Abstract
Scavenger receptor BI (SR-BI) is a selective uptake receptor for HDL cholesterol but is also involved in the catabolism of apolipoprotein (apo)B-containing lipoproteins. However, plasma levels of apoB-containing lipoproteins increase following hepatic SR-BI overexpression, suggesting that SR-BI not solely mediates their catabolism. We therefore tested the hypothesis that hepatic SR-BI impacts on VLDL production. On day 7 following adenovirus (Ad)-mediated overexpression of SR-BI, VLDL-triglyceride and VLDL-apoB production rates were significantly increased (P < 0.001), whereas VLDL production was significantly lower in SR-BI knockout mice compared with controls (P < 0.05). In mice injected with AdSR-BI, hepatic cholesterol content increased (P < 0.001), microsomal triglyceride transfer protein activity was higher (P < 0.01) and expression of sterol-regulatory element binding protein (SREBP)2 and its target genes was decreased (P < 0.01). Conversely, in SR-BI knockout mice, microsomal triglyceride transfer protein activity was lower and expression of SREBP2 target genes was increased (P < 0.01). Finally, we demonstrate in vitro in isolated primary hepatocytes as well as in vivo that cholesterol derived from HDL and taken up via SR-BI into the liver can be resecreted within VLDL. These data indicate that hepatic SR-BI expression is linked to VLDL production, and within liver, a metabolic shunt might exist that delivers HDL cholesterol, at least in part, to a pool from which cholesterol is mobilized for VLDL production. These results might have implications for HDL-based therapies against atherosclerotic cardiovascular disease, especially with SR-BI as target.
Keywords: triglycerides, cholesterol, liver, hepatocytes, labeling, high density lipoproteins, microsomal triglyceride transfer protein, apolipoprotein B
The scavenger receptor BI (SR-BI) is well characterized for its ability to mediate selective uptake of cholesteryl ester from HDL particles into cells (1, 2). Consistent with this role, SR-BI expression is highest in the liver and in steroidogenic tissues (1). Recently, SR-BI has also been implicated in the catabolism of apolipoprotein (apo)B-containing lipoproteins such as chylomicrons and β-VLDLs by hepatocytes (3–6). However, the first study demonstrating that adenovirus-mediated SR-BI overexpression increases the HDL catabolic rate indicated that plasma levels of apoB-containing lipoproteins actually increased significantly over time, whereas HDL levels remained low (7). Interestingly, hepatic LDL receptor (LDLR) expression was unchanged in this study during the course of the experiment (7). These data suggest, in our view, the possibility that SR-BI might be involved in hepatic cholesterol secretion.
Therefore, we hypothesized that SR-BI might facilitate hepatic VLDL production, providing a potential link between the HDL and the apoB-containing lipoprotein cholesterol pathways. Our data demonstrate that hepatic VLDL-triglyceride as well as VLDL-apoB production in vivo are decreased in SR-BI knockout mice and increased following SR-BI overexpression. The results of our study further indicate that HDL-derived cholesterol is to a certain extent resecreted by hepatocytes as a component of VLDL particles in a process dependent on SR-BI expression.
EXPERIMENTAL PROCEDURES
Animals
SR-BI knockout mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and backcrossed to the C57BL/6J genetic background for a total of eight generations. C57BL/6J control mice were purchased from Charles River (Sulzfeld, Germany). The animals were kept in animal rooms with alternating 12 h periods of light (from 7:00 AM to 7:00 PM) and dark (from 7:00 PM to 7:00 AM), with ad libitum access to water and mouse chow diet (Abdiets, Woerden, The Netherlands). Animal experiments were performed in accordance with national laws. All protocols were approved by the responsible ethics committees of the University of Groningen and the Landesamt für Gesundheit, Ernährung, und technische Sicherheit Berlin.
