Abstract
BACKGROUND
Early life development may influence the timing of natural menopause through association with size of the initial follicle pool or early follicular loss. This study examines the relationships of birthweight, gestational age and birthweight standardized by gestational age with early menopause in the 1958 British birth cohort study.
METHODS
Study participants were over 2900 women with data on birthweight, gestational age (obtained at birth), menopausal status at age 44–45 years and potential confounding factors. Logistic regression was used to study relationships of birthweight, gestational age and birthweight standardized by gestational age with post-menopausal status by 44–45 years, with and without adjustments for confounding factors.
RESULTS
There was a U-shaped association between birthweight and menopausal status at 44–45 years: women at either extremes of birthweight (<2.5 and ≥4.0 kg) had increased odds of post-menopausal status compared with those weighing 3.0–3.49 kg [odds ratio (OR) = 1.91, 95% confidence interval (CI) 1.08, 3.38; 1.81, 95% CI 1.11, 2.97, respectively]. Women with higher birthweight standardized by gestational age (which indicates faster fetal growth rate) also had increased odds of being post-menopausal by 44–45 years (OR for fastest quarter versus second fastest quarter = 1.80; 95% CI 1.16, 2.81). These associations persisted after adjustment for socioeconomic position at birth, adult smoking status and use of oral contraceptives.
CONCLUSIONS
These findings suggest that variations in fetal environment may be associated with the timing of menopause. Given that extremes of birthweight and higher birthweight standardized by gestational age were associated with earlier age at menopause, mechanisms related to these characteristics that also regulate ovarian function should be investigated further.
Keywords: menopause, fetal environment, birth cohort, birthweight, fetal growth
Introduction
Early life development may be critical for the timing of menopause. The primordial follicle pool size reaches its maximum at approximately 5 months gestation, with follicular loss already occurring by the onset of post-natal life (Ginsberg, 1991; Forabosco and Sforza, 2007). Disruptions to the creation of the follicle pool or accelerated in utero or post-natal follicle loss could result in earlier menopause due to an earlier exhaustion of the follicular pool. The developmental origins of adult disease hypothesis propose that early life environment alters the structure and function of an organism such that it has decreased the ability to respond to the adult environment (Gluckman et al., 2005). If this paradigm applies to menopause, poor fetal environment may signal to the body to favour earlier reproduction and earlier menopause. Early natural menopause is associated with future health outcomes, including increased risk of all-cause and cause-specific mortality, notably stroke and cardiovascular disease (Snowdon et al., 1989; Cooper and Sandler, 1998; Cooper et al., 2000; Mondul et al., 2005; Ossewaarde et al., 2005). As stroke and cardiovascular disease have also been shown to be related to fetal environment (Lawlor et al., 2005, 2007), it is possible that ovarian aging is part of the broader aging process (Dorland et al., 1998).
While autoimmune conditions, infections and X chromosome abnormalities (Beck-Peccoz and Persani, 2006) are established risk factors for premature ovarian failure (depletion of ovarian follicles prior to age 40), few risk factors for early menopause outside this premature age range have been identified. From a limited number of studies that have examined early life factors, shorter length at birth, higher ponderal index, larger placenta, low weight gain by age 1 year (Cresswell et al., 1997) and childhood social conditions (Hardy and Kuh, 2005; Mishra et al., 2007) have been found to be associated with earlier age at menopause. Only a few studies have examined associations with birthweight or gestational age, but none have demonstrated relationships with age at menopause (Cresswell et al., 1997; Treloar et al., 2000; Hardy and Kuh, 2002; Mishra et al., 2007). However, no studies have yet used birthweight standardized by gestational age, an indicator of fetal growth rate. Birthweight standardized by gestational age has been shown to be related to adult outcomes, including systolic blood pressure (Leon et al., 2000; Lawlor et al., 2007) and mortality from ischaemic heart disease (Leon et al., 1998). Among risk factors later in life, smoking and nulliparity have been shown to be related to early age at menopause, but results concerning the relationships with other adult risk factors, such as reproductive health and socioeconomic position, have been mixed (Whelan et al., 1990; Luoto et al., 1994; Torgerson et al., 1994; Cramer et al., 1995; Bromberger et al., 1997; Nilsson et al., 1997; van Noord et al., 1997; Gold et al., 2001; Mikkelsen et al., 2007).
