Abstract
Cold-induced adaptive (or nonshivering) thermogenesis in small mammals is produced primarily in brown adipose tissue (BAT). BAT has been identified in humans and becomes more active after cold exposure. Heat production from BAT requires sympathetic nervous system stimulation, T3, and uncoupling protein 1 (UCP1) expression. Our previous studies with a thyroid hormone receptor-β (TRβ) isoform-selective agonist demonstrated that after TRβ stimulation alone, adaptive thermogenesis was markedly impaired, although UCP-1 expression in BAT was normal. We used mice with a dominant-negative TRβ PV mutation (frameshift mutation in resistance to thyroid hormone patient PV) to determine the role of TRβ in adaptive thermogenesis and UCP1 expression. Wild-type and PV mutant mice were made hypothyroid and replaced with T3 (7 ng/g · d) for 10 d to produce similar serum thyroid hormone concentration in the wild-type and mutant mice. The thermogenic response of interscapular BAT, as determined by heat production during iv infusions of norepinephrine, was reduced in PVβ heterozygous and homozygous mutant mice. The level of UCP1, the key thermogenic protein in BAT, was progressively reduced in PVβ+/− and PVβ−/− mutant mice. Brown adipocytes isolated from PV mutant mice had some reduction in cAMP and glycerol production in response to adrenergic stimulation. Defective adaptive thermogenesis in TRβ PV mutant mice is due to reduced UCP1 expression and reduced adrenergic responsiveness. TRβ mediates T3 regulation of UCP1 in BAT and is required for adaptive thermogenesis.
Heat production from brown adipose tissue, which is important for regulation of metabolism and body weight, requires the expression of uncoupling protein under the regulation of a specific type of thyroid hormone receptor.
Thyroid hormone plays an important role in both obligatory and adaptive thermogenesis (1). Adaptive thermogenesis occurs in response to cold exposure or caloric intake and is regulated by hypothalamic centers that integrate environmental and visceral cues (2). The sympathetic nervous system mediates the signal to target tissues, primarily brown adipose tissue (BAT), and other sites such as skeletal muscle (3). Effective adaptive thermogenesis in BAT requires thyroid hormone, adrenergic stimulation, and uncoupling protein 1 (UCP1) expression (4,5,6,7,8).
A variety of genetic approaches have been used to determine the importance of the various elements that play a role in adaptive thermogenesis. UCP1 knockout mice were not obese when maintained at room temperature but had a deficit in adaptive thermogenesis from BAT (9). UCP1 knockout mice maintained at a thermoneutral temperature (approximately 30 C), however, were obese and had a further marked weight gain in response to a high-fat diet (10). The β3-adrenergic receptor knockout mouse had a very modest deficit of adaptive thermogenesis (11), but a mouse with inactivation of all three β-adrenergic receptors was obese and did not appropriately elevate body temperature in response to cold exposure (12). Previous studies established the importance of adrenergic signaling, UCP expression, and conversion of the prohormone T4 to the active hormone T3 for adaptive thermogenesis in the rat (13,14,15). In a mouse with inactivation of the type 2 5′-deiodinase (D2), the enzyme that activates T4 to T3 conversion, the local availability of T3 in BAT was reduced (16). These mice had normal circulating T3 concentration but a deficit in adaptive thermogenesis due to the absence of locally generated T3 in BAT (16,17).
Thyroid hormone mediation of adaptive thermogenesis involves both thyroid hormone receptor-α (TRα) and -β (TRβ). Genetic and pharmacological models have been used to identify their roles. The TRα knockout mouse was found to have a reduced basal body temperature and heart rate, neither of which normalized with T3 treatment (18). Adaptive thermogenesis, but not obligatory thermogenesis, was defective in mice lacking all TRα gene products (19). We have previously shown, using the TRβ isoform-selective ligand GC1, that TRα plays an essential role in mediating thyroid hormone effects on adrenergic sensitivity (20). In this model, UCP1 levels in BAT were normal, and defective adaptive thermogenesis was due to diminished adrenergic sensitivity (20). We have also shown in mice with an introduced TRα dominant-negative point mutation that adaptive thermogenesis is blunted, but UCP1 levels in BAT are normal (21). Because T3 regulates UCP1 expression and TRα is not involved, we predicted that T3 stimulation of UCP1 in BAT was primarily mediated by TRβ.
To determine the role of TRβ in adaptive thermogenesis, we used a mouse with a dominant-negative knock-in mutation in the TRβ gene, termed the PV mutation, a frameshift mutation in resistance to thyroid hormone patient PV. The PV mutation does not bind T3, and functions as a dominant-negative TR (22). Previous studies have characterized the TRβ PV mutant mouse, which has an RTH phenotype and defects in thyroid hormone regulation at the level of the pituitary as well as delayed growth (23). Because the heterozygote and homozygote mice have a marked increase in circulating thyroid hormone levels, we also studied animals after they were made hypothyroid and provided a fixed amount of thyroid hormone replacement to normalize the levels in control and mutant mice. We identified a defect in adaptive thermogenesis in response to catecholamine infusion. The level of UCP1 protein in BAT in the TRβ PV mutant mouse, however, was reduced. In vitro studies of BAT response to catecholamines showed some loss of adrenergic sensitivity. These studies demonstrate that expression of UCP1 in BAT is required for adaptive thermogenesis and is preferentially stimulated by TRβ.