Generation of recombinant adenoviruses
Generation of the murine SR-BI expressing adenovirus AdSR-BI as well as of the empty control adenovirus AdNull has been described (7). Recombinant adenoviruses were amplified and purified as reported previously (8). Most in vivo experiments described were carried out using a dose of 1 × 10E11 particles of each of these adenoviruses per mouse. In additional experiments, we determined the response of hepatic VLDL production to increasing dosages of the AdSR-BI adenovirus (1 × 10E10, 3 × 10E10, 1 × 10E11), whereby the total dose of adenovirus that each mouse received was kept constant at 1 × 10E11 particles/mouse by the addition of the control adenovirus AdNull. Please note that the use of AdSR-BI did not result in an altered proinflammatory response by the liver as assessed by hepatic interleukin-1β, interleukin-6, and tumor necrosis factor-α mRNA expression (data not shown).
Plasma lipid and lipoprotein analysis
Mice were bled from the retroorbital plexus after a 4 h fast using heparinized capillary tubes. Aliquots of plasma were stored at −20°C until analysis. Plasma total cholesterol, triglycerides, and phospholipids were measured enzymatically using commercially available reagents (Wako Pure Chemical Industries, Neuss, Germany). Pooled plasma samples were subjected to fast protein liquid chromatography (FPLC) gel filtration using a superose 6 column (GE Healthcare, Uppsala, Sweden) as described (9). Individual fractions were assayed for cholesterol concentrations as indicated above.
Determination of hepatic triglyceride and apoB secretion
Fasted mice were allowed to eat fat-free food for 2 h and then injected intraperitoneally with Poloxamer 407 (P-407) (1,000 mg/kg body weight) as a 75 mg/ml solution in saline as previously described (10, 11). Blood samples were drawn into heparinized tubes at 0, 30, and 180 min after injection, plasma was separated and assayed for triglycerides as described above. Hepatic triglyceride production rates were calculated from the slope of the curve and expressed as mg/kg/h assuming the plasma volume to be 3.5% of the body weight. The measurement of hepatic VLDL apoB secretion was carried out by endogenous labeling using 35S-methionine (PerkinElmer, Waltham, MA), essentially as described (12) with the exception that 500 µCi of 35S-methionine were mixed with the P-407 solution and administered intraperitoneally as detailed above. Briefly, blood samples were drawn at 5 min and 180 min following tracer injection. VLDLs were recovered by ultracentrifugation (d < 1.006 g/ml) using a TLA120 rotor in a TLX ultracentrifuge (Beckman, Fullerton, CA) and VLDL proteins were separated by linear gradient SDS PAGE (3–20%). ApoB48 and apoB100 bands were identified by autoradiography, the respective bands were cut out of the gel, rehydrated, and following incubation with Solvable (Packard, Meriden, CT) for 3 h at 50°C, counts were determined by scintillation counting (Beckman LS6500). The apoB counts were corrected for the injected dose of tracer in each mouse assessed as the plasma counts at 5 min after tracer injection. Therefore, individual counts in apoB were divided by the ratio of the respective 5 min plasma count and the highest 5 min plasma count in the experiment.
HDL isolation and labeling
Human HDL isolated by sequential ultracentrifugation (density: 1.063 < d < 1.21) was prepared from pooled plasma of healthy donors and labeled with 3H-cholesteryl ester (PerkinElmer) essentially as described (13), and 1 million dpm of the 3H- cholesteryl ester-HDL were injected via the tail vein into mice that had been given P-407 i.p. 30 min before as detailed above. Blood was drawn 4 h later by heart puncture, VLDL was isolated from 200 µl of plasma as described above, and counts within VLDL were determined by scintillation counting (Beckman LS6500, Palo Alto, CA).
Analysis of nascent VLDL composition
VLDL (d < 1.006) was isolated from 200 µl of plasma from each mouse obtained 180 min after P-407 injection as described (14). Enzymatic methods were used to quantitate total cholesterol, free cholesterol, triglycerides, and phospholipids as detailed above. The bicinchoninic acid method (Pierce, Rockford, IL) was used to quantitate protein.