The aim of this study was to examine the relationship between fetal environment and early age at menopause using a large British birth cohort study with risk factors measured prospectively over the life course. We hypothesized that poor fetal environment, as indicated by low birthweight, early gestational age and low birthweight standardized by gestational age (as an indicator of slow fetal growth) would be associated with earlier age at menopause.
Materials and Methods
Study population
The 1958 British birth cohort study consists of 17 638 males and females followed up since their births during 1 week in March 1958 in England, Scotland and Wales, with 920 immigrants with the same birth dates recruited into the study up to age 16 years (Power and Elliott, 2006). Of the 8522 females who have been cohort members since birth (437 female immigrants were not included in these analyses because they lacked data on birth characteristics), 4506 participated in a biomedical survey at age 44–45 years, when menopausal status was assessed, with 4403 women providing sufficient information for a valid menopausal status to be assigned. The South East Multi-Centre Research Ethics Committee granted ethical approval for this study.
Menopausal status
At age 44–45 years, women were asked to complete a series of questions about their menstrual cycles in the previous year, hormone therapy (HT) use and gynaecological surgery. Using this information menopausal status at age 44–45 years was defined as premenopausal (menstruation reported within the last 3 months), perimenopausal (3–12 months of amenorrhea or reports of periods becoming less regular in the absence of amenorrhea) or post-menopausal (at least 12 months of amenorrhea with no other reason reported to explain cessation). For the purposes of analyses, a binary outcome variable was created: post-menopausal versus premenopausal or perimenopausal. Women whose periods stopped because of surgery, for some other reason (including radiation therapy or chemotherapy), or who initiated HT use prior to their final menstrual period were considered in a separate category.
Fetal environment
Information on birthweight (measured in ounces and converted into kilograms) and gestational age (measured in completed weeks since the start of the mother's last menstrual period) was recorded at birth. Birthweight was standardized by gestational age as follows: the difference between the individual's birthweight and the mean birthweight was calculated for that gestational age (in weeks) divided by the standard deviation of birthweight for that gestational age (Leon et al., 1998; Lawlor et al., 2005). This birthweight for gestation z-score allows comparison of the relative variation in birthweight independent of gestational age, and has been interpreted as an indicator of fetal growth rate. In some analyses, these variables were considered as continuous terms. In addition, birthweight was categorized into five groups: < 2.5 kg, 2.5–2.9, 3.0–3.49 (reference group), 3.5–3.9, ≥ 4.0 kg; gestational age was categorized into six groups: < 37 weeks, 37, 38, 39, 40 (reference group), > 40 weeks and birthweight for gestation z-scores were categorized into quarters (second highest quarter as reference group). For birthweight, the reference group was selected because it contained the mean and for birthweight for gestation z-scores, a similar category in terms of relative position was selected.
Potential confounding factors
Childhood socioeconomic position was indicated by the father's occupation at the cohort member's birth and grouped as follows: I or II (professional and managerial), III non-manual (unskilled), III manual (skilled) and IV and V (semi-skilled and unskilled manual) or female head of the household. If the father's occupation at birth was missing, then his occupation when the cohort member was aged 7 years was utilized (n = 64). At age 42 years, the same categorizations of occupation were used to describe the socioeconomic position of the cohort member. At this age, the cohort member's highest level of educational attainment was also recorded, and her current smoking status was categorized as current, former or never smoker. At age 44–45 years, the respondent reported years of use of oral contraceptives (or contraception through an injection) and number of biological children, i.e. parity. BMI (kg/m2) at this age was calculated from measured heights and weights.
Statistical analyses
Separate logistic regression models were utilized to test the unadjusted relationships between each of the three indicators of fetal environment (birthweight, gestational age and birthweight standardized by gestational age) and the binary outcome of post-menopausal status at age 44–45 years. Nonlinearity was tested by including quadratic as well as linear continuous terms in models. Where there was no evidence against linearity, a continuous, linear term was included in subsequent models. Where there was evidence of nonlinearity, linear and quadratic terms were included in subsequent models, and variables were also modelled as categorical terms (using the categorizations described above).
In a second stage of analysis, adjustments were made for all potential confounders that were found to be associated with post-menopausal status at the 0.05 significance level based on χ2 tests.
