Abstract
FAs are mobilized from triglyceride (TG) stores during exercise to supply the working muscle with energy. Mice deficient for adipose triglyceride lipase (ATGL-ko) exhibit defective lipolysis and accumulate TG in adipose tissue and muscle, suggesting that ATGL deficiency affects energy availability and substrate utilization in working muscle. In this study, we investigated the effect of moderate treadmill exercise on blood energy metabolites and liver glycogen stores in mice lacking ATGL. Because ATGL-ko mice exhibit massive accumulation of TG in the heart and cardiomyopathy, we also investigated a mouse model lacking ATGL in all tissues except cardiac muscle (ATGL-ko/CM). In contrast to ATGL-ko mice, these mice did not accumulate TG in the heart and had normal life expectancy. Exercise experiments revealed that ATGL-ko and ATGL-ko/CM mice are unable to increase circulating FA levels during exercise. The reduced availability of FA for energy conversion led to rapid depletion of liver glycogen stores and hypoglycemia. Together, our studies suggest that ATGL-ko mice cannot adjust circulating FA levels to the increased energy requirements of the working muscle, resulting in an increased use of carbohydrates for energy conversion. Thus, ATGL activity is required for proper energy supply of the skeletal muscle during exercise.
Keywords: exercise, metabolism, lipids, glycogen
Carbohydrates and FAs are the major energy substrates in the working muscle. The use of FAs for energy conversion depends, among other things, on exercise intensity and duration. During prolonged exercise, when carbohydrate reserves get depleted, oxidation of FAs becomes increasingly important (1, 2). Under these conditions, FAs are imported from plasma sources or mobilized from intramyocellular stores. Most of the body's energy reserves are stored in white adipose tissue (WAT) implicating that the supply of the muscle with energy during prolonged exercise is largely dependent on adipose lipolysis. The mobilization of FAs in adipose tissue is tightly controlled by hormones. Catecholamines and other effectors activate lipases resulting in increased FA release into the circulation (3, 4). Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) are the major triglyceride (TG) lipases in this process. HSL most efficiently hydrolyses diglycerides (DGs), but is also capable of degrading several other lipid substrates including TGs, monoglycerides, cholesteryl ester, and retinyl ester (5). In HSL-deficient WAT, TG hydrolysis results in the accumulation of DG, suggesting that HSL is a major DG hydrolase in vivo (6). ATGL specifically performs the first step in lipolysis, generating DGs and FAs (7). ATGL-deficiency in mice results in obesity caused by severely reduced TG hydrolysis in adipose tissue. Increased deposition of TG is also observed in many other tissues (8). In the absence of both enzymes, hormone-induced FA release in WAT is decreased by more than 95% (9). Together, these observations suggest that efficient lipolysis is dependent on the coordinate action of acylglycerol lipases in sequential steps: ATGL generates DG, which is subsequently hydrolyzed by HSL. In the last step of lipolysis, monoglycerides are degraded by monoglyceride lipase leading to the formation of glycerol and FAs (10).
To date, limited information is available about the role of ATGL to exercise-induced TG hydrolysis. Because FAs are an important energy source in skeletal muscle, we hypothesized that defective lipolysis in ATGL-deficient mice is associated with changes in energy availability and energy substrate utilization in the working muscle. ATGL-ko mice develop cardiomyopathy due to massive accumulation of TGs in the heart. To circumvent this problem, we also investigated a mouse model lacking ATGL in all tissues except cardiac muscle (ATGL-ko/CM). Treadmill experiments using these mouse models revealed an important role of ATGL in exercise-induced FA mobilization. ATGL-ko and ATGL-ko/CM mice are not capable of mobilizing sufficient FAs during moderate exercise to maintain normal energy metabolism, which results in marked changes in energy substrate utilization. Moreover, TG accumulation in oxidative muscle fibers lacking ATGL suggests that, in addition to decreased adipose lipolysis, defective mobilization of intramyocellular TG reserves reduce the availability of FAs for energy conversion in the working muscle.
METHODS
Animals
Mice were maintained on a regular light-dark cycle (14 h light, 10 h dark) and kept on a standard laboratory chow diet (4.5% w/w fat). ATGL-ko mice were generated by targeted homologous recombination as described (6, 8). To achieve cardiac specific expression of ATGL, full-length murine ATGL was introduced into a minigene containing the cardiac-specific promoter of the α-myosin heavy chain (α-MHC) gene (GenBank: U71441) and transgenic mice were created as described (11). For generation of mice lacking ATGL in all tissues except cardiac muscle, mice were backcrossed on the ATGL-ko background. Mice used for experiments were 8 to 10 weeks of age, kept ad libitum, and backcrossed at least five times on the C57BL/6J background. Animals were anesthetized with IsoFlo/Isoflurane (Abbott, Animal Health, Queenborough, Kent, UK) and euthanized by cervical dislocation. The study was approved by the Austrian ethics committee, and is in accordance with the council of Europe Convention (ETS 123).
Animal experiments
Healthy male and female mice were exercised on a motorized treadmill (IITC Inc./Life Science, Woodland, CA). Before experiments, all mice were familiarized to the treadmill in a 5 min run at 14 m/min and 0° slope for 2 days. For the experimental run, normally fed mice ran for 60 min at a speed of 14 m/min and 0° slope in individual treadmill lanes. These settings were chosen because they were close to the maximal endurance capacity of ATGL-ko mice. Mice that were not capable of running the whole distance were excluded from the experiments. Treadmill experiments were performed between 9 and 11 AM. A mild electrical stimulus (100-120 V; 2 mA) was applied to mice that stepped off of the treadmill lane. To measure spontaneous physical activity, O2 consumption, CO2 production, and food intake, mice were housed in a laboratory animal monitoring system that allows the continuous measurement of these parameters (LabMaster, TSE Systems GmbH, Bad Homburg, Germany).
Blood and plasma parameters
Resting and exercised animals were anesthetized using isoflurane and blood samples were collected by retro-orbital puncture. Plasma levels of TGs, glycerol, and FAs were determined using commercial kits (Thermo Electron Corp., Victoria, Australia; Sigma, St. Louis, MO; Wako Chemicals, Neuss, Germany). Blood glucose and lactate concentrations were determined using Accu-Check glucometer and Accutrend® Plus, respectively (Roche Diagnostics, Vienna, Austria).