Materials and Methods
Animals and drugs
All studies performed were approved by the Animal Research Committee, Veterans Affairs Greater Los Angeles Health Care System. All drugs and reagents, unless otherwise specified, were purchased from Sigma Chemical Co. (St. Louis, MO). Male C57 mice were obtained from Harlan Sprague Dawley (Indianapolis, IN), and PV mutant mice were bred and genotyped as described (23). Mice were kept in cages with no more than five animals per cage with chow and tap water ad libitum at 23 C and light cycles of 12 h. All mice were 70–80 d old at the beginning of the experiments and weighed 22–28 g. Hypothyroidism was induced by feeding a low-iodine diet supplemented with 0.15% propylthiouracil, purchased from Harlan Teklad Co. (Indianapolis, IN), for 8–10 d. Mice were then treated daily for 10 d with ip injections of 7 ng/g body weight (BW) T3. After treatment, the interscapular BAT (IBAT) thermogenic response to norepinephrine (NE) infusion was measured.
IBAT thermal response to NE infusion
In these studies, we used a protocol as previously described for rats (4) and modified for use in mice (20). All animals were anesthetized with a mixture of urethane (560 mg/kg; ip) and chloralose (38 mg/kg body weight; ip) the morning of the experiment. Mice were kept on a warm pad (30 C) through the course of the experiment. A polyethylene (P-50) cannula was inserted into the left jugular vein and later used for NE infusion. IBAT temperatures were measured using a precalibrated thermistor probe YSI 427 (YSI, Yellow Springs, OH) secured under the brown fat pad connected to a high-precision thermometer (YSI precision 4000A thermometer). IBAT temperature was monitored during a period of 10 min to obtain a stable baseline, and then NE infusion was started. NE infusion (1075 pmol/min) was performed with an infusion pump (Harvard model 2274) at a rate of 0.459 μl/min for 30 min. Raw data were plotted over time and expressed in terms of maximum IBAT temperature. Heart rate was also monitored electrocardiographically during NE infusion.
Tissue sampling and processing
The whole IBAT pad, about 1 g liver, and the heart from individual mice were processed for mitochondrial isolation. Tissue samples were homogenized with a motor-driven homogenizer in 2 ml ice-cold 0.32 m sucrose, 2 mm EDTA, and 5 mm 2-mercaptoethanol, in 10 mm Tris buffer (pH 7.2). The homogenate was centrifuged at 10,000 × g for 10 min. The top fat layer of BAT homogenate was discarded, and the pellets were resuspended in 2 ml buffer and centrifuged for 10 min at 10,000 × g to sediment the mitochondria. The mitochondrial pellet was resuspended in 100 ml buffer and kept frozen at −70 C. Protein measurement was done by the Bradford method (24).
Western blot analysis
Protein (20 μg/lane) was size-fractionated on a 10% SDS-PAGE gel and electrophoretically transferred onto a nitrocellulose membrane (Immobilon, Millipore Co., Bedford. MA) using 0.17 m Tris base, 0.17 m glycine, and 15% methanol (pH 8.3) as transfer buffer. Protein loading was normalized by protein measurement (24) and confirmed by Coomassie blue staining of the gel. The membrane was then incubated overnight with Tris buffer and 0.5% blocking solution, containing anti-UCP1 antibody, 1:1000 (Calbiochem, San Diego CA) and processed further using a chemiluminescence Western blotting kit (Roche Molecular Biochemicals, Indianapolis, IN), according to the manufacturer’s protocol. Signal intensity was measured by densitometry using the NIH Image software (Scientific Computing Resource Center, Bethesda, MD).
RIA
Total T4 levels were measured in 25-μl serum samples by RIA in duplicate determinations (ImmunoChem coated tube-T4 iodine 125I RIA kit; ICN Pharmaceuticals, Costa Mesa, CA).
α-Glycerol phosphate dehydrogenase (α-GPD) assay
α-GPD was assayed as described previously (25) at 35 C in 65 mm sodium phosphate buffer (pH 7.4) containing 1 mm KCN, 50 mm α-glycerophosphate, 1.7 mm iodonitrotetrazolium violet, and 50–100 μg mitochondrial protein. Product formation was monitored spectrophotometrically at 550 nm. The results are expressed as increments in OD units per minute per milligram of mitochondrial protein.
cAMP and glycerol production in isolated brown adipocytes
Studies in brown adipocytes were performed as we have previously described (5). BAT was fragmented and digested with collagenase until adipocytes were released. Cells were kept alive for several hours in DMEM buffered with 44 mm Na2CO3 and 20 mm HEPES (pH 7.4) and supplemented with 2 mm glutamine, 10 mm glucose, 10 mm fructose, 1 mm pyruvic acid, and 4% albumin. Adenylyl cyclase activity was measured in cells that were incubated for 60 min with specific adrenergic stimulators plus 10 m Ro-20.1724 (a phosphodiesterase inhibitor) and 0.8 U/ml adenosine deaminase to minimize the A1 receptor inhibition of cAMP generation. Perchloric acid was added to a final concentration of 3% to terminate the incubation and pH adjusted to 5.5–6.0. cAMP production was measured by solid-phase RIA using a kit (NEN Life Science Products, Boston MA), according to the manufacturer’s recommendations. Adenylyl cyclase activity was expressed as picomoles of cAMP per hour per 105 cells. Glycerol was measured in supernatants of the perchloric acid precipitate by ELISA, using a commercially available kit (Sigma) (5).