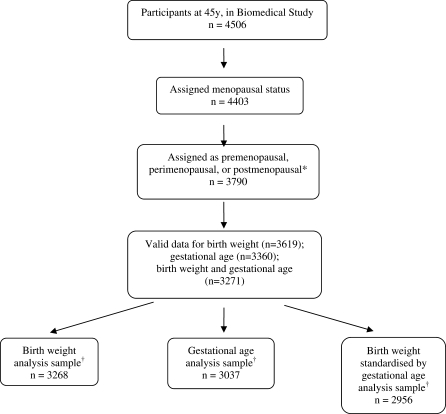
The main analyses presented were run on a sample excluding the women who initiated HT use prior to menopause (n = 158) or whose periods stopped due to surgery (n = 435) or for other reasons (n = 20). Women with missing data on covariates were also excluded from all analyses, giving analysis samples ranging from 3268 to 2956 women, depending on the exposure examined (Fig. 1). Sensitivity analyses were performed to check the effect on the findings of these exclusions. First, all analyses were repeated including women whose periods stopped due to surgery or other reasons or who initiated HT use prior to menopause as post-menopausal: results were similar to those presented here in which these women were excluded. Second, unadjusted analyses were rerun using maximum available samples and compared with those presented here excluding those with incomplete covariate information; the findings were again found to be similar.
Figure 1.
Study participants (numbers with data) to examine the relationships of birthweight, gestational age and birthweight standardized by gestational age with early menopause in the 1958 British birth cohort study. *Exclusions in this step: hormone therapy (n = 158), hysterectomy (n = 435) and other reasons for periods stopping (n = 20). †Excludes women with missing data on covariates.
All analyses were performed using Stata Version 9.
Results
Table I presents the characteristics of the study sample. At age 44–45 years, 73% of the female cohort members, included in analyses, were still premenopausal, 21% were perimenopausal and 6% were post-menopausal. In unadjusted analyses, women of higher socioeconomic position at birth (P = 0.04) and who had used oral contraceptives (P < 0.01) were less likely to be post-menopausal by age 44–45 years compared with their counterparts, where as current smokers at age 42 years were more likely to be post-menopausal at age 44–45 years compared with former or never smokers. Other covariates (i.e. socioeconomic position at age 42 years, age at menarche, parity by age 44–45 years, educational attainment by age 42 years or BMI at age 44–45 years) were not associated with menopausal status.
Table I.
Characteristics of the study population (including women with a valid ‘natural’ menopausal status and at least one measure of fetal environment).
| (%) | N | |
|---|---|---|
| Menopausal status at age 44–45 years | 3708 | |
| Premenopausal | 73.0 | |
| Perimenopausal | 21.0 | |
| Post-menopausal | 6.0 | |
| Birthweight (kg) | 3619 | |
| <2.5 | 5.3 | |
| 2.5–2.9 | 21.5 | |
| 3.0–3.49 | 40.4 | |
| 3.5–3.9 | 24.4 | |
| ≥ 4.0 | 8.4 | |
| Gestational age (weeks) | 3360 | |
| <37 | 3.7 | |
| 37 | 4.1 | |
| 38 | 9.5 | |
| 39 | 22.0 | |
| 40 | 27.5 | |
| > 40 | 33.2 | |
| Father's occupational class at birth | 3703 | |
| I or II (professional/managerial) | 19.9 | |
| III non-manual | 10.2 | |
| III manual | 48.1 | |
| IV, V, female head of household | 21.8 | |