Determination of liver glycogen content
Livers of resting and exercised mice were homogenized (Ultra Turrax®, Staufen, Germany) in 0.03 N HCl (20 µl/mg tissue) on ice. For the determination of liver total glucose concentration, aliquots of lysates were mixed with equal volumes of 2 N HCl and incubated for 2 h at 90°C. The hydrolysate was neutralized with 2 N NaOH. To determine free glucose concentration, aliquots of liver lysates were mixed with equal amounts of (2 N) HCl and immediately neutralized with 2 N NaOH. Glucose concentrations were measured using D-Glucose-HK-Kit (Megazyme International Ireland Ltd, Wicklow, Ireland). The amount of liver glycogen was determined by the difference of total glucose and free glucose concentrations (12).
Determination of skeletal muscle TG content
Skeletal muscle lipids were extracted by the method of Folch and the chloroform phase containing neutral lipids was brought to dryness under nitrogen. For determination of skeletal muscle TG content, the lipid pellet was solubilized in 2% Triton X-100 by sonication and TG concentrations were determined using Infinity Triglycerides Reagent (Thermo Electron Corp.).
Determination of TG hydrolase activity
WAT and skeletal and cardiac muscle from resting animals were homogenized in buffer A (0.25 M sucrose, 1 mM EDTA, 1 mM dithiothreitol, 20 µg/ml leupetine, 2 µg/ml antipain, 1 µg/ml pepstatin, [pH 7.0]) on ice using an Ultra Turrax®. Thereafter, the homogenate was centrifuged at 20,000 g, 4°C for 30 min and the fat-poor infranatant was used for TG hydrolase activity assays. For that purpose, tissue lysates were incubated in a total volume of 100 μl buffer A with 100 μl substrate in a water bath at 37°C for 60 min. After incubation, the reaction was terminated by adding 3.25 ml of methanol/chloroform/heptane (10:9:7) and 1 ml of 0.1 M potassium carbonate, 0.1 M boric acid, (pH 10.5). After centrifugation (800 g, 15 min), the radioactivity in 1 ml of the upper phase was determined by liquid scintillation counting. Stimulation of ATGL was achieved by coincubation of the tissue preparations with purified murine GST-CGI-58 (CGI-58) (9, 13). The substrate was prepared by emulsifying 330 μM triolein (40,000 cpm/nmol) and 45 μM phosphatidylcholine/phosphatidylinositol (3:1) in of 0.1 M potassium phosphate buffer (pH 7.0) by sonication, followed by the addition of 5% defatted BSA.
Histology
Muscles (Musculus gastrocnemius) were prepared from the hind leg and frozen in liquid nitrogen or fixed in 4% buffered formaldehyde and embedded in paraffin. To visualize neutral lipids, sections from frozen muscles were stained with Sudan III or Oil Red O (Sigma-Aldrich) and nuclei were counterstained with hemalaun (Roth, Karlsruhe, Germany) following standard protocols. To differentiate between oxidative and non-oxidative muscle fibers, sections of skeletal muscle were stained with NADH tetrazolium reductase or myosin adenosine triphosphatase (ATPase, pH 4.3) according to standard protocols.
Statistical analysis
Statistical significance was determined by the Student's unpaired t-test (two-tailed). Group differences were considered significant for p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
RESULTS
Locomotion and energy expenditure of ATGL-ko mice
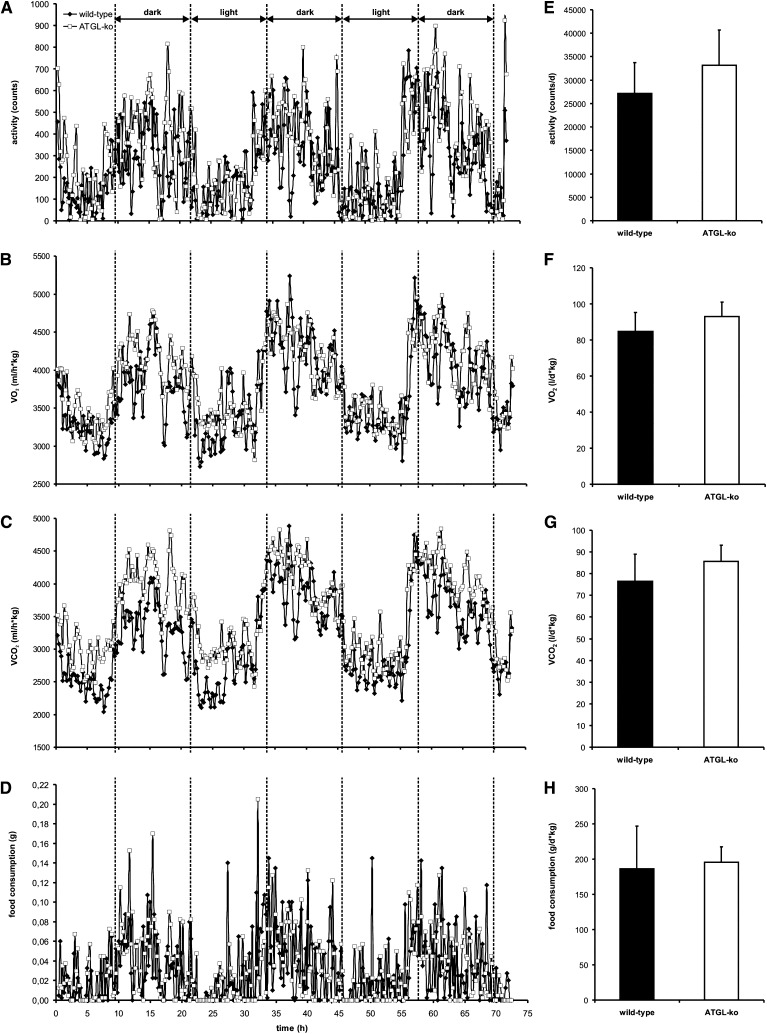
ATGL-ko mice were reported to exhibit reduced oxygen (O2) consumption and hypothermia in response to fasting (6). To investigate whether these mice also show changes in physical activity and energy expenditure under nonfasted conditions, mice were housed in an animal metabolism research system (Lab Master) for 72 h where they had free access to food. As shown in Fig. 1, we could not observe significant differences between wild-type and ATGL-ko animals in locomotion (Fig. 1A, E), O2 consumption (VO2; Fig. 1B, F), CO2 production (VCO2; Fig. 1C, G), and food consumption (Fig. 1D, H). Thus, ATGL-deficiency is not associated with severe changes in physical activity or energy expenditure when mice are fed ad libitum.
Fig. 1.
