Abstract
Previous studies have infused radiolabeled arachidonic acid (AA) into rat brains and followed AA esterification into phospholipids for up to 24 h; however, the half-life of AA in rat brain phospholipids is unknown. Eighteen day old rats were fed either an n-3 PUFA adequate or deprived diet for 15 weeks. Following the 15 weeks, 40 µCi of [3H] AA was injected intracerebroventricularly into the right lateral ventricle using stereotaxic surgery and returned to their dietary treatment. From 4–120 days after [3H] AA administration, brains were collected for chemical analyses. The half-life of AA in rat brain phospholipids was 44 ± 4 days for the n-3 PUFA adequate group and 46 ± 4 days for the n-3 PUFA deprived group, which closely approximates the predicted half-life previously reported, based on the rate of entry from the plasma unesterified pool, suggesting the plasma unesterified pool is a major contributor to brain uptake of AA. Furthermore, unlike a previous report in which the half-life of brain phospholipid docosahexaenoic acid (DHA) was increased in n-3 PUFA deprived rats, n-3 PUFA deprivation did not significantly alter the AA half-life, suggesting different mechanisms exist to maintain brain concentrations of AA and DHA.
Keywords: docosahexaenoic acid, phospholipase, intracerebroventricular, rat
The PUFAs docosahexaenoic acid (DHA; 22:6n-3) and arachidonic acid (AA; 20:4n-6) have been increasingly recognized as biochemically potent compounds in the human diet, playing an important role in the nervous system (1–3). DHA and AA may be consumed directly through the diet, or they may be synthesized de novo through hepatic desaturation and elongation of their precursors, α-linolenic acid (ALA; 18:3n-3) and linoleic acid (LA; 18:2n-6), respectively (4–8). The rate of brain desaturation and elongation of plasma unesterified ALA and LA into DHA and AA, respectively, has been shown to be <1% of the brain rate of uptake of preformed DHA and AA from plasma in vivo (7, 9). One area of interest in these PUFAs is with regards to brain development and function, as the brain is highly enriched in both DHA and AA (10). Currently, DHA is being investigated for its potential neuroprotective and anti-inflammatory properties (11–13). Alternatively, understanding AA-mediated signal transduction and metabolism within the brain may provide insight into a variety of neurological and neurodegenerative disorders. Several studies have shown that widely used mood stabilizers target turnover and enzymatic pathways of AA (14, 15). Other studies have implicated upregulated AA signaling cascades in Alzheimer's disease and vascular dementia (16), Multiple Sclerosis (17), and Parkinson's disease (18, 19).
AA entry into the brain may originate from several sources, including the plasma unesterified pool, or via lipoproteins, including HDL, LDL, and VLDL; lipoprotein receptors have been identified on the blood brain barrier (20). Chen et al. (21) examined the contribution of the LDL pathway of PUFA entry into the brain by comparing brain phospholipid PUFA levels in LDL-knockout and wild-type mice and found no significant difference. However, it is unknown whether other pathways of entry (HDL, VLDL, unesterified, lysophosphatidylcholine, etc.) compensated for the lack of influx from the LDL pool. Contreras et al. (22) infused unesterified radiolabeled AA into the femoral vein of awake third-generation n-3 PUFA deprived rats and calculated the rate of unesterified AA entry into brain phospholipids Jin,i(plasma unesterified AA). From this calculation, a mathematical model can be used to predict an AA half-life in rat brain phospholipids [equation 1: ln(2)*cbrain,i(phospholipid AA)/Jin,i(plasma unesterified AA)], where cbrain,i(phospholipid AA) is the concentration of AA in brain phospholipid i, and Jin,i(plasma unesterified AA) is the net rate of AA entry from the plasma unesterified pool into brain phospholipid i, which was determined to be 42 and 48 days for n-3 PUFA adequate and n-3 PUFA deprived animals, respectively. However, this rate of AA loss from brain phospholipids is predicted exclusively by the rate of entry of plasma unesterified AA into the brain. Similarly, positron emission tomography studies in humans using unesterified [1-11C] AA have calculated a brain AA incorporation rate Jin,i(plasma unesterified AA) of 17.8 mg/day (2, 23). However, these studies did not take into account other potential sources of AA, including lipoprotein-transported AA or an as yet unidentified source.
Two distinct brain phospholipid PUFA half-lives have been described (24, 25). In one method, upon infusion of a unesterified radiolabeled PUFA into the plasma, the net rate of entry from the plasma unesterified pool is used to calculate the half-life of replacement in brain phospholipids according to equation 1, ln(2)*cbrain,i(phospholipid PUFA)/(Jin,i(plasma unesterified PUFA)) (see above). Because brain phospholipid concentrations are relatively stable over time (24), if plasma unesterified PUFAs are a major contributor to brain phospholipids, then the Jin,i(plasma unesterified PUFA) should closely approximate the net rate of loss of PUFA from brain phospholipids Jout,i(brain phospholipid PUFA), which can be measured directly by labeling brain phospholipids with a PUFA radiotracer and measuring the loss over time (4, 24). Indeed, DHA's half-life from brain phospholipids (33 days) approximates the rate of replacement from the plasma unesterified DHA pool, suggesting that for DHA, Jin,i(plasma unesterified DHA) ≅ Jout,i(brain phospholipid DHA) (4, 24). By correcting for potential dilution from sources besides the plasma unesterified PUFA pool, Rapoport and colleagues (25–27) have developed a method to calculate the net rate of PUFA uptake into brain phospholipids from the PUFA-CoA pool [JFAi,(brain phospholipid PUFA)]. This is accomplished by correcting Jin,i(plasma unesterified PUFA) for the ratio of the specific activity of the brain PUFA-CoA pool to the specific activity of the plasma unesterified PUFA pool according to equation 2, JFAi,(phospholipid PUFA) = Jin,i(phospholipid PUFA)/([c*brain PUFA-CoA/cbrain PUFA-CoA] /[c*plasma unesterified PUFA/cplasma unesterified PUFA]), where c* is the radiolabeled brain PUFA-CoA or plasma unesterified PUFA. Because brain synthesis of PUFA in vivo is slow (5, 7), one assumption of JFA,i(phospholipid PUFA) is that if the plasma unesterified pool is a major contributor to brain uptake, the dilution would be largely attributed to Land's recycling (25, 28, 29) and not uptake from other plasma pools (i.e., lipoproteins) (21). In this model, 90–97% of PUFA released from brain phospholipids is recycled back into brain phospholipids through the PUFA-CoA pool, giving half-lives much more rapid (30–33-fold) than the net loss of PUFA from brain phospholipids. Furthermore, because PUFA are largely recycled (90–97%) and not lost in this model, the JFAi,(brain phospholipid PUFA) ≠ Jout,i(brain phospholipid PUFA), which as discussed above should ≅ Jin,i(plasma unesterified PUFA) if plasma unesterified PUFAs are a major pool of uptake into brain phospholipids (4, 28). Several experiments have, in regards to AA, demonstrated that JFAi,(brain phospholipid AA) can be regulated independently of Jin,i(plasma unesterified AA) (30, 31), but whether or not the Jin,i(plasma unesterified AA) ≅ Jout,i (brain phospholipid AA) has not been reported.