| Woman's occupational class at age 42 years | 3545 | |
| I or II (professional/managerial) | 38.2 | |
| III non-manual | 33.2 | |
| III manual | 7.6 | |
| IV or V | 21.0 | |
| Educational level attained by age 42 years | 3708 | |
| None | 7.9 | |
| Some qualifications | 15.1 | |
| O level | 34.0 | |
| A level | 10.5 | |
| Degree or higher | 32.5 | |
| Age at menarche (years) | 2816 | |
| ≤ 11 | 18.7 | |
| 12 | 23.3 | |
| 13 | 28.8 | |
| 14 | 20.1 | |
| ≥ 15 | 9.1 | |
| Parity at age 44–45 years | 3450 | |
| No children | 17.5 | |
| 1 | 16.1 | |
| 2 | 42.8 | |
| 3 | 17.9 | |
| ≥ 4 children | 5.7 | |
| Oral contraceptive use by age 44–45 years | 3449 | |
| Never used | 8.3 | |
| 0–4.9 | 20.8 | |
| 5–9.9 | 23.5 | |
| 10–14.9 | 21.7 | |
| ≥ 15 | 25.7 | |
| Smoking at 42 years | 3353 | |
| Current | 21.6 | |
| Former | 22.1 | |
| Never | 56.3 | |
| BMI (kg/m2) at 44–45 years | 3697 | |
| < 25.0 | 43.9 | |
| 25.0–30.0 | 33.0 | |
| > 30.0 | 23.1 |
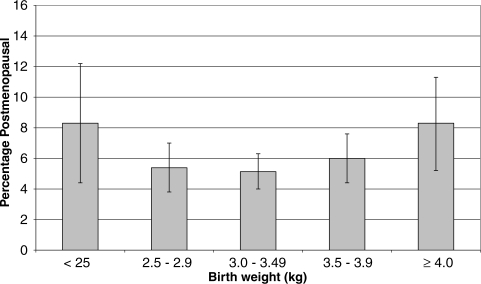
When examining the association between birthweight and menopausal status, there was evidence of a U-shaped relationship (Table II and Fig. 2). In the unadjusted model, women who weighed <2.5 kg at birth and women who weighed 4.0 kg or more were more likely to be post-menopausal at age 44–45 years than women who weighed between 3.0 and 3.49 kg. Women with the highest and lowest levels of birthweight continued to have increased odds of being post-menopausal by age 44–45 years after adjustment for potential confounders. In a model using continuous terms, the P-value for the quadratic term retained significance (P < 0.001).
Table II.
Associations of birthweight, gestational age and birthweight standardized by gestational age with post-menopausal status at age 44–45 years in the 1958 British birth cohort study.
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | |
|---|---|---|
| Birthweight (kg) (n = 3268) | ||
| < 2.5 | 1.91 (1.08, 3.38) | 1.81 (1.02, 3.22) |
| 2.5–2.9 | 1.10 (0.73, 1.67) | 1.08 (0.71, 1.63) |
| 3.0–3.49 | 1.00 | 1.00 |
| 3.5–3.9 | 1.27 (0.87, 1.86) | 1.28 (0.88, 1.88) |
| ≥ 4.0 | 1.81 (1.11, 2.97) | 1.84 (1.12, 3.03) |
| Gestational age (n = 3037) per 1 week increase | 0.98 (0.90, 1.08) | 0.98 (0.90, 1.08) |
| Birthweight standardized by gestational age (n = 2956) | ||
| Quarter 1 (lowest) | 1.27 (0.79, 2.03) | 1.21 (0.75, 1.95) |
| 2 | 1.15 (0.71, 1.88) | 1.14 (0.70, 1.87) |
| 3 | 1.00 | 1.00 |
| Quarter 4 (highest) | 1.80 (1.16, 2.81) | 1.84 (1.18, 2.88) |
OR, odds ratio; CI, confidence interval.
*Adjusted for socioeconomic position at birth, smoking status at age 42 years and oral contraceptive use up to age 44–45 years.
Figure 2.
Percentage (SE) post-menopausal by age 44–45 years by birthweight.
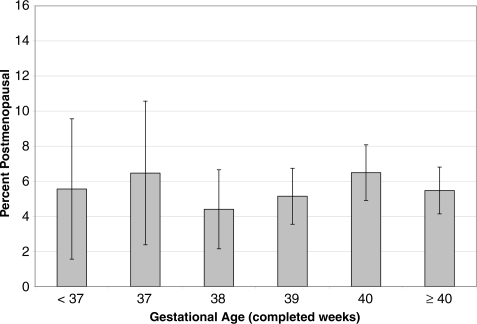
There was no evidence of a relationship between gestational age and post-menopausal status in unadjusted or adjusted analyses (Table II and Fig. 3).
Figure 3.
Percentage (SE) post-menopausal by age 44–45 years by gestational age (completed weeks).