Physical activity, energy expenditure, and food consumption of wild-type and ATGL-ko mice. Male mice were housed in a laboratory animal monitoring system (LabMaster, TSE Systems), which allows the simultaneous measurement of physical activity, O2 consumption (VO2), CO2 production (VCO2), and food consumption. A–D: Mean changes in these parameters during light and dark periods (n = 4 for each genotype). E–H: Daily activity, VO2, VCO2, and food consumption were calculated from data obtained from single mice during the whole 72 h monitoring period. Data are presented as mean ± SD.
Histological and biochemical characterization of mutant mice
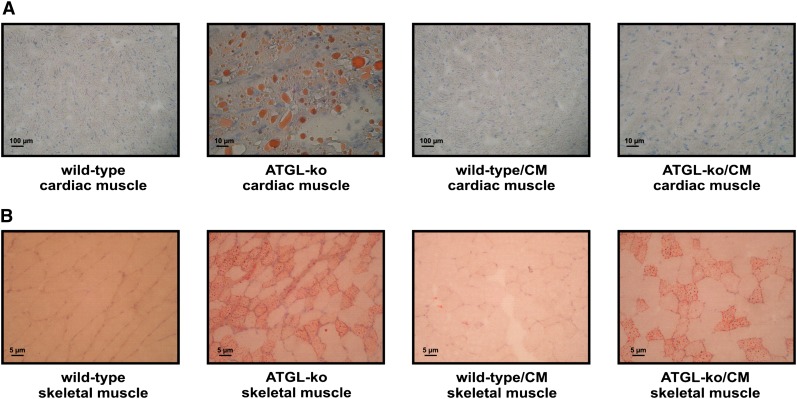
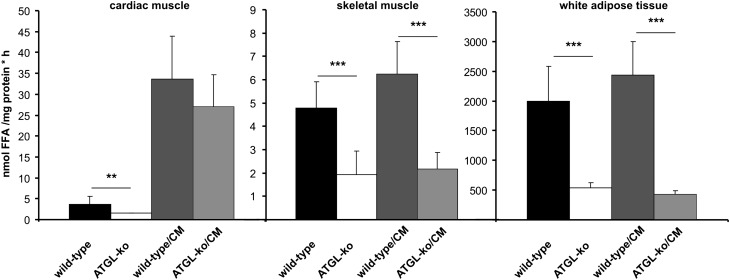
ATGL-ko mice exhibit reduced TG hydrolase activities in most tissues including heart, skeletal muscle, and adipose tissue (8). These mice accumulate large amounts of TG in cardiomyocytes, which causes cardiomyopathy starting at the age of ∼3 months. To exclude the possibility that heart abnormalities affect exercise performance, we generated mice overexpressing ATGL specifically in the heart (wild-type/CM) and crossed them on the ATGL-ko background (ATGL-ko/CM). In contrast to ATGL-ko mice, ATGL-ko/CM animals did not accumulate TG in cardiac muscle (Fig. 2A) whereas skeletal muscle sections of ATGL-ko/CM animals stained positive for neutral lipids to an extent comparable to ATGL-ko mice (Fig. 2B). TG hydrolase assays using tissue lysates of ATGL-ko mice revealed significantly reduced activities in cardiac muscle, skeletal muscle, and WAT compared with wild-type (Fig. 3). Wild-type/CM and ATGL-ko/CM animals exhibited an ∼10-fold increase in TG hydrolase activity in cardiac muscle compared with wild-type mice. In skeletal muscle and WAT, wild-type/CM mice exhibited TG hydrolase activities comparable to the values obtained for wild-type controls. ATGL-ko/CM animals exhibited reduced activities in skeletal muscle and WAT similar as observed in ATGL-ko animals. These data demonstrate that the ATGL transgene is active in vivo and specifically expressed in the heart.
Fig. 2.
Accumulation of neutral lipids in heart and skeletal muscle of ATGL-deficient mice. To visualize neutral lipids, cross-sections from frozen cardiac muscle (A) and skeletal muscle (M. gastrocnemius) (B) derived from wild-type and mutant mice were stained with Sudan III. Nuclei were stained with hemalaun.
Fig. 3.
TG hydrolase activity in tissues of wild-type and mutant mice. TG hydrolase activity was determined in lysates (20.000 g infranatant) of cardiac muscle, skeletal muscle, and white adipose tissue using an artificial radiolabeled triolein substrate. Data are presented as mean ± SD (n = 5 for each genotype; ** p < 0.01, *** p < 0.001).
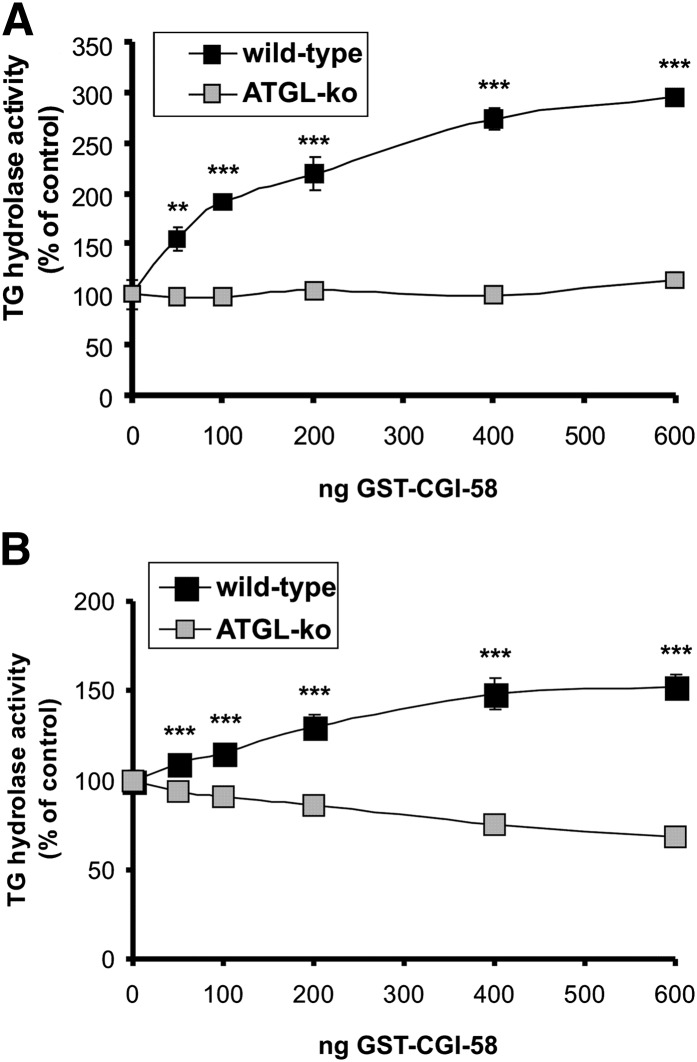
To further investigate the role of ATGL in skeletal muscle, we measured TG hydrolase activity in the presence of CGI-58 (comparative gene expression protein-58, also known as α/β hydrolase domain containing protein 5, ABHD5), the activator protein of ATGL (Fig. 4A) (13). The addition of CGI-58 to wild-type muscle lysates led to a dose-dependent increase in TG hydrolase activity up to ∼3-fold, implicating that ATGL in muscle lysates is incompletely activated by endogenous CGI-58. In ATGL-deficient muscle preparations, this increase was completely blunted, suggesting that ATGL is the sole CGI-58 activated lipase in skeletal muscle. For comparison, we also determined TG hydrolase activities of wild-type and ATGL-ko WAT in the presence of various concentrations of CGI-58 (Fig. 4B). The addition of CGI-58 resulted in an increase of lipolytic activity up to ∼1.5-fold in adipose tissue of wild-type mice whereas no stimulation was observed in ATGL-ko WAT lysates. The lower increase in TG hydrolase activity in WAT in response to CGI-58 addition can probably be explained by higher endogenous levels of CGI-58 in WAT than in skeletal muscle.