AA is recognized as a significant component in neuronal cell membranes not only due to its effects on phospholipid membrane fluidity, but through its role in signal transduction (2, 32). These signaling cascades may play a crucial role in the development and treatment of a variety of psychiatric and neurological disorders. Therefore, it is important to understand how AA enters the brain from the plasma, the dynamics of AA turnover, and kinetic control once in the brain. In this study, we measured the rate of AA loss from rat brain phospholipids directly, by injecting radiolabeled AA into the brains of rats consuming either an n-3 PUFA adequate or deprived diet (24, 33, 34). Brain phospholipid radiolabeled AA levels were measured over multiple time points to determine the rate of loss and compared with previously predicted half-lives based on the rate of entry from the plasma unesterified pool (22) in an attempt to determine if the unesterified AA pool is a significant contributor to brain phospholipid AA. Brain phospholipid AA half-lives calculated in this study closely match the predicted half-lives from the plasma unesterified fatty acid pool.
MATERIALS AND METHODS
Animals
The protocol was approved by the Department of Comparative Medicine Animal Ethics Committee at the University of Toronto. Eighteen day old male Long Evans pups and their dams were purchased from Charles River Laboratories (Saint-Constant, Quebec). The pups were allowed to nurse for 3 days. When they reached 21 days of age, they were removed from the dams and fed either an n-3 PUFA adequate or n-3 PUFA deprived diet, as described below. The pups were maintained on their assigned diet for the duration of the study. The rats were housed at 22°C under a 12 h light/dark cycle with ad libitum access to food and water.
n-3 PUFA adequate and deficient diets
Rodent diets (prepared by Dyets Inc., Bethlehem, PA) were designed around a standard AIN-93 formulation [AIN-93 custom-saturated fat level (product 101093) and AIN-93 custom low n-3 (product 101094), n-3 PUFA adequate and deprived diets, respectively], with carbohydrate, protein, fat, fiber, salt, and vitamin/essential amino acid contents at 60, 20, and 10, 5, 3.5, and 1.5% (by weight), respectively (22, 24, 35, 36). Dietary fat came from adding select amounts of hydrogenated coconut, safflower, or flaxseed oils. Hydrogenated coconut (6% and 6.6% by weight, n-3 PUFA adequate and deprived, respectively) and safflower oil (3.2% and 3.4% by weight, n-3 PUFA adequate and deprived, respectively) were added to both diets as a base. Flaxseed oil (0.8% by weight) was added to the n-3 PUFA adequate diet to provide ALA at 3.3% of total fatty acids. The n-3 PUFA deprived chow was very low in ALA (0.15% of total fatty acids). Lauric (12:0), myristic (14:0), palmitic (16:0), and stearic (18:0) saturated fatty acids comprised approximately 39, 14, 9, and 7% of the total fatty acids for the n-3 PUFA adequate chow, respectively. The n-3 PUFA deprived chow had a similar saturated fatty acid profile but contained a higher percentage of lauric acid (42%) compared with the n-3 PUFA adequate diet to compensate for the lack of n-3 fatty acids. Both diets contained safflower oil to provide LA as 21% of total fatty acids. All 20+ carbon chain fatty acids amounted to <0.05% of total fatty acids in both diets. LA and ALA were set at 6% and 1% of total caloric intake (3935 kcal/kg), giving a ratio of 6:1 in the n-3 PUFA adequate diet. Dietary fatty acid composition was confirmed by GC analysis (see below).
Radiotracer
5,6,8,9,11,12,14,15-3H Arachidonic acid ([3H] AA) in 100% ethanol, specific activity of 200 Ci/mM, was purchased from Moravek Biochemicals (Brea, CA). HPLC with liquid scintillation counting was used to verify radioactive purity at >95%. The [3H] AA was dissolved in 5 mM HEPES buffer (pH 7.4) containing 50 mg/ml fatty acid-free BSA and sonicated for 15 min (24, 37). The radioactivity of the perfusate was confirmed to be 94% 20:4n-6 with 6% of the radioactivity eluting at 45 min.
Intracerebroventricular injection of [3H] AA
After 15 weeks of feeding, each rat was weighed using a digital scale. Rats were then anesthetized by isofluorane inhalation, and the head was placed in a stereotaxic instrument (Stoelting, IL) and the skull exposed. A 33-gauge bevelled injection needle (World Precision Instruments, Sarasota, FL) was inserted into the right lateral cerebral ventricle (4 mm ventral to the dura) via a hole that was drilled in the cranium at 1 mm posterior and 1.5 mm lateral to the bregma (37, 38). An injection (5 µL total volume, 0.175 µL/min; quintessential stereotaxic injector; Stoelting, IL) was made of 40 µCi of [3H] AA. The needle was left in for 5 min following the end of the injection, after which it was removed at a rate of 1 mm/min and the hole sealed with cranioplastic cement. The wound was closed with self-dissolving sutures and swabbed with iodine. For pain control, 1% sensocaine solution was injected under the scalp and a subcutaneous injection of 1 ml 0.9% saline was given to prevent dehydration. Animals were placed in a recovery cage with a heat lamp and then returned to their respective n-3 PUFA adequate or deficient diets, where they remained until the end of the study.
Collection of brains
At 4, 6, 8, 12, 16, 36, 48, 60, 90, and 120 days after intracerebroventricular (i.c.v.) injection of [3H] AA, n-3 PUFA adequate (n = 4) and deprived rats (n = 4) were euthanized by CO2 inhalation and decapitation. Brains were removed and stored at −80°C.
Isolation of brain lipids
Total lipids from whole brain were extracted according to the method of Folch, Lees, and Sloane Stanley (39). Isolation of various lipid classes from the total lipid extract was achieved by TLC. TLC H-plates (Analtech, Newark, DE) were washed in chloroform and methanol (2:1) and activated by heating for 1 h at 100°C. Brain total lipid extracts were separated into total phospholipids and phospholipid classes [choline glycerophospholipid (ChoGpl), ethanolamine glycerophospholipid (EtnGpl), phosphatidylinositol (PtdIns), and phosphatidylserine (PtdSer)] using a solvent system of heptane:diethyl ether:glacial acetic acid (60:40:2 by volume) or chloroform:methanol:2-propanol:0.25% (w/v) M KCl:triethylamine (30:9:25:6:18 by volume), respectively. TLC plates were sprayed with 8-anilino-1-napthalene sulfonic acid (0.1% w/v), and lipid bands were visualized under UV light. The positions of brain total phospholipid, ChoGpl, EtnGpl, PtdIns, and PtsSer bands were identified using authentic phospholipid standards (Avanti, Alabaster, AL) run on the TLC plates.