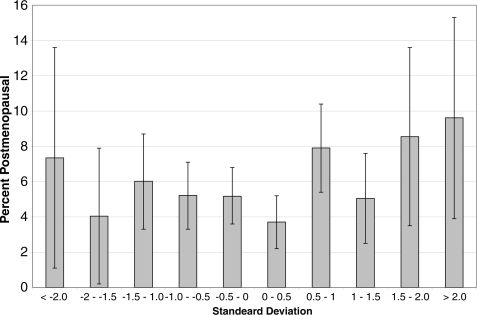
In unadjusted models, women in the highest quarter of birthweight standardized by gestational age were more likely to be post-menopausal when compared with women in the second highest quarter (Table II and Fig. 4). This relationship remained after adjustment for potential confounders: women with low birthweight standardized by gestational age showed an increased risk of being post-menopausal, although the difference from the reference category did not reach conventional statistical significance. However, in analysis of the continuous birthweight standardized by gestational age variable the quadratic term retained significance (P = 0.03), suggesting a curvilinear association.
Figure 4.
Percentage (SE) post-menopausal by age 44–45 years by birthweight standardized by gestational age (presented as a standard deviation score).
Discussion
This study tested the hypothesis that a poor fetal environment is related to early age at menopause. There was evidence of an increased risk of menopause by 44–45 year among women with low weight at birth, possibly indicating an effect of poor fetal environment. However, women who had the heaviest weight at birth or highest birthweight standardized by gestational age (which is an indicator of faster fetal growth rate) were also more likely to be post-menopausal by age 44–45 years than women with mid-range values of these measures. These results suggest that mechanisms relating fetal environment to menopause, additional to those originally proposed, may also influence risk of early age at menopause. Childhood socioeconomic position, use of oral contraceptives and smoking were related to early menopause but these factors did not account for the relationships between indicators of fetal environment and early menopause.
To our knowledge, this study is the first to observe a relationship between birthweight and increased risk of early menopause: previous studies did not find an association (Treloar et al., 2000; Hardy and Kuh, 2002). Our results are consistent with a previous study which found no relationship between gestational age (Cresswell et al., 1997) and age at menopause among a study group that included a wider range of age at menopause. Our results for birthweight standardized by gestational age suggest that growth rate, and not necessarily prematurity, is the characteristic of interest concerning early menopause. Evidence suggests that risk factors for early menopause differ from those for later menopause (Mishra et al., 2007). However, most studies have considered relationships between risk factors and age at menopause over a broader age range. Such an approach may obscure relationships between fetal environment and early menopause specifically. Further, some previous analyses that have used gestational age or birthweight have utilized small samples (Cresswell et al., 1997).
Study strengths and limitations
Strengths of this study include the use of a large population-based sample, thereby increasing study power to detect associations. We were also able to focus specifically on early onset of menopause. In addition, because this study is a prospective birth cohort and all women were born in the same week of the same year, we can be confident that the results are not explained by periods or cohort effects, and problems associated with recall are minimized.
One limitation of this study is that the actual age at menopause for those women post-menopausal by age 44–45 years was not recorded. Data on timing of menopause would have allowed the use of a method such as survival analysis that incorporates data on women who initiated HT use prior to the cessation of menstruation or whose periods stopped for other reasons. The exclusion of these women in the current analysis may have skewed the distribution of age at menopause towards a higher age, and bias may have been introduced. For example, young age at hysterectomy may be indicative of early ovarian aging (Cooper et al., 2008). Further, initiation of HT prior to the final menstrual period is likely to indicate a response to symptoms related to the menopausal transition (Dennerstein et al., 2000; Hardy and Kuh, 2002; Kravitz et al., 2008), suggesting that both these sets of women may have been likely to experience an early menopause. However, as detailed above, the results from sensitivity analyses showed that when these women were included in the post-menopausal group findings were similar.
A further limitation of not having information on timing of menopause is that it was not possible to exclude women with premature ovarian failure, who may have a different risk profile than women who undergo early menopause within the normal range. The Study of Women's Health Across the Nation found that among women who had experienced menopause by age 45 years, 35% had premature ovarian failure (Luborsky et al., 2003). Another potential limitation of the study concerns sample attrition and exclusion of women with missing data on covariates. Surviving study members who had low birthweight were less likely than those with higher birthweight to participate in the survey at 44-45 years (Atherton et al., 2008).