Statistical analysis
Results are expressed as mean ± sd throughout the text and figures. Multiple comparisons were performed by one-way ANOVA followed by the Student-Newman-Keuls test.
Results
Catecholamine responsiveness in wild-type and TRβ PV mutant mice
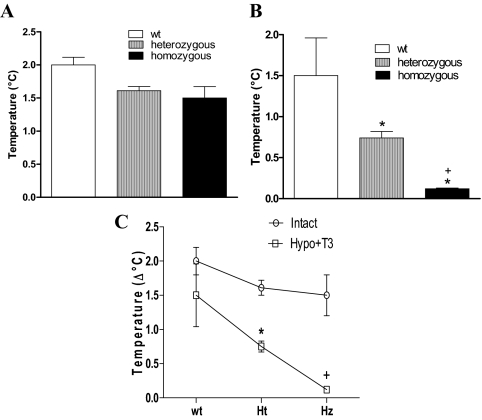
We have previously demonstrated increased IBAT temperature in a dose- and time-dependent response to catecholamine infusion in mice (20). This dynamic measurement of the thermogenic response of brown fat to catecholamines is modulated by thyroid hormone status. Hypothyroid mice do not have an increase in IBAT temperature in response to catecholamine infusion (20). The IBAT response to catecholamines was determined in mice heterozygous and homozygous for the TRβ PV mutation and compared with wild-type mice. Based on our previous studies of the IBAT thermal response in euthyroid mice, we used a NE dose of 1076 pmol/min NE for 30 min to achieve an intermediate increase in IBAT temperature in wild-type mice (2.2 C). The infusion of saline did not change the basal body temperature of the animals (data not shown). Progressive infusion of catecholamine produced no significant difference in the IBAT thermal response between wild-type and TRβ PV mutant mice. The increment in IBAT temperature after 30 min infusion of NE (1076 nmol/min) is shown for the three groups, and no significant differences were seen (Fig. 1A), although there was a trend toward reduced heat production in the TRβ PV mutant mice.
Figure 1.
Thermal response of BAT to catecholamine infusion. IBAT thermal response of TRβ PV mutant mice is shown after 30 min NE infusion (1076 mol/min). A, Wild-type (wt), heterozygous, and homozygous mice without any previous treatment; B, wild-type (wt), heterozygous, and homozygous, mice made hypothyroid by treatment with a low-iodine diet and propylthiouracil (0.15%) for 3 wk and then treated with daily ip doses of T3 (7 ng/g BW) or a saline vehicle control for 10 d. *, P < 0.01 vs. wild type; +, P < 0.01 vs. heterozygous. C, Comparison of heat production after NE infusion in animals as described in A and B, untreated (intact) or hypothyroid and treated with T3 (Hypo+T3). Ht, Heterozygous; Hz, homozygous; hypo, hypothyroid. *, P < 0.001 vs. TRβ PV mutant heterozygous intact; +, P < 0.001 vs. TRβ PV mutant homozygous intact. Values are mean ± sd of three to four mice.
Catecholamine responsiveness in wild-type and TRβ PV mutant mice made hypothyroid and replaced with a uniform dose of T3
Given the significant elevation in circulating thyroid hormone levels in the PV mutant mice, we postulated that these elevated levels were compensating for impaired thyroid hormone action in BAT and normalizing heat production. Wild-type and TRβ PV mutant mice, therefore, were made hypothyroid by treatment with a low iodine diet supplemented with the antithyroid agent propylthiouracil for 3 wk. Serum T4 concentrations were lower than 1 mg/dl, as we have previously shown using this protocol (20). Mice were treated with daily ip doses of T3 (7 ng/g BW) or a saline vehicle control for 10 d. In hypothyroid mice, even after 30 min of NE infusion, IBAT temperature increased only about 0.2 C (data not shown). Replacement of the hypothyroid wild-type mice with T3 restored the change in IBAT temperature in response to catecholamines (1.50 ± 0.46 C, change in temperature ± sem) to the level of the euthyroid group (2.00 ± 0.2 C). The heterozygous TRβ PV mutant mice had a significant deficit in the BAT thermal response to catecholamines (0.74 ± 0.08 C) that was further reduced in the homozygous TRβ PV mutant to the level of the hypothyroid animals (0.12 ± 0.01 C) (Fig. 1B). A direct comparison of the temperature change in response to catecholamine in the untreated mice and hypothyroid, T3-replaced mice is shown (Fig. 1C). The TRβ PV mutant mice made hypothyroid and treated with T3 had significantly lower heat production compared with the untreated mutant mice (both heterozygous and homozygous TRβ PV mutants, P < 0.001).