Quantitation of phospholipid radioactivity and fatty acid concentrations
Brain phospholipid bands were scraped from TLC plates into scintillation vials with 5 ml of scintillation cocktail (ASC; GE Healthcare Biosciences, Piscataway, NJ) and counted using a Packard TRI-CARB2900TR liquid scintillation counter (GMI, Ramsey, MN) with a detector efficiency of 47.7%. Radioactivities (dpm) were adjusted for counting efficiency and converted to curies (Ci). To determine fatty acid concentrations in each phospholipid, TLC scrapes were methylated using 14% boron trifluoride-methanol at 100°C for 1 h. Prior to methylation, di-17:0 phosphatidylcholine was added as an internal standard to brain phospholipids. Fatty acid methyl esters (FAMEs) from total brain phospholipids, phospholipid fractions, and rodent chow were analyzed using a Varian-430 gas chromatograph (Lake Forest, CA) equipped with a Varian FactorFour capillary column (VF-23 ms; 30 m × 0.25 mm i.d. × 0.25 µm film thickness) and a flame ionization detector. Samples were injected in splitless mode. The injector and detector ports were set at 250°C. FAMEs were eluted using a temperature program set initially at 50°C for 2 min, increased at 20°C/min and held at 170°C for 1 min, then at 3°C/min and held at 212°C for 5 min to complete the run at 28 min. The carrier gas was helium, set to a constant flow rate of 0.7 ml/min. Peaks were identified by retention times of FAME standards. Fatty acid concentrations (µmol/g wet weight brain) were calculated by proportional comparison of GC peak areas with the area of the 17:0 internal standard (40).
Confirmation of radiotracer identity
Radiotracer separation and identification were performed according to the method of Aveldano et al., with slight modifications (41–44). FAMEs from total phospholipids were separated by HPLC (Waters 2690; Boston, MS) with a Luna C18 reverse column (4.6 × 250 mm, 100 Å; Phenomenex, Torrance, CA) equipped with an in-line UV photodiode array detector (Waters 996) and monitored at 242 nm. Initial conditions were set at 1 ml/min gradient system consisting of (A) 100% water and (B) 100% acetonitrile. The gradient commenced with 85% (B) for 30 min, then increased to 100% (B) over a 10 min period, where it was maintained for 20 min before returning to 85% (B) over a 5 min period. All fractions were collected at 1 min intervals for a total of 55 min and then analyzed separately by liquid scintillation counting. Similar to what has been reported by Igarashi et al. (42), using authentic standards (Nu-Chek-Prep, Elysian, MN), we find that methyl esters of 20:4n-6, 22:4n-6, and 22:5n-6 eluted from the HPLC at 33.5, 42.5, and 38.5 min, respectively.
Calculations and statistics
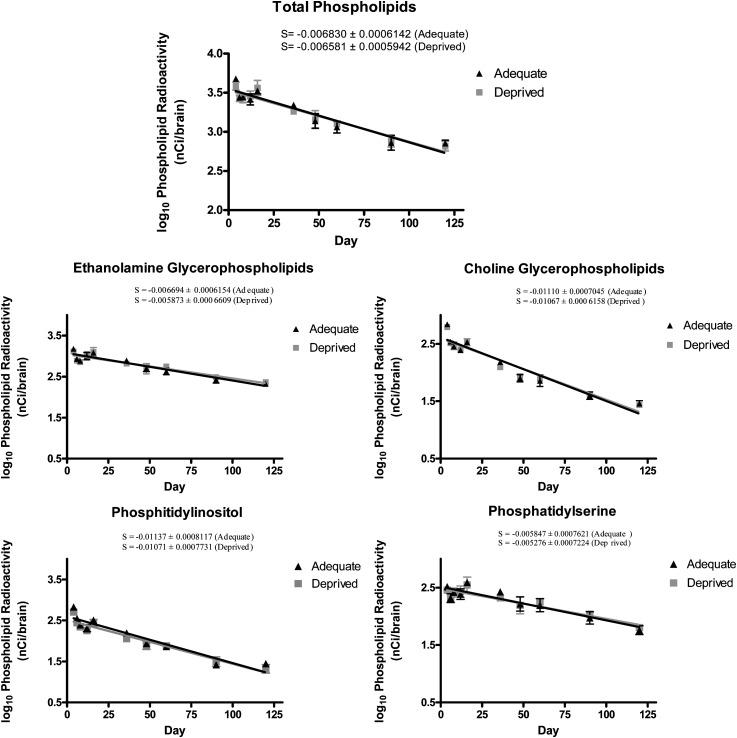
Data were expressed as means ± SE. Log10 radioactivity (in total or individual brain phospholipid fractions, nCi/brain) was plotted against time after i.c.v. injection of [3H] AA, and the data were fit by linear regression to provide slopes in (log10) nCi/brain/day (Fig. 1). Linear regression was used to determine if the slopes were significantly different from zero and was used to determine whether the slope in each phospholipid fraction differed significantly between n-3 PUFA deprived and adequate rats (GraphPad Prism 4; GraphPad Software, La Jolla, CA). Statistical significance was taken as P ≤ 0.05. Half-lives (days) of [3H] AA were calculated from the measured slopes in total and individual phospholipids by the following equation 3 (24, 45): T1/2 = log10 (2)/slope of regression line.
Fig. 1.
Linear regression slopes for (log10) phospholipid radioactivity (nCi/brain) over time (days). Adequate, n-3 PUFA adequate group; deprived, n-3 PUFA deprived group; day, time (days) following 15 weeks of feeding, after i.c.v. [3H] AA injection. Data are mean ± SE; n = 4 independent samples per group per time point (note differences in y axis scales.)
RESULTS
Bodyweights
Rat bodyweights (735 ± 57 and 716 ± 69 g, n-3 PUFA adequate and deprived groups, respectively) did not differ statistically (P = 0.2) after 15 weeks of n-3 PUFA adequate or deprived diet.
Tracer identification and brain phospholipid PUFA concentrations
Brain samples (day 4 and 120 time point, postsurgery, n-3 PUFA adequate and deprived diet) were analyzed by HPLC to confirm successful infusion of the perfusate into the brain; 20:4n-6 eluded at 33.5 min, and total phospholipid radioactivity was >94% AA. Trace amounts of radioactivity (<6%) were eluted at 45 min. Because this peak was also found in the perfusate, it is not possible to associate this to brain metabolism. We also analyzed the 20:4n-6 fraction obtained from the HPLC by GC/flame ionization detection and did not detect any other PUFA in this fraction.