Possible mechanisms for the association
Previous studies have found that impaired fetal growth, which low birthweight indicates, was related to detrimental changes to the pancreas (Fowden et al., 2005) and kidney (Schreuder et al., 2006), in support of an association between poor fetal environment and limitations in development. Because the ovaries are also located in the trunk section of the body and are related to these organs in the development process, it is possible that the ovaries are prone to similar effects (de Bruin et al., 2001). However, anatomical analyses of fetal ovaries do not suggest that slow fetal growth is associated with smaller follicle pool size or accelerated depletion (de Bruin et al., 1998, 2001). Previous papers have also found a relationship between size at birth and other reproductive measures. For example, small size at birth has been shown to be related to earlier menarche (Cooper et al., 1996; Gluckman and Hanson, 2006), and babies of low birthweight exhibit ovarian function suppression in response to energetic stress (Jasienska et al., 2006).
Gestational diabetes has been shown to be related to increased birthweight and fetal growth as well as adult outcomes, such as metabolic syndrome and atherosclerosis (Vohr and Boney, 2008). It is therefore possible that gestational diabetes could also explain the relationship between characteristics at birth and early age at menopause. However, because few mothers of our study members had gestational diabetes (n < 10) (Thomas et al., 2007), it was not possible to investigate this pathway. Another possible explanation of our findings concerning greater weight at birth and faster fetal growth is that these characteristics could be related to exposure to elevated hormonal levels in utero, which could also be associated with menopausal age. Animal models have suggested that in utero levels of estrogen and other hormones may play a key role in regulating the development of the ovary (Kezele and Skinner, 2003; Chen et al., 2007; Sotomayor-Zárate et al., 2008). Large size at birth is associated with higher levels of maternal concentrations of estrogen (Troisi et al., 2007). It is possible that large size at birth might indicate changes to the ovary related to increased levels of hormonal exposure in utero, as reflected by higher maternal concentrations. In addition, at the cellular level, fast early growth may be related to shorter telomere length or telomerase activity, which could affect the initial follicle pool size during mitosis (Aydos et al., 2005) or meiosis (Bekaert et al., 2004; Siderakis and Tarsounas, 2007) or rate of atresia (Yamagata et al., 2002). Further evidence is needed to clarify these hypothesized pathways. It is also possible that prenatal hormonal exposures might influence mechanisms acting later in life. For example, women who were larger at birth were more likely to have higher estradiol concentrations during adult menstrual cycling (Jasienska et al., 2006). Alternatively, size at birth may be an indicator for other future characteristics that implicate age at menopause.
Conclusions
There is evidence in the 1958 British birth cohort study of associations between markers of fetal environment and age at menopause, with women at the extremes of birthweight and women with higher birthweight standardized by gestational age, which indicates faster fetal growth rate, more likely than other women to have experienced menopause by age 44–45 years. Future studies are needed to confirm our study findings and to focus on mechanisms during ovarian development and later life that may explain these relationships. As the study cohort ages, it will be possible to examine whether the relationship with fetal environment is unique to early menopause or also extends to the broader distribution of natural age at menopause.
Authors' roles
S.E.T. designed the study, analysed and interpreted the data and drafted and revised the paper. R.C. analysed and interpreted the data and revised the paper. J.G. interpreted the data and revised the paper. R.H. guided analysis, interpreted the data and revised the paper. D.K. designed the study, interpreted the data and revised the paper. C.P. designed the study, guided analysis, interpreted the data and revised the paper.
Funding
S.E.T. and J.G. were funded by the Intramural Research Program at the National Institute on Aging, National Institutes of Health. R.C., D.K. and R.H. were funded by the Medical Research Council. The biomedical examination of the 1958 cohort was funded by the Medical Research Council(grant G0000934 awarded under the Health of the Public Initiative). The Great Ormond Street Hospital/UCL Institute of Child Health receives a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The Centre for Paediatric Epidemiology and Biostatistics also benefits from the funding support from the Medical Research Council in its capacity as the MRC Centre of Epidemiology for Child Health.