Fig. 4.
Stimulation of TG hydrolase activity by CGI-58 in skeletal muscle and adipose tissue. TG hydrolase activity was determined in lysates (20.000g infranatant) of skeletal muscle (A) and adipose tissue (B) using an artificial radiolabeled triolein substrate. Experiments were performed in the presence and in the absence of recombinant GST-tagged CGI-58. Values are means ± SD of triplicate determinations and are representative for three independent experiments (*** p < 0.001).
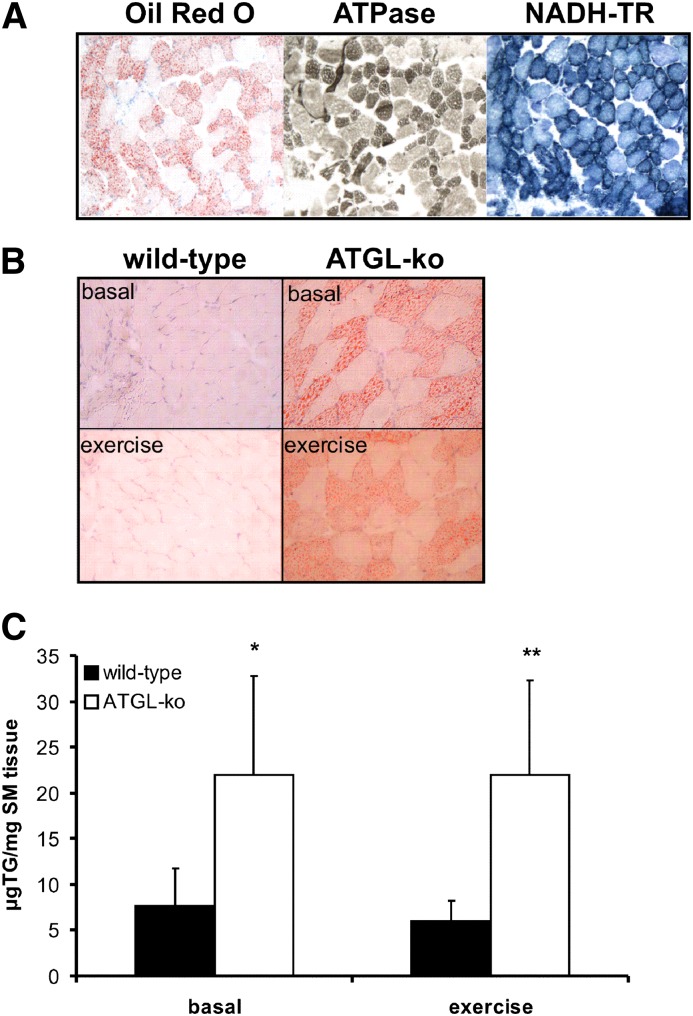
As shown above (Fig. 2B), substantial lipid accumulation was present in skeletal muscle of ATGL-ko mice whereas no accumulation was visible in wild-type mice. Neutral lipids were detected only in specific fibers whereas others seemed unaffected. Most of these lipid accumulating fibers also stained positive for ATPase (at pH 4.3) and for NADH-tetrazolium reductase (NADH-TR) (Fig. 5A) demonstrating that TG accumulation occurs predominantly in oxidative fibers. To investigate whether the reduced TG hydrolase activity in ATGL-deficient skeletal muscle affects the mobilization of endogenous TG sources, we compared neutral lipid accumulation in sections obtained from skeletal muscle of wild-type and mutant mice at rest and after exercise. Interestingly, TG accumulation in ATGL-ko mice was present in resting and exercised mice implicating that TG was not mobilized in response to exercise. Biochemical analysis of muscle TG levels revealed an ∼3-fold increase in muscle TG content in ATGL-ko mice compared with wild-type controls. TG levels in both genotypes were similar before and after exercise (Fig. 5C). Identical observations were made in ATGL-ko/CM mice (not shown). Together, our data indicate that the lack of ATGL substantially affects TG mobilization not only in adipose tissue (8) but also in oxidative fibers of skeletal muscle.
Fig. 5.
Neutral lipids accumulate in oxidative fibers of ATGL-ko mice and are not mobilized during exercise. A: Muscle sections obtained from an ATGL-ko animal were stained with Oil Red O (left) and with oxidative fiber-specific staining protocols. These fibers appear intensely dark following ATPase (at pH 4.3, middle) or NADH-TR (right) staining. B: Lipid accumulation in skeletal muscle (M. gastrocnemius) of wild-type and ATGL-ko mice at rest and after treadmill exercise (1 h, 14 m/min, 0% angle) was visualized using Sudan III staining. Nuclei were stained with hemalaun. Images are representative for cryosections obtained from three different mice. C: TG content of skeletal muscle (M. gastrocnemius) of wild-type and ATGL-ko mice at rest and after treadmill exercise.