Table 1 shows the values for esterified fatty acids in brain phospholipid classes, from n-3 PUFA adequate and deprived rats, measured after 15 weeks of feeding. DHA concentrations in total brain phospholipids were 27% lower in the n-3 PUFA deprived group compared with the n-3 PUFA adequate group. DHA was also lower in phospholipid fractions of the n-3 PUFA deprived rats (29% ChoGpl, 24% EtnGpl, 50% PtdIns, and 31% PtdSer), compared with the n-3 PUFA adequate group (P < 0.05). The n-6 PUFA docosapentaenoic acid (22:5n-6) was significantly elevated in the n-3 PUFA deprived group compared with the n-3 PUFA adequate group across all phospholipid classes (P < 0.001). AA was significantly higher in total brain phospholipids and ethanolamine glycerophospholipids from the n-3 PUFA deprived group compared with the n-3 PUFA adequate group, whereas myristic acid (14:0) was significantly higher in PtdSer in the n-3 PUFA deprived group (P < 0.05). See Table 1 for other fatty acids.
TABLE 1.
Esterified fatty acid concentrations in brain phospholipid classes from n-3 PUFA adequate and deprived rats, measured following 15 weeks of feeding
| Concentration, µmol/g Brain |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Esterified Fatty Acid | ChoGpl |
EtnGpl |
PtdIns |
PtdSer |
Total PL |
|||||
| Adq. | Dep. | Adq. | Dep. | Adq. | Dep. | Adq. | Dep. | Adq. | Dep. | |
| 14:0 | 0.1 ± 0.09 | 0.12 ± 0.02 | 0.3 ± 0.09 | 0.3 ± 0.17 | 0.02 ± 0.003 | 0.03 ± 0.004 | 0.03 ± 0.002 | 0.01 ± 0.005** | 0.5 ± 0.1 | 0.9 ± 0.2 |
| 16:0 | 17.7 ± 0.9 | 20.3 ± 1.8 | 3.7 ± 0.2 | 4.3 ± 0.2* | 0.6 ± 0.03 | 0.7 ± 0.06 | 0.35 ± 0.04 | 0.35 ± 0.06 | 21.7 ± 1.3 | 23.1 ± 0.2 |
| 18:0 | 5.8 ± 0.3 | 6.8 ± 0.6 | 8.1 ± 0.4 | 9.2 ± 0.4 | 1.8 ± 0.1 | 1.9 ± 0.1 | 6.1 ± 0.2 | 6.4 ± 0.4 | 22.1 ± 1.1 | 22.8 ± 0.3 |
| 20:0 | 0.1 ± 0.006 | 0.1 ± 0.01 | 0.2 ± 0.05 | 0.1 ± 0.009 | 0.02 ± 0.003 | 0.02 ± 0.002 | 0.07 ± 0.006 | 0.06 ± 0.004 | 0.7 ± 0.03 | 0.9 ± 0.1 |
| 22:0 | 0.1 ± 0.01 | 0.2 ± 0.02 | ND | 0.05 ± 0.005 | ND | ND | 0.09 ± 0.003 | 0.09 ± 0.009 | 0.78 ± 0.04 | 0.8 ± 0.04 |
| 16:1n-9 | 0.3 ± 0.08 | 0.60 ± 0.2 | 0.6 ± 0.2 | 0.8 ± 0.3 | 0.02 ± 0.008 | 0.07 ± 0.02 | 0.02 ± 0.004 | 0.02 ± 0.003 | 1.6 ± 0.2 | 2.1 ± 0.3 |
| 18:1n-(7+9) | 11.3 ± 1 | 13.0 ± 1.6 | 8.0 ± 0.9 | 9.8 ± 0.9 | 0.7 ± 0.05 | 0.7 ± 0.03 | 3.5 ± 0.3 | 3.5 ± 0.2 | 21.5 ± 1.0 | 21 ± 0.3 |
| 20:1n-9 | 0.5 ± 0.01 | 0.6 ± 0.05 | 1.4 ± 0.09 | 1.5 ± 0.06 | 0.06 ± 0.009 | 0.06 ± 0.002 | 0.4 ± 0.05 | 0.4 ± 0.03 | 1.1 ± 0.3 | 1.4 ± 0.3 |
| 22:1n-9 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.005 | 0.09 ± 0.005 | 0.04 ± 0.01 | 0.09 ± 0.03 | 0.07 ± 0.005 | 0.08 ± 0.006 | 0.3 ± 0.1 | 0.4 ± 0.05 |
| 24:1n-9 | 0.1 ± 0.009 | 0.2 ± 0.02* | 0.03 ± 0.002 | 0.06 ± 0.006** | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.03 ± 0.001 | 0.06 ± 0.007** | 2.0 ± 0.16 | 2.3 ± 0.07 |
| 18:2n-6 | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.13 ± 0.01 | 0.27 ± 0.04** | 0.02 ± 0.002 | 0.02 ± 0.003 | 0.04 ± 0.004 | 0.04 ± 0.004 | 0.7 ± 0.16 | 0.6 ± 0.06 |
| 18:3n-3 | 0.027 ± 0.004 | 0.05 ± 0.008* | 0.02 ± 0.0007 | 0.04 ± 0.007* | ND | ND | 0.02 ± 0.006 | 0.02 ± 0.004 | 0.04 ± 0.02 | 0.02 ± 0.004 |
| 18:3n-6 | ND | ND | 0.008 ± 0.001 | 0.01 ± 0.006 | ND | ND | 0.004 ± 0.0001 | ND | 0.04 ± 0.009 | ND |
| 20:3n-3 | 0.05 ± 0.004 | 0.06 ± 0.007 | 0.09 ± 0.01 | 0.07 ± 0.004 | ND | 0.006 ± 0.001 | 0.02 ± 0.002 | 0.02 ± 0.004 | 0.07 ± 0.03 | 0.1 ± 0.02 |
| 20:4n-6 | 2.0 ± 0.1 | 2.4 ± 0.2 | 4.4 ± 0.03 | 5.2 ± 0.03 * | 1.5 ± 0.1 | 1.7 ± 0.04 | 0.5 ± 0.07 | 0.6 ± 0.1 | 7.8 ± 0.3 | 8.9 ± 0.1* |
| 22:4n-6 | 0.3 ± 0.01 | 0.4 ± 0.04** | 1.9 ± 0.1 | 2.5 ± 0.09** | 0.09 ± 0.015 | 0.1 ± 0.01 | 0.5 ± 0.04 | 0.6 ± 0.04 | 1.5 ± 0.3 | 1.3 ± 0.05 |
| 22:5n-6 | 0.04 ± 0.003 | 0.6 ± 0.05*** | 0.1 ± 0.01 | 2.3 ± 0.1*** | 0.01 ± 0.001 | 0.06 ± 0.006*** | 0.08 ± 0.04 | 1.2 ± 0.09*** | 0.2 ± 0.006 | 4.0 ± 0.2*** |
| 22:5n-3 | 0.03 ± 0.0001 | 0.01 ± 0.002*** | 0.09 ± 0.005 | 0.04 ± 0.001*** | ND | ND | 0.03 ± 0.001 | 0.02 ± 0.002*** | 0.14 ± 0.02 | 0.12 ± 0.02 |
| 22:6n-3 | 1.5 ± 0.1 | 1.0 ± 0.09** | 6.2 ± 0.5 | 4.5 ± 0.09** | 0.2 ± 0.04 | 0.1 ± 0.007* | 2.6 ± 0.1 | 1.7 ± 0.07*** | 9.9 ± 0.5 | 7.3 ± 0.2*** |
| Totalsa | 40.3 ± 2.7 | 46.8 ± 4.5 | 35.3 ± 2.6 | 41.1 ± 1.9 | 5.1 ± 0.4 | 5.6 ± 0.3 | 14.5 ± 0.9 | 15.2 ± 0.9 | 92.7 ± 5.7 | 98.0 ± 2.0 |
Data are means ± SE (n = 5 independent samples per group). Total PL, total phospholipids; ND, not detected. *P < 0.05, **P < 0.01, ***P < 0.001; significant difference between n-3 PUFA deprived (Dep.) and n-3 PUFA adequate (Adq.) means. Fatty acids: 14:0, myristic; 16:0, palmitic; 18:0, stearic; 20:0, arachidic; 22:0, docosanoic; 16:1n-9, palmitoleic; 18:1n-(7+9), vaccenic/oleic; 20:1n-9, eicosenoic; 22:1n-9, erucic; 24:1n-9, nervonic; 1 8:2n-6, linoleic 18:3n-3, α-linolenic; 18:3n-6, γ-linolenic; 20:3n-3, eicosatrienoic; 20:4n-6, arachidonic; 22:4n-6, docosatetraenoic; 22:5n-6, docosapentaenoic; 22:5n-3, docosapentaenoic 22:6n-3, docosahexaenoic.