References
- Atherton K, Fuller E, Shepherd P, Strachan DP, Power C. Loss and representativeness in a biomedical survey at age 45 years: 1958 British birth cohort. J Epidemiol Community Health. 2008;62:216–223. doi: 10.1136/jech.2006.058966. [DOI] [PubMed] [Google Scholar]
- Aydos SE, Elhan AH, Tukun A. Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet. 2005;272:113–116. doi: 10.1007/s00404-004-0690-2. [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1:9. doi: 10.1186/1750-1172-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaert S, Derradji H, Baatout S. Telomere biology in mammalian germ cells and during development. Dev Biol. 2004;274:15–30. doi: 10.1016/j.ydbio.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. Childhood growth and age at menarche. Br J Obstet Gynaecol. 1996;103:814–817. doi: 10.1111/j.1471-0528.1996.tb09879.x. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Baird DD, Weinberg CR, Ephross SA, Sandler DP. Age at menopause and childbearing patterns in relation to mortality. Am J Epidemiol. 2000;151:620–623. doi: 10.1093/oxfordjournals.aje.a010250. [DOI] [PubMed] [Google Scholar]
- Cooper R, Mishra G, Clennell S, Guralnik J, Kuh D. Menopausal status and physical performance in midlife: findings from a British birth cohort study. Menopause. 2008;15:1079–1085. doi: 10.1097/gme.0b013e31816f63a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DW, Xu H, Harlow BL. Does ‘incessant' ovulation increase risk for early menopause? Am J Obstet Gynecol. 1995;172:568–573. doi: 10.1016/0002-9378(95)90574-x. [DOI] [PubMed] [Google Scholar]
- Cresswell JL, Egger P, Fall CH, Osmond C, Fraser RB, Barker DJ. Is the age of menopause determined in-utero? Early Hum Dev. 1997;49:143–148. doi: 10.1016/s0378-3782(97)00028-5. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Dorland M, Bruinse HW, Spliet W, Nikkels PG, te Velde ER. Fetal growth retardation as a cause of impaired ovarian development. Early Hum Dev. 1998;51:39–46. doi: 10.1016/s0378-3782(97)00073-x. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Nikkels PG, Bruinse HW, van Haaften M, Looman CW, te Velde ER. Morphometry of human ovaries in normal and growth-restricted fetuses. Early Hum Dev. 2001;60:179–192. doi: 10.1016/s0378-3782(00)00118-3. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351–358. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- Dorland M, van Kooij RJ, te Velde ER. General ageing and ovarian ageing. Maturitas. 1998;30:113–118. doi: 10.1016/s0378-5122(98)00066-8. [DOI] [PubMed] [Google Scholar]
- Forabosco A, Sforza C. Establishment of ovarian reserve: a quantitative morphometric study of the developing human ovary. Fertil Steril. 2007;88:675–683. doi: 10.1016/j.fertnstert.2006.11.191. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81:723–734. doi: 10.1016/j.earlhumdev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ginsberg J. What determines the age at the menopause? Br Med J. 1991;302:1288–1289. doi: 10.1136/bmj.302.6788.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. The consequences of being born small—an adaptive perspective. Horm Res. 2006;65(Suppl. 3):5–14. doi: 10.1159/000091500. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutrition. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- Hardy R, Kuh D. Does early growth influence timing of the menopause? Evidence from a British birth cohort. Hum Reprod. 2002;17:2474–2479. doi: 10.1093/humrep/17.9.2474. [DOI] [PubMed] [Google Scholar]
- Hardy R, Kuh D. Social and environmental conditions across the life course and age at menopause in a British birth cohort study. Br J Obstet Gynaecol. 2005;112:346–354. doi: 10.1111/j.1471-0528.2004.00348.x. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Thune I, Ellison PT. Fatness at birth predicts adult susceptibility to ovarian suppression: an empirical test of the predictive adaptive response hypothesis. Proc Natl Acad Sci USA. 2006;103:12759–12762. doi: 10.1073/pnas.0605488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesteronee: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MR. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–990. [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen children of the 1950s prospective cohort study. Circulation. 2005;112:1414–1418. doi: 10.1161/CIRCULATIONAHA.104.528356. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Hübinette A, Tynelius P, Leon DA, Smith GD, Rasmussen F. Associations of gestational age and intrauterine growth with systolic blood pressure in a family-based study of 386,485 men in 331,089 families. Circulation. 2007;115:562–568. doi: 10.1161/CIRCULATIONAHA.106.646661. [DOI] [PubMed] [Google Scholar]
