment and vice versa (36).
One of the shortcomings of the current design is that it includes only morbidly obese women, and more information is needed about gender differences. Although in this study adiposity was associated with chronodisruption, even in morbidly obese women, another aspect to consider is whether the rhythms may be different for less obese individuals. However, to obtain enough AT sample from normal-weight individuals may be challenging, and it could be also an ethical problem, considering the difficulties in obtaining enough fat from visceral and sc locations within the same patients to perform four different adipose tissue explants cultures.
In conclusion, we have found for the first time that expression of adiponectin and its receptors (ADIPOR1 and ADIPOR2) showed 24-h rhythmicity in human visceral and sc AT explants from morbid obese patients. Interestingly, this rhythmic adiponectin expression is in phase with its receptors, and there is a zenith delay of 6 h in omental AT with respect to sc fat.
Acknowledgments
We thank Ana Lorente and Juan José Hernández-Morante for their excellent technical assistance.
Footnotes
This work was supported in part by National Institutes of Health Grant DK075030 and Contracts 53-K06-5-10 and 58-1950-9-001 from the U.S. Department of Agriculture Research Service; the Government of Education, Science, and Research of Murcia (Project BIO/FFA 07/01-0004); the Spanish Government of Science and Innovation (Project AGL2008-01655/ALI); the Spanish Ministry of Education and Science (Project BFU2007-60658/BFI); Seneca Foundation (PI/05700/07); the Institute of Health Carlos III (RETICEF, RD06/0013/0019); and Línea Especial of University of Navarra (LE/97).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 3, 2009
Abbreviations: ADIPOR, Adiponectin receptor; AT, adipose tissue; BMI, body mass index; Ct, cycle threshold; IDF, International Diabetes Federation; MetS, metabolic syndrome; PR, percent rhythm; SA, sc area; T, time; VA, visceral area; WHR, waist to hip ratio.
References
- Froy O 2007 The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol 28:61–71 [DOI] [PubMed] [Google Scholar]
- Garaulet M, Madrid JA 2009 Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol 20:127–134 [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Bass J 2007 A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab 18:4–11 [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR 2002 Coordination of circadian timing in mammals. Nature 418:935–941 [DOI] [PubMed] [Google Scholar]
- Gómez-Abellán P, Hernández-Morante JJ, Luján JA, Madrid JA, Garaulet M 2008 Clock genes are implicated in the human metabolic syndrome. Int J Obes 32:121–128 [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB 2006 Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab 8:264–280 [DOI] [PubMed] [Google Scholar]
- Gómez-Santos C, Gómez-Abellán P, Madrid JA, Hernández- Morante JJ, Luján JA, Ordovás JM, Garaulet M 2009 Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring) 17:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE 2003 Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46:459–469 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Scherer PE 2003 Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep 3:207–213 [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H 2004 Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323:630–635 [DOI] [PubMed] [Google Scholar]
- Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M 2005 Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res 97:1245–1252 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T 2003 Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769 [DOI] [PubMed] [Google Scholar]
- Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B 2006 Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 14:28–35 [DOI] [PubMed] [Google Scholar]
- Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS 2003 Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab 88:2838–2843 [DOI] [PubMed] [Google Scholar]
- Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A 2005 Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 146:5631–5636 [DOI] [PubMed] [Google Scholar]
- Garaulet M, Hernández-Morante JJ, Tébar FJ, Zamora S, Canteras M 2006 Two-dimensional predictive equation to classify visceral obesity in clinical practice. Obesity (Silver Spring) 14:1181–1191 [DOI] [PubMed] [Google Scholar]
- Hernandez-Morante JJ, Milagro FI, Lujan JA, Martinez JA, Zamora S, Garaulet M 2008 Insulin effect on adipose tissue (AT) adiponectin expression is regulated by the insulin resistance status of the patients. Clin Endocrinol (Oxf) 69:412–417 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U 2000 Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289:2344–2347 [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J 2006 Metabolic syndrome a new wold-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23:469–480 [DOI] [PubMed] [Google Scholar]
- Gil-Campos M, Cañete RR, Gil A 2004 Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr 23:963–974 [DOI] [PubMed] [Google Scholar]
- Hernandez-Morante JJ, Milagro FI, Larque E, Lujan J, Martinez JA, Zamora S, Garaulet M 2007 Relationship among adiponectin, adiponectin gene expression and fatty acids composition in morbidly obese patients. Obes Surg 17:516–524 [DOI] [PubMed] [Google Scholar]
- Després JP, Lemieux I 2006 Abdominal obesity and metabolic syndrome. Nature 444:881–887 [DOI] [PubMed] [Google Scholar]
- Blüher M, Williams CJ, Klöting N, Hsi A, Ruschke K, Oberbach A, Fasshauer M, Berndt J, Schön MR, Wolk A, Stumvoll M, Mantzoros CS 2007 Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care 30:3110–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campión J, Martínez JA, Zamora S, Garaulet M 2009 Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obes (Lond) 33:473–480 [DOI] [PubMed] [Google Scholar]
- Morínigo R, Musri M, Vidal J, Casamitjana R, Delgado S, Lacy AM, Ayuso C, Gomis R, Corominola H 2006 Intra-abdominal fat adiponectin receptors expression and cardiovascular metabolic risk factors in obesity and diabetes. Obes Surg 16:745–751 [DOI] [PubMed] [Google Scholar]
- Oliver P, Ribot J, Rodríguez AM, Sánchez J, Picó C, Palou A 2006 Resistin as a putative modulator of insulin action in the daily feeding/fasting rhythm. Pflugers Arch 452:260–267 [DOI] [PubMed] [Google Scholar]
- Seaman GV, Engel R, Swank RL, Hissen W 1965 Circadian periodicity in some physicochemical parameters of circulating blood. Nature 207:833–835 [DOI] [PubMed] [Google Scholar]
- Malherbe C, De Gasparo M, De Hertogh R, Hoet JJ 1969 Circadian variations of blood sugar and plasma insulin levels in man. Diabetologia 5:397–404 [DOI] [PubMed] [Google Scholar]
- Gagliardino JJ, Hernández RE 1971 Circadian variation of the serum glucose and immunoreactive insulin levels. Endocrinology 88:1532–1534 [PubMed] [Google Scholar]
- Schlierf G, Dorow E 1973 Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J Clin Invest 52:732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Hernández-Morante JJ, de Heredia FP, Tébar FJ 2007 Adiponectin, the controversial hormone. Public Health Nutr 10:1145–1150 [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM 2001 A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 50:1237–1243 [DOI] [PubMed] [Google Scholar]
- Erren TC, Reiter RJ 2009 Defining chronodisruption. J Pineal Res 46:245–247 [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J 2005 Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J 2004 Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA 101:10434–10439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Owens JA, Voultsios A, Boden MJ, Varcoe TJ 2007 Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am J Physiol Regul Integr Comp Physiol 293:R1528–R1537 [DOI] [PubMed] [Google Scholar]