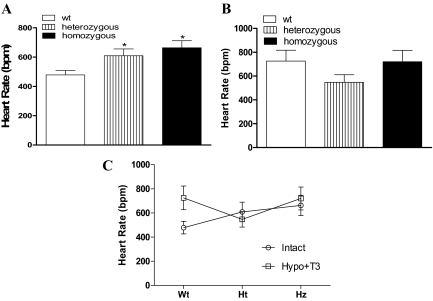
Heart rate in wild-type and TRβ PV mutant mice
Thyroid hormone regulation of heart rate is mediated by TRα, the predominant TR isoform expressed in the heart (20,26,27). Patients with RTH, which is associated with TRβ mutations, have tachycardia due to elevated serum thyroid hormone levels and largely unopposed T3 action on wild-type TRα (26,27,28). Consistent with the findings in patients with RTH, basal heart rate in anesthetized mice was significantly increased in the untreated heterozygous and homozygous TRβ PV mutant mice, when compared with untreated wild-type animals (Fig. 2A). Because the TRβ mutation in these animals is associated with high levels of circulating T4, we made wild-type and PV mutant animals hypothyroid and replaced them to a uniform thyroid status with T3. The basal heart rate was not significantly different in the TRβ PV mutant mice compared with wild-type mice after the serum levels of T3 were normalized (Fig. 2B) or comparing between the TRβ PV mutant mice untreated and hypothyroid treated with T3 groups (Fig. 2C). This is consistent with TRα predominantly expressed in pacemaker cells of the sinoatrial node influenced only by thyroid hormone levels and unaffected by a mutation in TRβ (26).
Figure 2.
Basal heart rate of anesthetized mice with the TRβ PV mutation. A, Wild-type (wt) and TRβ PV mutant heterozygous and homozygous mice without any previous treatment (intact). *, P < 0.05 vs. wt. B, Wild-type (wt), heterozygous, and homozygous made hypothyroid by treatment with a low-iodine diet and propylthiouracil (0.15%) for 3 wk and then treated with daily ip doses of T3 (7 ng/g BW) or a saline vehicle control for 10 d. C, Comparison of the basal heart rate of the same animals as described in A and B, untreated (intact) or hypothyroid and treated with T3 (Hypo+T3).
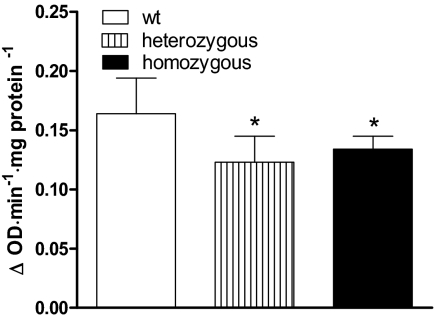
α-GPD activity in the heart
TRβ is expressed in the ventricle and mediates some T3-dependent gene expression (26,27). α-GPD is a thyroid hormone-regulated enzyme expressed in the heart (25). We determined whether the TRβ PV mutant receptor interfered with its expression in mice made hypothyroid and replaced with T3, as previously described. α-GPD activity was significantly reduced in heterozygous (∼25%) and in homozygous TRβ PV mutant animals (∼20%) (Fig. 3).
Figure 3.
α-GPD in the heart. α-GPD activity in heart of TRβ PV wild-type (wt), heterozygous, and homozygous mice made hypothyroid by treatment with a low-iodine diet and propylthiouracil (0.15%) for 3 wk and then treated with daily ip doses of T3 (7 ng/g BW) or a saline vehicle control for 10 d. *, P < 0.05 vs. wild type. Values are the mean ± sd of three to four mice.
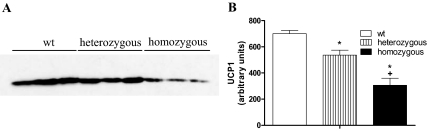
UCP1 protein levels in IBAT
We have previously shown, using the TRβ-selective agonist GC1, that mice with only TRβ stimulation have defective adaptive thermogenesis, but normal levels of UCP1 protein in BAT (20). We postulated that T3 stimulation was TRβ dependent and that mutations in the TRβ gene would impair UCP1 expression. We, therefore, compared the levels of brown fat UCP1 protein in wild-type and TRβ PV mutant mice. UCP1 protein levels, as determined by Western blot analysis (Fig. 4A), were reduced in TRβ PV heterozygotes (24% reduction) and further reduced in TRβ PV homozygous mutant mice (56% reduction) (Fig. 4B).
Figure 4.
Mitochondrial UCP1 in BAT. Protein from IBAT of wild-type (wt), heterozygous, and homozygous TRβ PV mutant mice made hypothyroid by treatment with a low-iodine diet and propylthiouracil for 3 wk and then treated with daily ip doses of T3 (7 ng/g BW) or a saline vehicle control for 10 d. A, Western blot of mitochondrial protein using anti-UCP1 antibody; B, blots were analyzed by densitometry, and the results are shown. *, P < 0.01 vs. wild type; +, P < 0.01 vs. heterozygous. Values are the mean ± sd of three to four mice.