MTP activity assay
Assessment of microsomal triglyceride transfer protein (MTP) activity in mouse livers was performed using a fluorigenic assay essentially as described (15, 16). Briefly, to prepare microsomes, mouse liver pieces were washed with PBS, homogenized in 50 mM Tris-Cl, pH 7.4, 5 mM EDTA, 250 mM sucrose, and 0.02% sodium azide using a Polytron homogenizer, and centrifuged (10,900 rpm, 30 min, 4°C; Beckman microcentrifuge). Supernatants were adjusted to pH 5.1 with concentrated HCl, mixed in the cold for 30 min, and centrifuged (13,000 rpm, 30 min, 4°C; Beckman microcentrifuge). Pellets were resuspended in 1 mM Tris-Cl, pH 7.6, 1 mM EGTA, and 1 mM MgCl2, vortexed, incubated for 30 min at 4°C, and ultracentrifuged (50,000 rpm, 4°C, 1 h; SW55 Ti rotor). Supernatants were used for protein determination and MTP activity measurements in a reaction mixture (100 µl) containing 3 µl of donor vesicles [1.2 nmol of phosphatidylcholine (PC) and 100 pmol of fluorescent lipids], 3 µl of acceptor vesicles (7.2 nmol of PC) in 10 mM Tris, pH 7.4, 0.1% BSA, and 150 mM NaCl buffer. To determine the percentage of lipid transfer, fluorescence values obtained from control assays containing no MTP source (blanks) were subtracted from sample values and then divided by the total fluorescence present in the vesicles reduced by blanks.
Analysis of gene expression by real-time quantitative PCR
Total RNA from mouse livers was isolated using trizol (Invitrogen), and quantified with a NanoDrop ND-100 UV-Vis spectrophotometer. cDNA synthesis was performed from 1 μg of total RNA using reagents from Applied Biosystems (Darmstadt, Germany). Real-time quantitative PCR was carried out on an ABI-Prism 7700 (Applied Biosystems) sequence detector with the default settings. PCR primers and fluorogenic probes were designed with the Primer Express Software (Applied Biosystems) and synthesized by Eurogentec (Seraing, Belgium). mRNA expression levels presented were calculated relative to the average of the housekeeping gene cyclophilin and further normalized to the relative expression level of the respective controls (9).
Western blotting
Western blots for SR-BI were essentially performed as previously described using a commercially available goat anti-mouse SR-BI first antibody (Novus Biologicals, Littleton, CO) (17). Samples to detect nuclear Srebp2 were prepared as follows: livers were homogenized in homogenization buffer containing Complete (Roche, Basel, Switzerland) protease inhibitor cocktail, 10 mM EGTA, 10 mM EDTA, 0.25 M sucrose, 2 mM MgCl2, 20 mM Tris-HCl (pH 7.4) and centrifuged for 5 min at 2,500 g at 4°C. Supernatants were aspirated, pellets were dissolved in homogenization buffer followed by centrifugation for 5 min at 1,000 g at 4°C. The supernatant was aspirated and pellets were dissolved for 60 min at 4°C in buffer containing 1 mM EGTA, 1 mM EDTA, 1.5 mM MgCl2, 0.42 M NaCl, 2.5% v/v glycerol, 20 mM Hepes-KOH, pH 7.6. The nuclear suspension was then centrifuged for 30 min at 100,000 g at 4°C using an ultracentrifuge. The supernatant was used for Western blots. Equal amounts of protein were resolved by SDS-PAGE. Srebp2 was detected using a commercially available antibody from Abcam (Cambridge, UK).
Analysis of liver lipid composition
To determine contents of cholesterol, phospholipids, and triglycerides in the liver, liver tissue was homogenized as described (9), and triglycerides, free cholesterol, and total cholesterol were measured using commercial kits (Roche Diagnostics and Wako Chemicals, Neuss, Germany) after extracting lipids according to Bligh and Dyer and redissolving the lipids in water containing 2% Triton X-100. Phospholipid content of the liver was determined after lipid extraction essentially as described (9).