- Leon DA, Lithell HO, Vâgerö D, Koupilová I, Mohsen R, Berglund L, Lithell UB, McKeique PM. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915–29. Br Med J. 1998;317:241–245. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon DA, Johansson M, Rasmussen F. Gestational age and growth rate of fetal mass are inversely associated with systolic blood pressure in young adults: an epidemiologic study of 165,136 Swedish men aged 18 years. Am J Epidemiol. 2000;152:597–604. doi: 10.1093/aje/152.7.597. [DOI] [PubMed] [Google Scholar]
- Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18:199–206. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139:64–76. doi: 10.1093/oxfordjournals.aje.a116936. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TF, Graff-Iversen S, Sundby J, Bjertness E. Early menopause, association with tobacco smoking, coffee consumption and other lifestyle factors: a cross-sectional study. BMC Public Health. 2007;7:149. doi: 10.1186/1471-2458-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra G, Hardy R, Kuh D. Are the effects of risk factors for timing of menopause modified by age? Results from a British birth cohort study. Menopause. 2007;14:717–724. doi: 10.1097/GME.0b013e31802f3156. [DOI] [PubMed] [Google Scholar]
- Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–1097. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Möller L, Köster A, Hollnagel H. Social and biological predictors of early menopause: a model for premature aging. J Intern Med. 1997;242:299–305. doi: 10.1046/j.1365-2796.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobee DE, van der Schow YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16:556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study) Int J Epidemiol. 2006;35:34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- Schreuder M, Delemarre-van de Waal H, van Wijk A. Consequences of intrauterine growth restriction for the kidney. Kidney Blood Press Res. 2006;29:108–125. doi: 10.1159/000094538. [DOI] [PubMed] [Google Scholar]
- Siderakis M, Tarsounas M. Telomere regulation and function during meiosis. Chromosome Res. 2007;15:667–679. doi: 10.1007/s10577-007-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Kane RL, Beeson WL, Burke GL, Sprafka JM, Potter J, Iso H, Jacobs DR, Jr, Phillips RL. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79:709–714. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor-Zárate R, Dorfman M, Paredes A, Lara HE. Neonatal exposure to estradiol valerate programs ovarian sympathetic innervation and follicular development in the adult rat. Biol Reprod. 2008;78:673–680. doi: 10.1095/biolreprod.107.063974. [DOI] [PubMed] [Google Scholar]
- Thomas C, Hyppönen E, Power C. Prenatal exposures and glucose metabolism in adulthood: are effects mediated through birth weight and adiposity? Diabetes Care. 2007;30:918–924. doi: 10.2337/dc06-1881. [DOI] [PubMed] [Google Scholar]
- Torgerson DJ, Avenell A, Russell IT, Reid DM. Factors associated with onset of menopause in women aged 45–49. Maturitas. 1994;19:83–92. doi: 10.1016/0378-5122(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Treloar SA, Sadrzadeh S, Do KA, Martin NG, Lambalk CB. Birth weight and age at menopause in Australian female twin pairs: exploration of the fetal origin hypothesis. Hum Reprod. 2000;15:55–59. doi: 10.1093/humrep/15.1.55. [DOI] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Hoover RN. Exploring the underlying hormonal mechanisms of prenatal risk factors for breast cancer: a review and commentary. Cancer Epidemiol Biomarkers Prev. 2007;16:1700–1712. doi: 10.1158/1055-9965.EPI-07-0073. [DOI] [PubMed] [Google Scholar]
- van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68:95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21:149–157. doi: 10.1080/14767050801929430. [DOI] [PubMed] [Google Scholar]
- Whelan EA, Sandler DP, McConnaughey DR, Weinberg CR. Menstrual and reproductive characteristics and age at natural menopause. Am J Epidemiol. 1990;131:625–632. doi: 10.1093/oxfordjournals.aje.a115546. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Nakamura Y, Umayahara K, Harada A, Takayama H, Sugino N, Kato H. Changes in telomerase activity in experimentally induced atretic follicles of immature rats. Endocr J. 2002;49:589–595. doi: 10.1507/endocrj.49.589. [DOI] [PubMed] [Google Scholar]