Plasma lipid profile, carbohydrate levels, and liver glycogen content at rest and after exercise
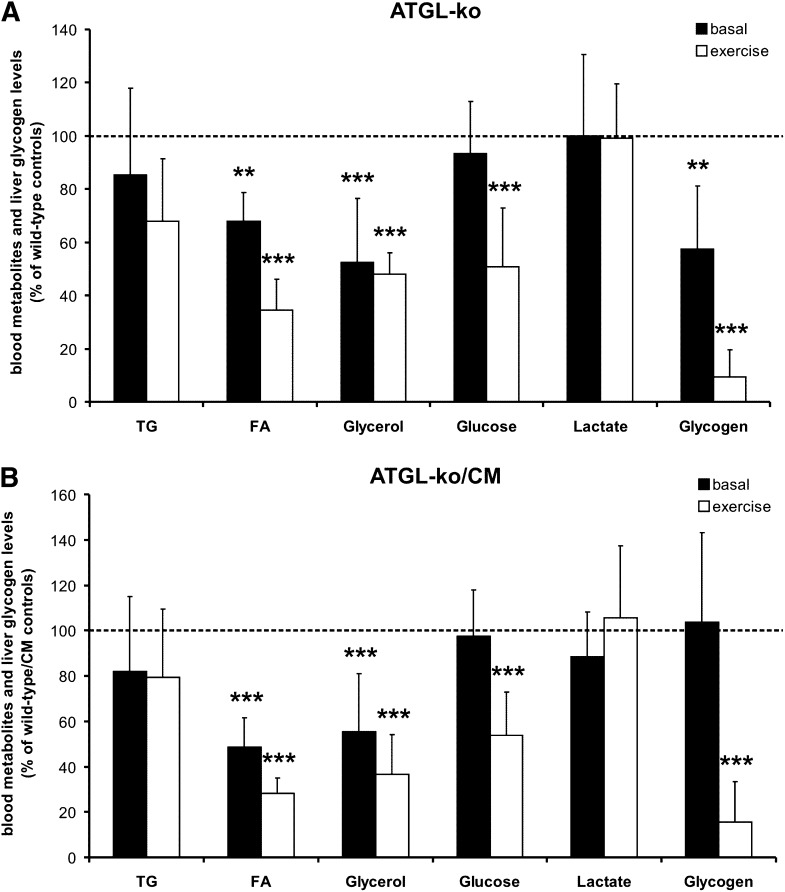
To investigate how the lack of ATGL affects lipolysis and energy substrate mobilization/utilization in response to exercise, we determined blood metabolites and glycogen levels in resting and exercised animals. The relative changes in mutant mice in comparison to their respective controls were calculated from the values shown in Table 1 and are summarized in Fig. 6. In the basal state, ATGL-ko mice exhibited reduced plasma FA, plasma glycerol, and liver glycogen levels (−32%, −48%, and −42%, respectively). Plasma TG, blood glucose, and blood lactate values were not different (Fig. 6A). In exercised animals, the differences between wild-type and mutant mice were more pronounced. Plasma FA and glycerol levels in ATGL-ko were reduced by 65% and 52%, respectively. Liver glycogen was almost depleted (−91%) and blood glucose levels were decreased by 49% (Fig. 6A). We also determined muscle glycogen concentrations at rest and after exercise. In the basal state, we observed a reduction by 30% in ATGL-ko animals (p < 0.05). In exercised animals, glycogen levels were below the detection limit of our method and we could not detect glycogen in wild-type and ATGL-ko skeletal muscle (not shown). Values shown in Fig. 6 were calculated from data obtained from male and female mice. Very similar changes in blood parameters and liver glycogen content of mutant mice were observed independent of sex. The values for male and female mice are shown separately in Table 1.
TABLE 1.
Blood energy metabolites and liver glycogen stores at rest and after exercise
| Male Resting | Wild-Type (n = 6) | ATGL-ko (n = 6) | Wild-Type/CM (n = 5) | ATGL-ko/CM (n = 9) |
| TG (mg/dl) | 89+/−30 | 81+/−34 | 66+/−9 | 52+/−27 |
| FA (mmol/L) | 0.39+/−0.12 | 0.28+/−0.05 | 0.54+/−0.22 | 0.22+/−0.04*** |
| Glycerol (mmol/L) | n.d. | n.d. | 0.52+/−0.17 | 0.24+/−0.09** |
| Glucose (mg/dl) | 164+/−30 | 164+/−31 | 164+/−29 | 160+/−42 |
| Lactate (mmol/L) | 4.4+/−1.7 | 4.3+/−0.7 | 4.5+/−0.9 | 3.9+/−1.1 |
| Glycogen (mg glucose/g) | 31.5+/−12.8 | 18.1+/−9.7 | 36.4+/−11.8 | 32.5+/−13 |
| Male Exercise | Wild-Type (n = 5) | ATGL-ko (n = 4) | Wild-Type/CM (n = 7) | ATGL-ko/CM (n = 7) |
| TG (mg/dl) | 41+/−16 | 27+/−9 | 71+/−19 | 44+/−16*** |
| FA (mmol/L) | 0.52+/−0.16 | 0.19+/−0.05** | 0.97+/−0.28 | 0.25+/−0.07*** |
| Glycerol (mmol/L) | n.d. | n.d. | 0.70+/−0.21 | 0.23+/−0.07*** |
| Glucose (mg/dl) | 185+/−26 | 75+/−25*** | 198+/−26 | 103+/−27*** |
| Lactate (mmol/L) | 4.1+/−0.4 | 3.6+/−0.4 | 2.9+/−1.3 | 3.3+/−1.1 |
| Glycogen (mg glucose/g) | 26+/−13.2 | 2+/−2 ** | 28+/−12.1 | 4.7+/−5.4*** |
| Female Resting | Wild-Type (n = 6) | ATGL-ko (n = 6) | Wild-Type/CM (n = 6) | ATGL-ko/CM (n = 7) |
| TG (mg/dl) | 85+/−29 | 66+/−23 | 55+/−20 | 47+/−12 |
| FA (mmol/L) | 0.40+/−0.11 | 0.26+/−0.04* | 0.54+/−0.12 | 0.27+/−0.07*** |
| Glycerol (mmol/L) | 0.28+/−0.07 | 0.14+/−0.08** | 0.51+/−0.09 | 0.33+/−0.16* |
| Glucose (mg/dl) | 146+/−32 | 125+/−28 | 203+/−32 | 197+/−27 |
| Lactate (mmol/L) | 4.2+/−0.9 | 4.2+/−1.5 | 3.7+/−0.5 | 3.4+/−0.3 |
| Glycogen (mg glucose/g) | 31.4+/−7 | 18.1+/−5** | 23.9+/−8.3 | 29.2+/−9.4 |
| Female Exercise | Wild-Type (n = 6) | ATGL-ko (n = 4) | Wild-Type/CM (n = 7) | ATGL-ko/CM (n = 7) |
| TG (mg/dl) | 47+/−11 | 33+/−13 | 76+/−20 | 35+/−9*** |
| FA (mmol/L) | 0.57+/−0.09 | 0.19+/−0.08*** | 0.99+/−0.13 | 0.30+/−0.05*** |
| Glycerol (mmol/L) | 0.38+/−0.06 | 0.17+/−0.02*** | 0.68+/−0.19 | 0.28+/−0.15*** |
| Glucose (mg/dl) | 146+/−26 | 89+/−38* | 168+/−15 | 94+/−43*** |
| Lactate (mmol/L) | 3.3+/−0.9 | 3.7+/−0.7 | 2.5+/−0.8 | 2.3+/−0.3 |
| Glycogen (mg glucose/g) | 31+/−12 | 3.4+/−4.1** | 19+/−3 | 2.6+/−3.4*** |
Blood metabolites and liver glycogen concentrations were determined in resting and exercised male and female mice (1 h, 14 m/min, 0% angle). Values of ATGL-ko and ATGL-ko/CM mice were compared with the values obtained for wild-type and wild-type/CM animals, respectively. Data are presented as mean ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001); (n.d., not determined). Relative differences between control animals and mice lacking ATGL as well as exercise-induced changes are summarized in Figs. 6 and 7, respectively.