No significant difference between respective n-3 PUFA adequate and deprived lipid pools.
Brain phospholipid AA half-lives
Total brain phospholipid and phospholipid fraction radioactivity was plotted over time (days) following surgery (Fig. 1). Slopes for all phospholipid classes among both dietary groups were significantly different from zero (P < 0.0001). Linear regression analysis of slopes showed no statistical difference between n-3 PUFA adequate and deprived groups across all phospholipid classes (P > 0.05). Half-lives are shown in Table 2, ranging from 26–51 days for the n-3 PUFA adequate group and from 28–57 days for the n-3 PUFA deprived group. No significant differences were found for the half-lives between the groups (P > 0.05). Within the dietary groups, the half-lives of AA in both EtnGpl and PtdSer were significantly longer than the half-lives of AA in ChoGpl and PtdIns (P < 0.0001). No other differences in half-life were observed between phospholipid fractions with a dietary group. The rate of AA loss from brain phospholipids (Jout ) was calculated using equation 4 (24, 46):
and ranged from 0.014 (PtdSer) to 0.149 µmol/brain/day (EtnGpl) for the n-3 PUFA adequate group and from 0.015 (PtdSer) to 0.149 µmol/brain/day (EtnGpl) for the n-3 PUFA deprived group (Table 2).
TABLE 2.
[3H]AA half-lives in rat brain phospholipids
| n-3 PUFA Diet Group | Brain Lipid | Slope, Days | SE, Days | P Value | T1/2 (days) | Jout (µmol/brain/day) | Loss/Brain/Day (%) |
|---|---|---|---|---|---|---|---|
| Adequate | |||||||
| Total PL | −0.006830 | 0.0006142 | <0.0001 | 44 | 0.248 | 1.6 | |
| ChoGpl | −0.01110 | 0.0007045 | <0.0001 | 27 | 0.103 | 2.6 | |
| EtnGpl | −0.006694 | 0.0006154 | <0.0001 | 45 | 0.149 | 1.5 | |
| PtdIns | −0.01137 | 0.0008117 | <0.0001 | 26 | 0.080 | 2.6 | |
| PtdSer | −0.005847 | 0.0007621 | <0.0001 | 51 | 0.014 | 1.4 | |
| Deprived | |||||||
| Total PL | −0.006581 | 0.0005942 | <0.0001 | 46 | 0.281 | 1.5 | |
| ChoGpl | −0.01067 | 0.0006158 | <0.0001 | 28 | 0.123 | 2.4 | |
| EtnGpl | −0.005873 | 0.0006609 | <0.0001 | 51 | 0.149 | 1.4 | |
| PtdIns | −0.01071 | 0.0007731 | <0.0001 | 28 | 0.088 | 2.5 | |
| PtdSer | −0.005276 | 0.0007224 | <0.0001 | 57 | 0.015 | 1.0 |
Slopes for decline of [3H] AA radioactivity in brain phospholipids and corresponding calculated half-lives, equation 3, (T1/2) = log10 (2)/slope of regression line and rates of AA loss; equation 4, Jout (µmol/brain/day) = ln(2)CAA/T1/2. p-values designate significance of difference of slope from 0. ChoGpl and PtdIns were significantly different compared with EtnGpl and PtdSer within the same dietary group; P < 0.05. Mean ± SE brain weight; 2.02 ± 0.34 and 2.10 ± 0.52 g, n-3 PUFA adequate and deprived groups, respectively. See Table 1 for abbreviations.
DISCUSSION
Dietary deprivation of n-3 PUFA in rats has previously been shown to result in a 27–37% decrease in brain phospholipid DHA following 15 weeks of feeding, compared with the n-3 PUFA adequate diet (24, 33, 34); we observed a significant total brain phospholipid DHA decrease of 27% in our n-3 PUFA deprived group compared with the n-3 PUFA adequate group (from 9.9–7.3 µmol/ g brain). Furthermore, after 15 weeks, total phospholipid AA was significantly increased in the n-3 PUFA deprived group; this effect has been previously reported (47, 48) and brain PUFA concentrations appear to be stable upon 15 weeks of adequate or deprived feeding (24). We also observed a 2,000% increase in total phospholipid n-6 docosapentaenoic acid in the n-3 PUFA deprived group compared with the n-3 PUFA adequate group (from 0.2–4 µmol/ g brain), which is consistent with observations from other studies (22, 24, 33). Because the brain appears limited in its ability to desaturate/elongate 18:2n-6 (7), it is possible that 22:5n-6 was formed in the liver and transported to the brain. Past studies have examined brain AA metabolism for ≤24 h following i.c.v. injection in the lateral ventricle (37, 49), giving us the opportunity to measure brain phospholipid AA loss over a greater time period (4–120 days). We calculated the half-life of AA in rat brain total phospholipids to be 44 ± 4 and 46 ± 4 days for the n-3 PUFA adequate and deprived groups, respectively; these half-lives represent the rate of AA metabolic consumption, and not the more rapid kinetic rate of recycling into the phospholipid membrane. The half-lives for phospholipid fractions ranged from 26 (PtdIns) to 51 (PtdSer) days in the n-3 PUFA adequate group and 28 (ChoGpl, PtdIns) to 57 (PtdSer) days in the n-3 PUFA deprived group; however, the differences were not significant between the dietary groups. This finding is consistent with slower rates of brain AA incorporation into EtnGpl and PtdSer, compared with ChoGpl and PtdIns, observed in other studies (50, 51). It should be noted, that i.c.v. infusion can induce inflammation and damage to the blood-brain barrier (52). To minimize this, we used a 33 gauge needle and allowed the animal to recover for 4–120 days prior to measuring brain phospholipid radioactivity. This procedure has been widely used to deliver compounds including fatty acids to the brain (24, 37, 49, 53–55).