Adrenergic responsiveness of primary brown adipocytes in vitro
cAMP
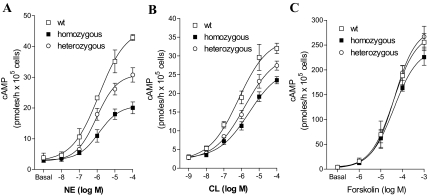
To evaluate the adrenergic stimulation pathway in the BAT, we measured cAMP generation in response to adrenergic stimulation in isolated brown fat adipocytes (Fig. 5). Mice were made hypothyroid as previously described for the in vivo studies and treated with saline or T3 for 10 d. Brown adipocytes were isolated, cultured, and treated with adrenergic stimuli for 60 min. Cells obtained from hypothyroid animals generated about 25% less cAMP than controls (P < 0.05). Brown adipocytes were incubated with various concentrations of NE, CL316,243 (a selective β3-adrenergic receptor agonist), and forskolin, and the generation of cAMP was measured. A dose-response increase in cAMP production was seen in brown adipocytes taken from T3-treated mice, with each of the adrenergic stimuli. In contrast, primary brown adipocytes from TRβ PV mutant mice showed reduced responsiveness to NE and CL316.243 but not to forskolin stimulation. NE-induced cAMP production is reduced by about 50% in the cells obtained from homozygous animals, whereas an intermediate response between wild-type and homozygous was observed in cells from the heterozygous TRβ PV mice. Direct stimulation of adenylyl cyclase with forskolin resulted in no differences in cAMP generation among the various genotypes, indicating that interference by the TRβ PV mutant is likely to be restricted to the precyclase level. Adrenergic sensitivity, therefore, is impaired by the dominant-negative PV mutation in the TRβ receptor. The cAMP response of brown adipocytes from hypothyroid T3-replaced mice was similar to euthyroid controls (data not shown).
Figure 5.
In vitro catecholamine-stimulated cAMP production in BAT. Dose-response curve of cAMP accumulation in isolated brown adipocytes from wild-type (wt), heterozygous, and homozygous TRβ PV mutant mice made hypothyroid by treatment with a low-iodine diet and propylthiouracil for 3 wk and then treated with daily ip doses of T3 (7 ng/d) or a saline vehicle control for 10 d. Brown adipocytes were incubated with the indicated doses of NE (A), the β3-adrenergic receptor-selective agonist CL316,243 (CL) (B), or forskolin (C), and cAMP accumulation was measured. Values are the mean ± sd of three tubes.
Glycerol release
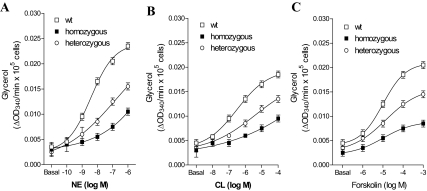
Lipolysis is a cAMP-dependent process, and a glycerol molecule is released for each triglyceride molecule that is hydrolyzed. We measured glycerol release in brown fat adipocytes in vitro to evaluate a biological action of cAMP production (Fig. 6). Brown fat adipocytes from the TRβ PV mutant mice had a blunted lipolytic response with all adrenergic stimulators compared with control, which was lowest in cells from the TRβ PV homozygous and intermediate in cells from the TRβ PV heterozygous mice. These results indicate that the TRβ PV mutation interferes with cAMP generation and downstream targets of thyroid-adrenergic synergism involved in protein kinase A activation.
Figure 6.
In vitro catecholamine-stimulated glycerol production in BAT. Dose-response curve of glycerol accumulation in isolated brown adipocytes from wild-type (wt), heterozygous, and homozygous TRβ PV mutant mice made hypothyroid by treatment with a low-iodine diet and propylthiouracil for 3 wk and then treated with daily ip doses of T3 (7 ng/d) or a saline vehicle control for 10 d. Brown adipocytes were incubated with the indicated doses of NE (A), the β3-adrenergic receptor-selective agonist CL316,243 (CL) (B), or forskolin (C), and glycerol accumulation was measured. Values are the mean ± sd of three tubes.
Discussion
There are several examples of TR isoform-specific functions developmentally and in adult tissues (28). Specific TR isoforms are important for thyroid hormone action, in the brain, the regulation of TRH and TSH, and sensory development (29,30,31). A spatial and temporal pattern of TR isoform expression has been especially well described for development of the inner ear and retina (30). There are also key contributions from expression of specific deiodinase enzymes, especially D2, which converts T4 to the active form T3, and the type 3 5-deiodinase enzyme (D3), which converts T3 to the inactive compound rT3 (30,32). The deiodinase enzymes also have temporal and spatial patterns of expression that are required for normal development of many tissues (30,32,33).
Thyroid hormone regulates a wide range of metabolic functions and alterations in thyroid status have long been associated with changes in resting energy expenditure, adaptive thermogenesis, body fat, and serum lipoproteins (2,28,34). TR isoform-specific agonists have been developed to exploit isoform-specific functions in the heart and lipoprotein metabolism (35,36). Significant success has been achieved with these agents to reduce cholesterol, triglycerides, and with at least one agent, a reduction in body weight (37,38). The action of these agonists is related to TR isoform specificity as well as greater concentration in specific tissues, especially the liver (35,36).