Fig. 6.
Blood energy metabolites and liver glycogen stores at rest and after exercise. Values were obtained at rest and after treadmill exercise (1 h, 14 m/min, 0% angle). Relative changes were calculated from the absolute values of male and female mice presented in Table 1. A: Differences between wild-type controls and ATGL-ko mice at rest and after exercise. B: Differences between wild-type/CM and ATGL-ko/CM mice at rest and after exercise. Data are presented as mean ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001).
Similar observations as shown for ATGL-ko animals were made in ATGL-ko/CM mice (Fig. 6B). In comparison to the wild-type/CM controls, these animals exhibited decreased plasma FA (−52%) and glycerol (−45%) levels in the basal state. Plasma TG, blood glucose, and blood lactate levels were unchanged. In contrast to ATGL-ko animals, basal liver glycogen content was not decreased, which possibly reflects the recovered ability of the heart to use FAs from TG stores for energy conversion. In exercised ATGL-ko/CM animals, plasma FA, plasma glycerol, and blood glucose levels were decreased by 72%, 63%, and 46%, respectively. No differences were observed in TG and lactate values. Liver glycogen was again almost depleted (−84%).
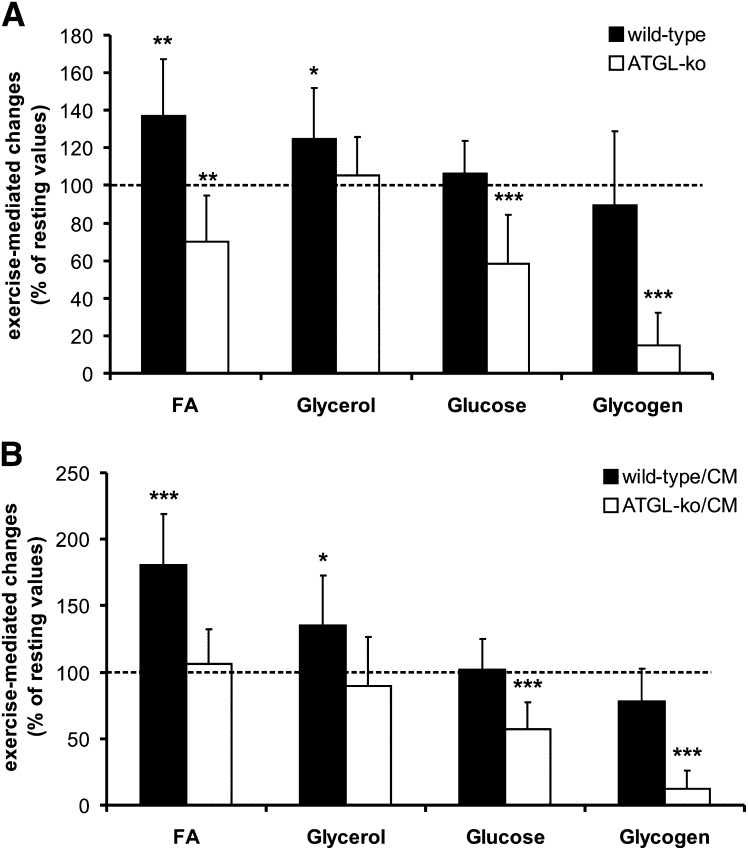
The effect of exercise on the lipolytic parameters FA and glycerol as well as on blood glucose and liver glycogen levels in these animal models is summarized in Fig. 7. Compared with resting conditions, FA and glycerol levels were significantly increased in wild-type (37% and 25%, respectively; Fig. 7A) as well as in wild-type/CM mice (80% and 35%, respectively; Fig. 7B). Conversely, plasma FA and glycerol levels were decreased or unchanged in ATGL-ko or ATGL-ko/CM animals. This implicates that adipose tissue lacking ATGL cannot adjust circulating FA levels to the increased energy requirements during exercise. The low exercise intensity settings in this experiment did not affect liver glycogen and blood glucose levels of wild-type and wild-type/CM animals. Yet under these moderate conditions, liver glycogen and blood glucose were substantially reduced in ATGL-ko (−85% and −42%, respectively; Fig. 7A) and ATGL-ko/CM animals (−88% and −43%, respectively; Fig. 7B). An increase in working intensity to 16 m/min (5% inclination) resulted in an ∼40% decrease in liver glycogen of wild-type animals (not shown). However, these settings were not chosen because only a few ATGL-ko animals were capable of running the whole distance under these conditions. Together, our observations strongly indicate that the lack of ATGL in adipose tissue leads to an undersupply of the muscle with FA, which is compensated by an increased use of carbohydrates for energy conversion. The data obtained for ATGL-ko/CM mice largely resembled the values obtained for mice totally lacking ATGL. Thus, heart abnormalities do not affect exercise performance of ATGL-ko mice, at least under the applied moderate working intensity conditions.
Fig. 7.
Exercise-mediated changes of blood energy metabolites and liver glycogen stores. Values were obtained at rest and after treadmill exercise (1 h, 14 m/min, 0% angle). Relative changes were calculated from the absolute values of male and female mice presented in Table 1. A: Exercise-mediated changes in comparison to resting conditions in wild-type and ATGL-ko mice. B: Exercise-mediated changes in comparison to resting conditions in wild-type/CM and ATGL-ko/CM mice. Data are presented as mean ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001).