With growing evidence implicating brain AA signaling in neurological and psychiatric disorders (2, 16–18), manipulation of brain phospholipid AA concentration appears to be a likely direction for future research (2, 56), and our half-life estimate offers insight into brain phospholipid AA depletion over time. One caveat to the use of these calculated half-lives, however, is that the brain appears to be capable of conserving a minimal concentration of both AA and DHA. DeMar et al. (24) measured the rate of loss of i.c.v.-infused radiolabeled DHA from rat brain phospholipids following 15 weeks of n-3 PUFA adequate or deprived feeding. The rates of loss were used to calculate half-lives for the two dietary groups, which were reported as 33 and 90 days for the n-3 PUFA adequate and deprived group, respectively. The prolonged half-life of the n-3 PUFA deprived group was interpreted as brain conservation of DHA. Conversion of ALA to DHA in the brain is slow (<1%) and is not increased in periods of n-3 PUFA deprivation (47). Conservation of brain DHA may be occurring, as the enzyme thought to be responsible for cleaving DHA from brain phospholipids, calcium-independent phospholipase A2, has been shown to be downregulated during n-3 PUFA deprivation (34). We did not observe a similar conservation of AA in our study, using the same n-3 PUFA dietary model for 15 weeks, suggesting DHA, but not AA, is selectively conserved in situations of n-3 PUFA deprivation. The difference between AA and DHA may be explained by the specificity of enzymes known to release AA and DHA from brain phospholipids and/or other selective catabolic reactions, including β-oxidation and eicosanoid/docosanoid synthesis (57–59). Several studies suggest AA and DHA are preferentially released from the sn-2 position of brain phospholipids by fatty acid selective phospholipase A2 (for review, see Ref. 58). Confirming enzyme specificity would be beneficial for pharmacological interventions of neurodegenerative disorders originating from an upregulation of the AA signaling cascade, by targeting AA turnover without altering phospholipid DHA release. n-3 PUFA deprivation has also been shown to upregulate calcium-dependant cytosolic phospholipase A2 activity (34); therefore, brain phospholipid AA turnover may be increased in this study. We did not observe a significantly different brain phospholipid AA half-life between the n-3 PUFA adequate and deprived group; however, only absolute loss of brain phospholipid AA was measured, and under normal conditions ∼97% of brain phospholipid AA cleaved from the phospholipid membrane is reesterified after being released (2, 58); thus, brain phospholipid AA turnover may be increased but recycled back into brain phospholipids. Furthermore, we tested the half-life of AA loss in rodents receiving 21% of fatty acids as LA. It would be of interest to repeat this study with chow consuming lower levels of n-6 PUFA (60) to see if n-3 PUFA deprivation increases AA loss when the AA supply to the brain is limited.
To our knowledge, direct measurement of the AA half-life in rat brain phospholipids has not been calculated before; however, brain uptake rates for AA have been calculated (using a similar dietary model) based on the rate of radiolabeled AA entry into brain phospholipids from the plasma unesterified pool (22). This study predicted a brain phospholipid AA half-life of approximately 42 and 48 days for n-3 PUFA adequate and deprived groups, respectively, which closely approximates the direct half-life found in our study. The similarity between this predicted AA half-life and our direct measure of AA half-life support findings suggesting the plasma unesterified pool may contribute a significant percentage of brain phospholipid AA (26, 28, 61). If the unesterified pool is supplying the brain with AA, attempts to alter the accretion and/or turnover of brain phospholipid AA through pharmacological manipulation of blood-brain barrier endothelium lipoprotein receptors may prove ineffective. Consequently, plasma unesterified AA influx to the brain could be decreased by lowering plasma unesterified AA concentrations. However, it is also possible that lipoprotein lipase action on plasma phospholipids contributes to plasma unesterified AA. Furthermore, investigations using unesterified [1-11C] AA for positron emission tomography imaging have calculated human brain AA uptake of 17.8 mg/day (58.5 μmol/day) based on the rate of AA incorporation from the plasma unesterified AA pool (2, 62). However, if other pools (LDL, HDL, VLDL, lysophospholipid, etc.) are also contributing significantly to brain AA concentrations, current estimations of brain AA uptake rate would be underestimated, and the brain phospholipid AA half-life therefore overestimated. Future studies are needed to confirm which plasma pool(s) contributes to brain phospholipid AA uptake.
CONCLUSIONS
In conclusion, we infused [3H] AA in the right lateral ventricle of male Long Evans rats, following 15 weeks of n-3 PUFA adequate or deprived diet, to determine the half-life of AA in rat brain phospholipids. We calculated a half-life of 44 and 46 days for the n-3 PUFA adequate and deprived groups, respectively, which is similar to the half-life predicted from the incorporation rate of plasma unesterified [3H] AA into brain phospholipids (22). This similarity suggests that the unesterified AA pool is a significant contributor to brain phospholipids. The calculated half-lives may be used as a guideline in future studies attempting to manipulate brain phospholipid AA using a dietary strategy; however, our half-lives may represent the upper-limit rate of loss, as a study using an n-6 PUFA adequate and deprived diet for 15 weeks reported a much slower rate of loss (56). Finally, we did not observe a conservation of AA as was observed for DHA in a similar n-3 PUFA adequate and deprived 15 week feeding model (24). This result could be explained by the selectivity of enzymes (calcium-independent phospholipase A2 and calcium-dependant cytosolic phospholipase A2) known to release AA and DHA from brain phospholipids; a better understanding of selectivity will be useful for pharmacological targeting of brain AA turnover without disruption of the brain DHA signaling cascade. This may prove beneficial for the treatment of various neurological and psychiatric diseases, such as bipolar disorder, in which evidence points to a pathological upregulation of brain AA signaling (14).
Acknowledgments
The authors thank Chuck T. Chen and Rainer de Guzman for their assistance with the project.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ALA
- α-linolenic acid
- ChoGpl
- choline glycerophospholipid
- DHA
- docosahexaenoic acid
- EtnGpl
- ethanolamine glycerophospholipid
- FAME
- fatty acid methyl ester
- i.c.v.
- intracerebroventricular
- LA
- linoleic acid
- PtdIns
- phosphatidylinositol
- PtdSer
- phosphatidylserine
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to R.P.B.