Isolation of primary mouse hepatocytes and pulse-chase experiments
C57BL/6 mice were injected with the control adenovirus AdNull or the SR-BI expressing adenovirus AdSR-BI. SR-BI knockout mice were administered AdNull. On day 3 following injection with the respective adenoviruses, mice were anesthetized by the ip injection of hypnorm (fentanyl/fluanisone, 1 ml/kg) and diazepam (10 mg/kg), livers were perfused, and hepatocytes isolated essentially as previously described (18). Viability of isolated hepatocytes was >85% as determined by the trypan blue exclusion method. Hepatocytes were resuspended in Williams E medium containing 10% FBS/1% antibiotics, plated in 12-well plates precoated with collagen, and allowed to attach for 4 h. Following a wash with PBS, new medium was added and the cells were kept overnight before the described experiments were carried out. HDL was isolated and labeled with 3H-cholesteryl ester as detailed above. Labeled HDL was added to the hepatocytes in Williams E medium without FBS containing either 0.25 mM BSA alone or 1 mM oleate complexed to 0.25 mM BSA, freshly prepared by sonication. Cells were pulsed for 1 h with labeled HDL then the medium was removed, cells were washed three times with PBS and followed by a 3 h chase in Williams E medium containing either 0.25 mM BSA alone or 1 mM oleate/0.25 mM BSA as indicated. At the end of this period, an aliquot of the medium was used to precipitate apoB-containing lipoproteins with 0.36% phosphotungstic acid (Sigma, St. Louis, MO) after adding unlabeled human LDL at a cholesterol concentration of 2 mg/ml as a carrier to improve precipitation efficacy. Cellular 3H-cholesterol content was determined by liquid scintillation counting following extraction of cellular lipids in isopropanol. Aliquots of medium as well as of the precipitates representing secreted apoB-containing lipoproteins were also subjected to liquid scintillation counting. Counts obtained from precipitates were expressed as a percentage of the total counts taken up by the cells during the 1 h pulse period, i.e., the sum of counts within the medium at the end of the chase and counts recovered from cells.
Statistical analysis
Statistical analyses were performed using the statistical package for social sciences (SPSS, SPSS Inc., Chicago, IL). Data are presented as means ± SEM. Statistical differences between two groups were assessed with either an independent samples or, where appropriate, a paired samples Student's t-test. To compare more than two groups, ANOVA followed by a Bonferroni post test was used. Statistical significance for all comparisons was assigned at P < 0.05.
RESULTS
SR-BI expression impacts on plasma lipid and lipoprotein levels
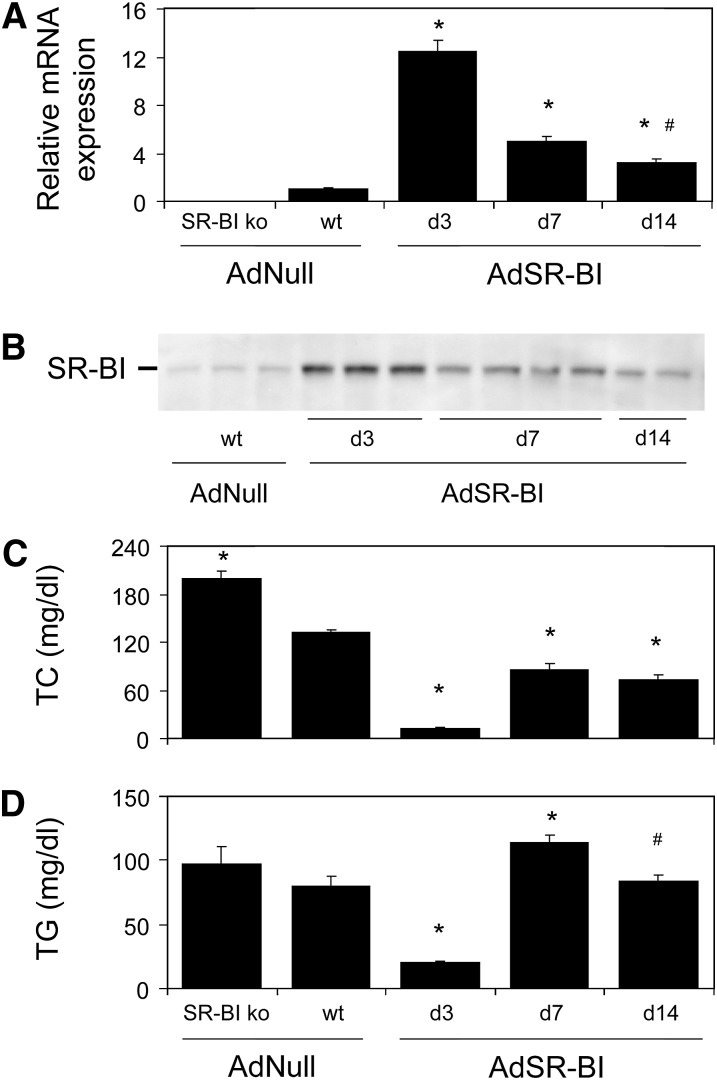