DISCUSSION
The present study demonstrates that mice lacking ATGL cannot adjust circulating FA levels to the increased energy consumption of the body during exercise. So far, most of the published experimental work on exercise-induced lipolysis was focused on the role of HSL in this process. A number of studies demonstrate that this enzyme is involved in the mobilization of FAs in muscle and adipose tissue during exercise (14–17). Moreover, in a very recent study, HSL was shown to be important for normal mobilization of FAs during exercise (18). Treadmill experiments with HSL-ko mice revealed decreased plasma FA levels, a faster depletion of liver carbohydrate stores, and reduced endurance capacity compared with wild-type controls. These findings demonstrate that HSL contributes to exercise-induced lipolysis in adipose tissue (6). However, observations in mice (8, 9) and recent human data (19) strongly suggest that ATGL initiates TG hydrolysis in adipose tissue of rodents as well as in humans. The resulting DGs are subsequently hydrolyzed by HSL implicating that HSL-mediated FA mobilization is largely dependent on ATGL activity.
Very recently, a manuscript was published that compares energy metabolism and exercise performance of ATGL-ko and HSL-ko mice (20). This study demonstrates that maximal running velocity and endurance capacity were reduced in ATGL-ko mice but not HSL-ko mice. In accordance with our observations, authors also demonstrate that the reduced availability of FAs results in more rapid depletion of glycogen stores in ATGL-ko animals. However, mice totally lacking ATGL accumulate excess TGs in the heart, which leads to cardiac abnormalities and premature death starting at the age of ∼3 months. Particularly during exercise, cardiomyopathy could prevent the cardiac output from rising sufficiently to meet tissue needs. To exclude the possibility that heart abnormalities or cardiac stress affect energy metabolism in resting or exercised animals, we generated mice overexpressing ATGL in cardiac muscle. These mice are protected from TG accumulation specifically in the heart and have normal life expectancy (not shown). However, the data obtained for ATGL-ko/CM mice largely resembled the values of mice totally lacking ATGL, implicating that cardiac abnormalities do not substantially affect blood metabolite concentrations at rest or under the applied moderate working conditions. In both animal models, the lack of ATGL activity has severe implications on energy metabolism. Notably, the working intensity settings in this study (14 m/min, 0% grade, 60 min) were very low in comparison to the maximal endurance capacity of wild-type mice. At a speed of 20 m/min and 5% inclination, C57Bl6 mice become exhausted after approximately 85 min (21). Yet, under the low intensity conditions applied in this study that did not affect liver glycogen levels in wild-type animals, liver glycogen stores in ATGL-ko and ATGL-ko/CM mice were almost depleted. These results implicate that the reduced availability of FA is compensated by an increased use of liver carbohydrates for energy conversion. Very similar metabolic changes as observed in exercised animals have been made in fasted ATGL-ko mice (8). It has been shown that fasting increases plasma FA in wild-type mice but not in ATGL-ko mice. With increasing fasting time, a gradual decrease in oxygen (O2) consumption was observed in ATGL-ko mice starting after ∼8 h, which suggests reduced energy expenditure presumably caused by insufficient FA supply. Simultaneously, the respiratory quotient of ATGL-ko mice was elevated in comparison to wild-type mice indicative of an increased use of carbohydrates for energy conversion. Thus, mice lacking ATGL cannot mobilize sufficient energy during exercise or under fasting conditions to maintain normal energy metabolism. Conversely, physical activity and energy expenditure of fed ATGL-ko mice are within the normal range.
In humans, mutations in the ATGL gene (PNPLA2) are associated with a rare inherited disorder annotated as neutral lipid storage disease with myopathy (NLSD-M) (22–24). Affected individuals exhibit systemic TG accumulation and suffer from myopathy, cardiac abnormalities, and hepatomegaly. Similar as observed in ATGL-ko mice, excess accumulation of TGs in the heart causes cardiac abnormalities in humans (25). Most of the mutations reported so far lead to the expression of truncated versions of ATGL, which are enzymatically active in vitro (24, 26). However, truncated variants of the enzyme are predominantly localized in the cytoplasm and show less association with lipid droplets, suggesting that the lipolytic defect in these patients is caused by mislocalization of ATGL (24). Mutations in CGI-58 in humans are also associated with systemic TG accumulation, mild myopathy, and hepatomegaly (27). A common feature also associated with CGI-58 mutations is ichthyosis, which is not present in NLSD-M. This disease is long known as Chanarin-Dorfman syndrome or NLSD with ichthyosis (NLSD-I) (28, 29). CGI-58 functions as activator protein of ATGL and exhibits no intrinsic TG hydrolase activity (13). Mutations associated with NLSD-I lead to the expression of mutant forms of CGI-58 that are not capable of stimulating ATGL activity implying that fat deposition in tissues occurs due to defective ATGL function (13).
In human skeletal muscle, reduced ATGL activity caused by mutations in either the ATGL or CGI-58 gene is always associated with increased fat deposition in myocytes suggesting an important function of these proteins not only in WAT but also in muscle lipolysis. Recently, it was demonstrated that ATGL is expressed in type I fibers, which generates energy mainly through aerobic metabolism (30) and that the enzyme is upregulated in human skeletal muscle by exercise training (31). Consistent with these observations in humans, we found that skeletal muscles of ATGL-ko mice accumulate TGs mostly in oxidative fibers. Moreover, we show that the stimulatory effect of CGI-58 on TG hydrolase activity is completely blunted in ATGL-deficient skeletal muscle, implicating that ATGL is the sole TG lipase activated by CGI-58 in this tissue. Notably, accumulation of TGs in muscle fibers of ATGL-ko mice was present in resting as well as in exercised animals, indicating that TG stores in muscle are not mobilized during exercise. Together, these observations indicate that ATGL-deficiency is associated with impaired hydrolysis of intramyocellular TG stores. On the other hand, TG content was not decreased in wild-type mice during exercise, either. However, in these mice, exercise resulted in increased circulating FA levels, suggesting that availability of plasma FA was sufficient to cover lipid utilization in muscle.
In summary, our data demonstrate that ATGL-ko mice are not capable of mobilizing sufficient energy in the form of FAs to maintain normal energy substrate utilization during moderate exercise. These changes are presumably caused by defective lipolysis in adipose tissue and skeletal muscle. The reduced availability of FAs is compensated by an increased use of carbohydrates for energy conversion leading to rapid depletion of liver glycogen stores during exercise. Our observations are consistent with the supposed role of ATGL as a major TG lipase, which is activated under conditions of increased energy demand like exercise or fasting.