REFERENCES
- 1.Salem N., Jr., Litman B., Kim H. Y., Gawrisch K. 2001. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 36: 945–959. [DOI] [PubMed] [Google Scholar]
- 2.Rapoport S. I. 2008. Arachidonic acid and the brain. J. Nutr. 138: 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessandri J. M., Guesnet P., Vancassel S., Astorg P., Denis I., Langelier B., Aid S., Poumes-Ballihaut C., Champeil-Potokar G., Lavialle M. 2004. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 44: 509–538. [DOI] [PubMed] [Google Scholar]
- 4.Rapoport S. I., Rao J. S., Igarashi M. 2007. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids. 77: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demar J. C., Jr., Ma K., Chang L., Bell J. M., Rapoport S. I. 2005. Alpha-linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 94: 1063–1076. [DOI] [PubMed] [Google Scholar]
- 6.Scott B. L., Bazan N. G. 1989. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. USA. 86: 2903–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMar J. C., Jr., Lee H. J., Ma K., Chang L., Bell J. M., Rapoport S. I., Bazinet R. P. 2006. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim. Biophys. Acta. 1761: 1050–1059. [DOI] [PubMed] [Google Scholar]
- 8.Mohrhauer H., Holman R. T. 1963. The effect of dose level of essential fatty acids upon fatty acid composition of the rat liver. J. Lipid Res. 4: 151–159. [PubMed] [Google Scholar]
- 9.Igarashi M., DeMar J. C., Jr., Ma K., Chang L., Bell J. M., Rapoport S. I. 2007. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J. Lipid Res. 48: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 10.Diau G. Y., Hsieh A. T., Sarkadi-Nagy E. A., Wijendran V., Nathanielsz P. W., Brenna J. T. 2005. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukiw W. J., Bazan N. G. 2008. Docosahexaenoic acid and the aging brain. J. Nutr. 138: 2510–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orr S. K., Bazinet R. P. 2008. The emerging role of docosahexaenoic acid in neuroinflammation. Curr. Opin. Investig. Drugs. 9: 735–743. [PubMed] [Google Scholar]
- 13.Kim H. Y. 2007. Novel metabolism of docosahexaenoic acid in neural cells. J. Biol. Chem. 282: 18661–18665. [DOI] [PubMed] [Google Scholar]
- 14.Rao J. S., Lee H. J., Rapoport S. I., Bazinet R. P. 2008. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol. Psychiatry. 13: 585–596. [DOI] [PubMed] [Google Scholar]
- 15.Rapoport S. I., Bosetti F. 2002. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch. Gen. Psychiatry. 59: 592–596. [DOI] [PubMed] [Google Scholar]
- 16.Yagami T. 2006. Cerebral arachidonate cascade in dementia: Alzheimer's disease and vascular dementia. Curr. Neuropharmacol. 4: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbige L. S., Sharief M. K. 2007. Polyunsaturated fatty acids in the pathogenesis and treatment of multiple sclerosis. Br. J. Nutr. 98(Suppl 1): S46–S53. [DOI] [PubMed] [Google Scholar]
- 18.Minghetti L. 2004. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 63: 901–910. [DOI] [PubMed] [Google Scholar]
- 19.Julien C., Berthiaume L., Hadj-Tahar A., Rajput A. H., Bedard P. J., Di Paolo T., Julien P., Calon F. 2006. Postmortem brain fatty acid profile of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem. Int. 48: 404–414. [DOI] [PubMed] [Google Scholar]
- 20.Meresse S., Delbart C., Fruchart J. C., Cecchelli R. 1989. Low-density lipoprotein receptor on endothelium of brain capillaries. J. Neurochem. 53: 340–345. [DOI] [PubMed] [Google Scholar]
- 21.Chen C. T., Ma D. W., Kim J. H., Mount H. T., Bazinet R. P. 2008. The low density lipoprotein receptor is not necessary for maintaining mouse brain polyunsaturated fatty acid concentrations. J. Lipid Res. 49: 147–152. [DOI] [PubMed] [Google Scholar]
- 22.Contreras M. A., Chang M. C., Rosenberger T. A., Greiner R. S., Myers C. S., Salem N., Jr., Rapoport S. I. 2001. Chronic nutritional deprivation of n-3 alpha-linolenic acid does not affect n-6 arachidonic acid recycling within brain phospholipids of awake rats. J. Neurochem. 79: 1090–1099. [DOI] [PubMed] [Google Scholar]
- 23.Giovacchini G., Lerner A., Toczek M. T., Fraser C., Ma K., DeMar J. C., Herscovitch P., Eckelman W. C., Rapoport S. I., Carson R. E. 2004. Brain incorporation of 11C-arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J. Nucl. Med. 45: 1471–1479. [PubMed] [Google Scholar]
- 24.DeMar J. C., Jr., Ma K., Bell J. M., Rapoport S. I. 2004. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J. Neurochem. 91: 1125–1137. [DOI] [PubMed] [Google Scholar]
- 25.Robinson P. J., Noronha J., DeGeorge J. J., Freed L. M., Nariai T., Rapoport S. I. 1992. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res. Brain Res. Rev. 17: 187–214. [DOI] [PubMed] [Google Scholar]
- 26.Rapoport S. I., Chang M. C., Spector A. A. 2001. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J. Lipid Res. 42: 678–685. [PubMed] [Google Scholar]
- 27.Grange E., Deutsch J., Smith Q. R., Chang M., Rapoport S. I., Purdon A. D. 1995. Specific activity of brain palmitoyl-CoA pool provides rates of incorporation of palmitate in brain phospholipids in awake rats. J. Neurochem. 65: 2290–2298. [DOI] [PubMed] [Google Scholar]
- 28.Chen C. T., Green J. T., Orr S. K., Bazinet R. P. 2008. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot. Essent. Fatty Acids. 79: 85–91. [DOI] [PubMed] [Google Scholar]
- 29.Lee H. J., Rao J. S., Rapoport S. I., Bazinet R. P. 2007. Antimanic therapies target brain arachidonic acid signaling: lessons learned about the regulation of brain fatty acid metabolism. Prostaglandins Leukot. Essent. Fatty Acids. 77: 239–246. [DOI] [PubMed] [Google Scholar]
- 30.Bazinet R. P., Rao J. S., Chang L., Rapoport S. I., Lee H. J. 2006. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol. Psychiatry. 59: 401–407. [DOI] [PubMed] [Google Scholar]
- 31.Lee H. J., Rao J. S., Chang L., Rapoport S. I., Bazinet R. P. 2008. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J. Lipid Res. 49: 162–168. [DOI] [PubMed] [Google Scholar]
- 32.Axelrod J. 1990. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem. Soc. Trans. 18: 503–507. [DOI] [PubMed] [Google Scholar]