To assess the effect of SR-BI on plasma lipid levels, wild-type mice injected with either an empty control adenovirus (AdNull) or a SR-BI-expressing adenovirus (AdSR-BI) as well as SR-BI knockout mice were investigated. To allow better comparability between the groups, the SR-BI knockout mice were also injected with the control virus AdNull. As summarized in Fig. 1A, injection with AdSR-BI resulted in a more than 12-fold increase in hepatic SR-BI mRNA expression on day 3 compared with AdNull injected controls (12.43 ± 0.95 vs. 1.00 ± 0.11, P < 0.001) that subsequently decreased on day 7 (4.99 ± 0.38, P < 0.001), but was still significantly elevated on day 14 (3.17 ± 0.41, P < 0.001). As shown in Fig. 1B, the time course of hepatic SR-BI protein expression in response to adenovirus-mediated overexpression largely followed the mRNA expression pattern.
Fig. 1.
Plasma lipid levels in SR-BI knockout mice, wild-type controls and on different days after adenovirus-mediated SR-BI overexpression. A: Hepatic SR-BI mRNA expression levels determined by real-time quantitative PCR as detailed in Experimental Procedures. B: Hepatic SR-BI protein expression assessed by Western blot as described in Experimental Procedures. C: Plasma total cholesterol (TC) levels, (D) plasma triglyceride (TG) levels. n = 6–8, except for plasma TG levels (n = 12), data are presented as means ± SEM. * indicates statistically significant differences compared with wild-type controls (at least P < 0.05) as assessed by ANOVA followed by posttest analysis and # indicates statistically significant differences between day 7 and day 14 values.
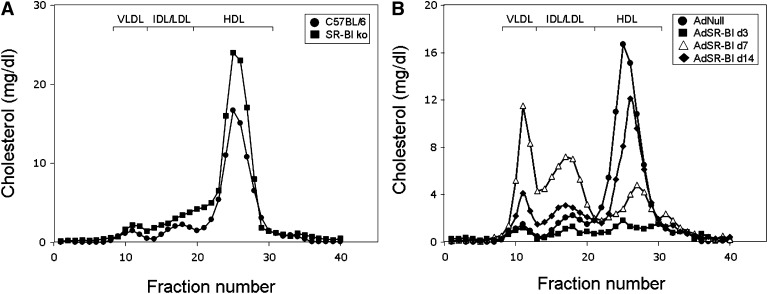
In SR-BI knockout mice, plasma total cholesterol (199 ± 10 vs. 133 ± 3 mg/dl, Fig. 1C, P < 0.01) was significantly elevated compared with wild-type mice whereas triglyceride levels were not different (96 ± 10 vs. 82 ± 5 mg/dl, Fig. 1D, n.s., not significant). FPLC analysis revealed an increase in HDL cholesterol as well as a slight increase in VLDL cholesterol levels in SR-BI knockout mice (Fig. 2A) consistent with previously published data (5, 6). On day 3 after AdSR-BI injection, plasma total cholesterol (12 ± 2 mg/dl, Fig. 1C) as well as triglyceride levels (20 ± 1 mg/dl, Fig. 1D) were significantly decreased (each P < 0.001). Although hepatic SR-BI expression showed a constant decline from day 3 to day 14, plasma total cholesterol and triglyceride levels did not follow this trend. On day 7, plasma total cholesterol (86 ± 8 mg/dl, Fig. 1C) and triglycerides (114 ± 6 mg/dl, Fig. 1D) increased significantly compared with day 3 values (P < 0.01). However, this was followed by a decrease on day 14 [cholesterol: 73 ± 6 mg/dl, triglycerides: 84 ± 5 mg/dl (P < 0.05 compared with day 7), Fig. 1C, D]. FPLC analysis revealed a striking increase in levels of apoB-containing lipoproteins on day 7 compared with day 3 and day 14 (Fig. 2B). These results led us to hypothesize that SR-BI might be involved in VLDL production in addition to functioning as an uptake receptor for cholesterol (3–6). Further experiments therefore focused on exploring the metabolic effects of SR-BI using adenovirus-mediated overexpression on day 7 after AdSR-BI injection as well as SR-BI knockout mice.