Footnotes
Abbreviations:
- ATGL
- adipose triglyceride lipase
- ATGL-ko
- ATGL-knockout
- CGI-58
- comparative gene identification protein-58
- DG
- diglyceride
- HSL
- hormone-sensitive lipase
- NLSD
- neutral lipid storage disease
- TG
- triglyceride
- WAT
- white adipose tissue
This research was supported by the grants P18434-B05, F3002-B05 SFB LIPOTOX, and W901-B05 DK (Molecular Enzymology), which are funded by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (FWF) and “GOLD - Genomics of Lipid-Associated Disorders”, which is part of the Austrian Genome Project “GEN-AU Genome research in Austria” and is funded by the Austrian Ministry for Education, Research, and Culture.
REFERENCES
- 1.Kiens B. 2006. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 86: 205–243. [DOI] [PubMed] [Google Scholar]
- 2.Spriet L. L., Watt M. J. 2003. Regulatory mechanisms in the interaction between carbohydrate and lipid oxidation during exercise. Acta Physiol. Scand. 178: 443–452. [DOI] [PubMed] [Google Scholar]
- 3.Holm C. 2003. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31: 1120–1124. [DOI] [PubMed] [Google Scholar]
- 4.Zechner R., Strauss J. G., Haemmerle G., Lass A., Zimmermann R. 2005. Lipolysis: pathway under construction. Curr. Opin. Lipidol. 16: 333–340. [DOI] [PubMed] [Google Scholar]
- 5.Yeaman S. J., Smith G. M., Jepson C. A., Wood S. L., Emmison N. 1994. The multifunctional role of hormone-sensitive lipase in lipid metabolism. Adv. Enzyme Regul. 34: 355–370. [DOI] [PubMed] [Google Scholar]
- 6.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., Zechner R. 2002. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 277: 4806–4815. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 8.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 9.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281: 40236–40241. [DOI] [PubMed] [Google Scholar]
- 10.Fredrikson G., Tornqvist H., Belfrage P. 1986. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 876: 288–293. [DOI] [PubMed] [Google Scholar]
- 11.Levak-Frank S., Hofmann W., Weinstock P. H., Radner H., Sattler W., Breslow J. L., Zechner R. 1999. Induced mutant mouse lines that express lipoprotein lipase in cardiac muscle, but not in skeletal muscle and adipose tissue, have normal plasma triglyceride and high-density lipoprotein-cholesterol levels. Proc. Natl. Acad. Sci. USA. 96: 3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passonneau J. V., Lauderdale V. R. 1974. A comparison of three methods of glycogen measurement in tissues. Anal. Biochem. 60: 405–412. [DOI] [PubMed] [Google Scholar]
- 13.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3: 309–319. [DOI] [PubMed] [Google Scholar]
- 14.Langfort J., Ploug T., Ihlemann J., Holm C., Galbo H. 2000. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem. J. 351: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donsmark M., Langfort J., Holm C., Ploug T., Galbo H. 2005. Hormone-sensitive lipase as mediator of lipolysis in contracting skeletal muscle. Exerc. Sport Sci. Rev. 33: 127–133. [DOI] [PubMed] [Google Scholar]
- 16.Prats C., Donsmark M., Qvortrup K., Londos C., Sztalryd C., Holm C., Galbo H., Ploug T. 2006. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J. Lipid Res. 47: 2392–2399. [DOI] [PubMed] [Google Scholar]
- 17.Watt M. J., Heigenhauser G. J., Spriet L. L. 2003. Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle. J. Physiol. 547: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez C., Hansson O., Nevsten P., Holm C., Klint C. 2008. Hormone-sensitive lipase is necessary for normal mobilization of lipids during submaximal exercise. Am. J. Physiol. Endocrinol. Metab. 295: E179–E186. [DOI] [PubMed] [Google Scholar]
- 19.Bezaire V., Mairal A., Ribet C., Lefort C., Girousse A., Jocken J., Laurencikiene J., Anesia R., Rodriguez A. M., Ryden M., Stenson B. M., Dani C., Ailhaud G., Arner P., Langin D. 2009. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in human hMADS adipocytes. J. Biol. Chem. 284: 18282–18291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huijsman E., van de Par C., Economou C., van der Poel C., Lynch G. S., Schoiswohl G., Haemmerle G., Zechner R., Watt M. J. 2009. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am. J. Physiol. Endocrinol. Metab. In press. [DOI] [PubMed] [Google Scholar]
- 21.Camacho R. C., Donahue E. P., James F. D., Berglund E. D., Wasserman D. H. 2006. Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 290: E405–E408. [DOI] [PubMed] [Google Scholar]
- 22.Fischer J., Lefevre C., Morava E., Mussini J. M., Laforet P., Negre-Salvayre A., Lathrop M., Salvayre R. 2007. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 39: 28–30. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama M., Sakai K., Ogawa M., McMillan J. R., Sawamura D., Shimizu H. 2007. Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve. 36: 856–859. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K., Inoguchi T., Maeda Y., Nakashima N., Kuwano A., Eto E., Ueno N., Sasaki S., Sawada F., Fujii M., Matoba Y., Sumiyoshi S., Kawate H., Takayanagi R. 2008. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J. Clin. Endocrinol. Metab. 93: 2877–2884. [DOI] [PubMed] [Google Scholar]
- 25.Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., et al. 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118: 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweiger M., Schoiswohl G., Lass A., Radner F. P., Haemmerle G., Malli R., Graier W., Cornaciu I., Oberer M., Salvayre R., et al. 2008. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J. Biol. Chem. 283: 17211–17220. [DOI] [PubMed] [Google Scholar]
- 27.Lefevre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J. L., Weissenbach J., et al. 2001. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 69: 1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorfman M. L., Hershko C., Eisenberg S., Sagher F. 1974. Ichthyosiform dermatosis with systemic lipidosis. Arch. Dermatol. 110: 261–266. [PubMed] [Google Scholar]
- 29.Chanarin I., Patel A., Slavin G., Wills E. J., Andrews T. M., Stewart G. 1975. Neutral-lipid storage disease: a new disorder of lipid metabolism. BMJ. 1: 553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jocken J. W., Smit E., Goossens G. H., Essers Y. P., van Baak M. A., Mensink M., Saris W. H., Blaak E. E. 2008. Adipose triglyceride lipase (ATGL) expression in human skeletal muscle is type I (oxidative) fiber specific. Histochem. Cell Biol. 129: 535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsted T. J., Nybo L., Schweiger M., Fledelius C., Jacobsen P., Zimmermann R., Zechner R., Kiens B. 2009. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am. J. Physiol. Endocrinol. Metab. 296: E445–E453. [DOI] [PubMed] [Google Scholar]