- 33.DeMar J. C., Jr., Ma K., Bell J. M., Igarashi M., Greenstein D., Rapoport S. I. 2006. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 47: 172–180. [DOI] [PubMed] [Google Scholar]
- 34.Rao J. S., Ertley R. N., DeMar J. C., Jr., Rapoport S. I., Bazinet R. P., Lee H. J. 2007. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry. 12: 151–157. [DOI] [PubMed] [Google Scholar]
- 35.Reeves P. G., Nielsen F. H., Fahey G. C., Jr. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123: 1939–1951. [DOI] [PubMed] [Google Scholar]
- 36.Moriguchi T., Loewke J., Garrison M., Catalan J. N., Salem N., Jr. 2001. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J. Lipid Res. 42: 419–427. [PubMed] [Google Scholar]
- 37.Gatti C., Noremberg K., Brunetti M., Teolato S., Calderini G., Gaiti A. 1986. Turnover of palmitic and arachidonic acids in the phospholipids from different brain areas of adult and aged rats. Neurochem. Res. 11: 241–252. [DOI] [PubMed] [Google Scholar]
- 38.Noble E. P., Wurtman R. J., Axelrod J. 1967. A simple and rapid method for injecting H3-norepinephrine into the lateral ventricle of the rat brain. Life Sci. 6: 281–291. [DOI] [PubMed] [Google Scholar]
- 39.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 40.Chen C. T., Liu Z., Ouellet M., Calon F., Bazinet R. P. 2009. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot. Essent. Fatty Acids. 80: 157–163. [DOI] [PubMed] [Google Scholar]
- 41.Aveldano M. I., VanRollins M., Horrocks L. A. 1983. Separation and quantitation of free fatty acids and fatty acid methyl esters by reverse phase high pressure liquid chromatography. J. Lipid Res. 24: 83–93. [PubMed] [Google Scholar]
- 42.Igarashi M., Ma K., Chang L., Bell J. M., Rapoport S. I., DeMar J. C., Jr. 2006. Low liver conversion rate of alpha-linolenic to docosahexaenoic acid in awake rats on a high-docosahexaenoate-containing diet. J. Lipid Res. 47: 1812–1822. [DOI] [PubMed] [Google Scholar]
- 43.Lee H. J., Rao J. S., Chang L., Rapoport S. I., Bazinet R. P. 2007. Chronic lamotrigine does not alter the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat: implications for the treatment of bipolar disorder. Psychopharmacology (Berl.). 193: 467–474. [DOI] [PubMed] [Google Scholar]
- 44.Lee H. J., Rao J. S., Ertley R. N., Chang L., Rapoport S. I., Bazinet R. P. 2007. Chronic fluoxetine increases cytosolic phospholipase A(2) activity and arachidonic acid turnover in brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl.). 190: 103–115. [DOI] [PubMed] [Google Scholar]
- 45.Stinson A. M., Wiegand R. D., Anderson R. E. 1991. Recycling of docosahexaenoic acid in rat retinas during n-3 fatty acid deficiency. J. Lipid Res. 32: 2009–2017. [PubMed] [Google Scholar]
- 46.Purdon A. D., Rosenberger T. A., Shetty H. U., Rapoport S. I. 2002. Energy consumption by phospholipid metabolism in mammalian brain. Neurochem. Res. 27: 1641–1647. [DOI] [PubMed] [Google Scholar]
- 47.Igarashi M., Ma K., Chang L., Bell J. M., Rapoport S. I. 2007. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J. Lipid Res. 48: 2463–2470. [DOI] [PubMed] [Google Scholar]
- 48.Mathieu G., Denis S., Lavialle M., Vancassel S. 2008. Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins Leukot. Essent. Fatty Acids. 78: 391–401. [DOI] [PubMed] [Google Scholar]
- 49.Sun G. Y., Yau T. M. 1976. Incorporation of (1-14C)oleic acid and (1-14C)arachidonic acid into lipids in the subcellular fractions of mouse brain. J. Neurochem. 27: 87–92. [DOI] [PubMed] [Google Scholar]
- 50.Sun G. Y., Su K. L. 1979. Metabolism of arachidonoyl phosphoglycerides in mouse brain subcellular fractions. J. Neurochem. 32: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 51.DeGeorge J. J., Noronha J. G., Bell J., Robinson P., Rapoport S. I. 1989. Intravenous injection of [1–14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J. Neurosci. Res. 24: 413–423. [DOI] [PubMed] [Google Scholar]
- 52.Choi Y. H., Fletcher P. J., Wong C. C., Anderson G. H. 1999. Measurement of blood-brain barrier permeability of rats with alpha-aminoisobutyric acid during microdialysis: possible application to behavioral studies. Physiol. Behav. 67: 587–598. [DOI] [PubMed] [Google Scholar]
- 53.Thakker D. R., Weatherspoon M. R., Harrison J., Keene T. E., Lane D. S., Kaemmerer W. F., Stewart G. R., Shafer L. L. 2009. Intracerebroventricular amyloid-beta antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice. Proc. Natl. Acad. Sci. USA. 106: 4501–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golovko M. Y., Murphy E. J. 2006. Uptake and metabolism of plasma-derived erucic acid by rat brain. J. Lipid Res. 47: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 55.Choi S. H., Langenbach R., Bosetti F. 2008. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 22: 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Igarashi M., Gao F., Kim H. W., Ma K., Bell J. M., Rapoport S. I. 2009. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim. Biophys. Acta. 1791: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cunnane S. C., Ryan M. A., Nadeau C. R., Bazinet R. P., Musa-Veloso K., McCloy U. 2003. Why is carbon from some polyunsaturates extensively recycled into lipid synthesis? Lipids. 38: 477–484. [DOI] [PubMed] [Google Scholar]
- 58.Green J. T., Orr S. K., Bazinet R. P. 2008. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. J. Lipid Res. 49: 939–944. [DOI] [PubMed] [Google Scholar]
- 59.Gavino G. R., Gavino V. C. 1991. Rat liver outer mitochondrial carnitine palmitoyltransferase activity towards long-chain polyunsaturated fatty acids and their CoA esters. Lipids. 26: 266–270. [DOI] [PubMed] [Google Scholar]
- 60.Bazinet R. P., Douglas H., Cunnane S. C. 2003. Whole-body utilization of n-3 PUFA in n-6 PUFA-deficient rats. Lipids. 38: 187–189. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton J. A., Brunaldi K. 2007. A model for fatty acid transport into the brain. J. Mol. Neurosci. 33: 12–17. [DOI] [PubMed] [Google Scholar]
- 62.Esposito G., Giovacchini G., Der M., Liow J. S., Bhattacharjee A. K., Ma K., Herscovitch P., Channing M., Eckelman W. C., Hallett M., et al. 2007. Imaging signal transduction via arachidonic acid in the human brain during visual stimulation, by means of positron emission tomography. Neuroimage. 34: 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]