BAT in rodents has been well characterized with respect to the elements required for adaptive thermogenesis, including adrenergic stimulation via the β3-adrenergic receptor, local activation of thyroid hormone via D2, and UCP1 expression (39,40,41). We have previously shown that the TRα isoform is required for adrenergic stimulation of BAT as well as heat production in response to cold, although BAT UCP1 levels remained normal (20,21). The current study extends these findings to show that TRβ has an essential role in regulating UCP1 gene expression.
Brown fat is required for rodents to defend against cold exposure, but its role in humans has been thought to be limited. Several recent studies using PET scans to show areas of hypermetabolism and computerized tomography to show fat density corresponding to the area of hypermetabolism, however, have identified a significant amount of BAT in human adults (42,43,44). These studies showed that BAT in humans was increased by cold ambient temperature and that the greatest amount of BAT was found in individuals that were younger age and lean and did not use β-blockers. BAT, however, was not found in most adults, and the amount was relatively modest. The physiological role of BAT in human adults is not known, but the finding of increased BAT in response to cold and reduction with β-blockade indicates regulation similar to that seen in rodents.
UCP1 and UCP3 gene expression is stimulated by thyroid hormone (40,41,45). The UCP1 regulatory region in the rat gene has been extensively characterized. An enhancer region, located approximately 2.5 kb upstream of the transcription start site, contains cAMP response elements, two thyroid hormone response elements (TREs), and regions that confer response to retinoic acid and peroxisome proliferator-activated receptor-γ. There are at least two TREs that bind TR and confer T3 responsiveness; one is an everted palindrome, which preferentially binds TR homodimers, and the other is a direct repeat with a 3-bp gap that binds TR/retinoid X receptor heterodimers (46,47,48). Although a comparison of TR isoform preference for these TREs has not been reported, TRβ has been shown to bind and transactivate both TREs. Pharmacological and genetic approaches to assess the role of specific TR isoforms in gene regulation have, in general, been much more sensitive tools to assess TR isoform specificity than transient transfection or binding assays (28,49,50).
The TRβ PV mutant does not bind T3 and additionally exhibits a dominant-negative effect on thyroid hormone signaling by competing with wild-type TRs (22,49,50). The TRβ PV mutant competes for binding to TRE in vivo as a heterodimeric partner with wild-type TR and with retinoid X receptor (50). As a result, the influence of the mutation on thyroid hormone action varies according to the native TR isoform expression in the tissue. Decreased thyroid hormone feedback signaling in the TRβ-predominant pituitary gland leads to elevated TSH secretion and increased thyroid hormone production (23,29). The resulting phenotype is a combination of increased thyroid hormone action in tissues such as the heart with TRα1 predominance (26) and reduced thyroid hormone action in tissues such as liver, in which the TRβ isoform predominates (50). This is illustrated in the present study by the fact that the heart rate of the TRβ PV animals was significantly higher than wild-type animals but was normalized when the animals were made hypothyroid and replaced with uniform levels of T3. The myocardial α-GPD activity was significantly lower in the T3-treated TRβ PV mice, demonstrating some reduction in thyroid hormone signaling in the ventricle, which expresses TRβ (26). This is likely due to reduced TRβ signaling but may also be due to TRβ PV antagonism of TRα (49).
The in vitro lipolytic response in BAT (Figs. 5 and 6) reflects the complex interaction of thyroid hormone action and adrenergic signaling. Thyroid hormone has been reported to act at many levels of adrenergic signaling including adrenergic receptor type, number, receptor affinity, and signal transduction pathways (34). Studies using the TRβ-selective agonist GC1 and a TRα point mutant mouse have shown that TRα is required for adrenergic-stimulated lipolysis in brown fat and white fat (20,21,51). Hormone-sensitive lipase expression, a final pathway of adrenergic signaling, is reduced in TRα mutant mice (21). Our findings with brown fat from mice with the TRβ PV mutation show a decrement in brown fat lipolysis. Although there was a reduction in glycerol production in response to catecholamines, the magnitude of the reduction is not as great as that seen with TRα mutation or with only GC1 stimulation (20,21). There are a number of possible mechanisms, which may all contribute. TRβ is likely to play a role in adrenergic signaling at one of the multiple sites of thyroid hormone influence (34). The mutant TRβ preferentially antagonizes wild-type TRβ but can also inhibit wild-type TRα action (49).
BAT expresses both TR isoforms, and adaptive thermogenesis requires thyroid hormone action. Distinct pathways have previously been linked to the TRα isoform in whole-animal BAT and in isolated brown adipocytes (20,21,51). BAT, therefore, provides an excellent model to define specific TR isoform actions within a tissue. We have shown that TRβ activation is essential for UCP1 expression, whereas TRα and TRβ mediate the synergism between thyroid hormone and adrenergic signaling that results in increased cAMP production and downstream actions, such as lipolysis (20,21,51). The thermogenic capacity of BAT from the TRβ PV mutant mice in response to NE infusion was reduced when serum T3 levels were normalized in the mutant mice. There was moderately reduced BAT thermal response in the heterozygous animals and an almost completely abolished response in the homozygous mice. This finding is in agreement with the reduction in UCP1 protein levels in heterozygous animals and nearly absent expression in the homozygous mice. These studies demonstrate TR isoform specificity for UCP1 regulation in BAT, which is required for adaptive thermogenesis. The availability of selective TR isoform pharmacological agonists and antagonists will allow for further probing of the mechanisms of these pathways and potential therapeutic applications in metabolic disorders and obesity.
Footnotes
This work was supported by NIH RO1 DK43714 (G.A.B.), a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) in Brazil (M.O.R.), Veterans Affairs Medical Research Funds (G.A.B.), and NIH RO1 DK65055 (A.C.B.).
Current address for M.O.R.: Curso de Ciências Biológicas, Centro de Ciências Biológicas e da Saúde, Universidade Presbiteriana Mackenzie, São Paulo, Brazil 01302-907.
Disclosure Summary: M.O.R., S.D.C.B., M.K., J.J.S., S.-Y.C., A.C.B., and G.A.B. have nothing to declare.
First Published Online November 11, 2009
Abbreviations: BAT, Brown adipose tissue; BW, body weight; D2, type 2 5′-deiodinase; α-GPD, α-glycerol phosphate dehydrogenase; IBAT, interscapular BAT; NE, norepinephrine; PV, frameshift mutation in resistance to thyroid hormone patient PV; RTH, resistance to thyroid hormone; TRα, thyroid hormone receptor-α; TRE, thyroid hormone response element; UCP1, uncoupling protein 1.
References
- Lowell BB, Spiegelman BM 2000 Towards a molecular understanding of adaptive thermogenesis. Nature 404:652–660 [DOI] [PubMed] [Google Scholar]
- Silva JE 2005 Thyroid hormone and the energetic cost of keeping body temperature. Biosci Rep 25:129–148 [DOI] [PubMed] [Google Scholar]
- Curcio C, Lopes AM, Ribeiro MO, Francoso Jr OA, Carvalho SD, Lima FB, Bicudo JE, Bianco AC 1999 Development of compensatory thermogenesis in response to overfeeding in hypothyroid rats. Endocrinology 140:3438–3443 [DOI] [PubMed] [Google Scholar]
- Ribeiro MO, Lebrun FL, Christoffolete MA, Branco M, Crescenzi A, Carvalho SD, Negrão N, Bianco AC 2000 Evidence of UCP1-independent regulation of norepinephrine-induced thermogenesis in brown fat. Am J Physiol Endocrinol Metab 279:E314–E322 [DOI] [PubMed] [Google Scholar]
- Carvalho SD, Bianco AC, Silva JE 1996 Effects of hypothyroidism on brown adipose tissue adenylyl cyclase activity. Endocrinology 137:5519–5529 [DOI] [PubMed] [Google Scholar]
- Rubio A, Raasmaja A, Maia AL, Kim KR, Silva JE 1995 Effects of thyroid hormone on norepinephrine signaling in brown adipose tissue. I. β1- and β2-adrenergic receptors and cyclic adenosine 3′,5′-monophosphate generation. Endocrinology 136:3267–3276 [DOI] [PubMed] [Google Scholar]
- Rubio A, Raasmaja A, Silva JE 1995 Thyroid hormone and norepinephrine signaling in brown adipose tissue. II. Differential effects of thyroid hormone on β3-adrenergic receptors in brown and white adipose tissue. Endocrinology 136:3277–3284 [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Locke RM 1984 Thermogenic mechanisms in brown fat. Physiol Rev 64:1–64 [DOI] [PubMed] [Google Scholar]
- Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP 1997 Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387:90–94 [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J 2009 UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9:203–209 [DOI] [PubMed] [Google Scholar]
- Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB 1995 Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem 270:29483–29492 [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB 2002 β-Adrenergic receptor signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Silva JE 1987 Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 79:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Silva JE 1987 Optimal response of key enzymes and uncoupling protein to cold in BAT depends on local T3 generation. Am J Physiol 253:E255–E263 [DOI] [PubMed] [Google Scholar]
- Silva JE, Larsen PR 1983 Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305:712–713 [DOI] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC 2001 The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Germain DL, Galton VA, Hernandez A 2009 Defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology 150:1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B 1998 Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrif H, Schifman A, Stepanyan Z, Gillis MA, Calderone A, Weiss RE, Samarut J, Silva JE 2005 Temperature homeostasis in transgenic mice lacking thyroid hormone receptor-α gene products. Endocrinology 146:2872–2884 [DOI] [PubMed] [Google Scholar]
- Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA 2001 Thyroid hormone-sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J Clin Invest 108:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Schultz JJ, Brent GA 2003 A thyroid hormone α gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem 278:38913–38920 [DOI] [PubMed] [Google Scholar]
- Bhat MK, McPhie P, Ting YT, Zhu XG, Cheng SY 1995 Structure of the carboxy-terminal region of thyroid hormone nuclear receptors and its possible role in hormone-dependent intermolecular interactions. Biochemistry 34:10591–10599 [DOI] [PubMed] [Google Scholar]
- Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S 2000 Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97:13209–13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Silva E, Schwartz HL, Surks MI 1977 Stimulation of hepatic mitochondrial α-glycerophosphate dehydrogenase and malic enzyme by l-triiodothyronine: characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest 59:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson EA, Gloss B, Belke DD, Kaneshige M, Cheng SY, Dillmann WH 2003 Cardiac expression and function of thyroid hormone receptor and its β PV mutant. Endocrinology 144:4820–4825 [DOI] [PubMed] [Google Scholar]
- Kahaly GJ, Dillmann WH 2005 Thyroid hormone action in the heart. Endocr Rev 26:704–728 [DOI] [PubMed] [Google Scholar]
- Brent GA 2000 Tissue-specific actions of thyroid hormone: insights from animal models. Rev Endocr Metab Disord 1:27–33 [DOI] [PubMed] [Google Scholar]
- Chiamolera MI, Wondisford FE 2009 Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology 150:1091–1096 [DOI] [PubMed] [Google Scholar]
- Nunez J, Celi FS, Ng L, Forrest D 2008 Multigenic control of thyroid hormone functions in the nervous system. Mol Cell Endocrinol 287:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano J, Morte B, Scanlan TS, Bernal J 2003 Differential effects of triiodothyronine and the thyroid hormone receptor β-specific agonist GC-1 on thyroid hormone target genes in the brain. Endocrinology 144:5480–5487 [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC 2008 Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MH, Martinez de Mena R, Obregon MJ, Marinkovic D, Howatson A, Visser TJ, Hume R, Morreale de Escobar G 2004 Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab 89:3117–3128 [DOI] [PubMed] [Google Scholar]
- Silva JE, Bianco SD 2008 Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 18:157–165 [DOI] [PubMed] [Google Scholar]
- Baxter JD, Webb P, Grover G, Scanlan TS 2004 Selective activation of thyroid hormone signaling pathways by GC-1: a new approach to controlling cholesterol and body weight. Trends Endocrinol Metab 15:154–157 [DOI] [PubMed] [Google Scholar]
- Brenta G, Danzi S, Klein I 2007 Potential therapeutic applications of thyroid hormone analogs. Nat Clin Pract Endocrinol Metab 3:632–640 [DOI] [PubMed] [Google Scholar]
- Berkenstam A, Kristensen J, Mellström K, Carlsson B, Malm J, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjöberg F, Angelin B, Baxter JD 2008 The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA 105:663–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover GJ, Mellström K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennström B, Mookhtiar K, Horvath R, Speelman J, Egan D, Baxter JD 2003 Selective thyroid hormone receptor-β activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci USA 100:10067–10072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JE 2001 The multiple contributions of thyroid hormone to heat production. J Clin Invest 108:35–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JE, Rabelo R 1997 Regulation of the uncoupling protein gene expression. Eur J Endocrinol 136:251–264 [DOI] [PubMed] [Google Scholar]
- Reitman ML, He Y, Gong DW 1999 Thyroid hormone and other regulators of uncoupling proteins. Int J Obesity 23(Suppl 6):S56–S59 [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR 2009 Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ 2009 Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508 [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P 2009 Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525 [DOI] [PubMed] [Google Scholar]
- Lanni A, Moreno M, Lombardi A, Goglia F 2003 Thyroid hormone and uncoupling proteins. FEBS Lett 543:5–10 [DOI] [PubMed] [Google Scholar]
- Rabelo R, Schifman A, Rubio A, Sheng X, Silva JE 1995 Delineation of thyroid hormone-responsive sequences within a critical enhancer in the rat uncoupling protein gene. Endocrinology 136:1003–1013 [DOI] [PubMed] [Google Scholar]
- Rabelo R, Reyes C, Schifman A, Silva JE 1996 Interactions among receptors, thyroid hormone response elements, and ligands in the regulation of the rat uncoupling protein gene expression by thyroid hormone. Endocrinology 137:3478–3487 [DOI] [PubMed] [Google Scholar]
- Rabelo R, Reyes C, Schifman A, Silva JE 1996 A complex retinoic acid response element in the uncoupling protein gene defines a novel role for retinoids in thermogenesis. Endocrinology 137:3488–3496 [DOI] [PubMed] [Google Scholar]
- Zavacki AM, Harney JW, Brent GA, Larsen PR 1993 Dominant negative inhibition by mutant thyroid hormone receptors is thyroid hormone response element and receptor isoform specific. Mol Endocrinol 7:1319–1330 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kaneshige M, Kamiya Y, Kaneshige K, McPhie P, Cheng SY 2002 Differential expression of thyroid hormone receptor isoforms dictates the dominant negative activity of mutant β receptor. Mol Endocrinol 16:2077–2092 [DOI] [PubMed] [Google Scholar]
- Liu YY, Heymann RS, Moatamed F, Schultz JJ, Sobel D, Brent GA 2007 A mutant thyroid hormone receptor α antagonizes peroxisome proliferator-activated receptor α signaling in vivo and impairs fatty acid oxidation. Endocrinology 148:1206–1217 [DOI] [PubMed] [Google Scholar]