Fig. 2.
Cholesterol distribution over the different subfractions determined by FPLC analysis either in (A) SR-BI knockout mice and wild-type controls administered the empty control adenovirus AdNull, or (B) wild-type mice on different days after injection with AdSR-BI compared with AdNull injected wild-type controls. FPLC was performed on pooled plasma samples (n = 6 mice each) from the respective groups of mice and cholesterol content within each fraction was measured as detailed in Experimental Procedures.
Hepatic SR-BI expression promotes VLDL triglyceride and apoB production
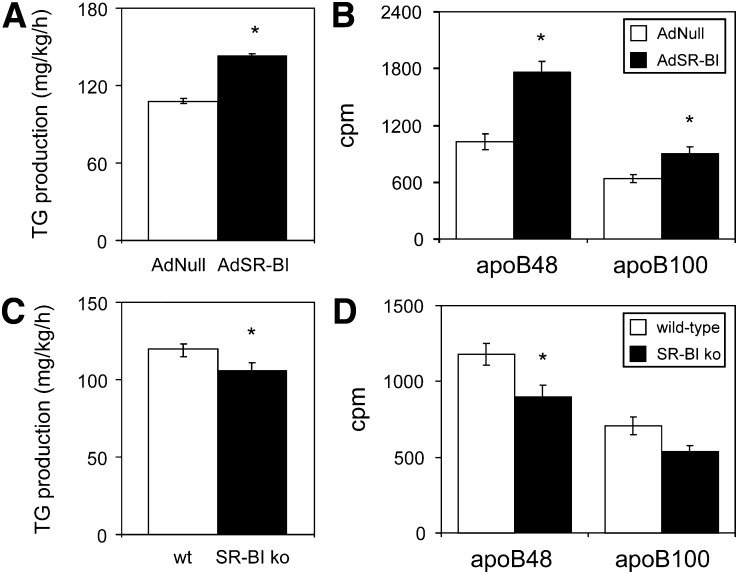
Because the finding of increased levels of apoB-containing lipoproteins on day 7 after AdSR-BI administration in the presence of high levels of hepatic SR-BI expression cannot be explained by SR-BI increasing the catabolic rate of VLDL, we first determined hepatic VLDL production rates. In mice overexpressing SR-BI, hepatic VLDL triglyceride production rates were significantly increased compared with controls (143 ± 2 vs. 108 ± 2 mg/kg/h, P < 0.001, Fig. 3A). In addition, the production of VLDL apoB48 (1760 ± 121 vs. 1028 ± 81 cpm, P < 0.001, Fig. 3B) as well as apoB100 (905 ± 75 vs. 642 ± 44 cpm, P < 0.05, Fig. 3B) were significantly higher in AdSR-BI injected mice. Complementing these results in SR-BI overexpressing mice, VLDL triglyceride production rates in SR-BI knockouts were significantly lower than in wild-type controls (106 ± 6 vs. 120 ± 3 mg/kg/h, P < 0.05, Fig. 3C). Also, production of VLDL apoB48 (1178 ± 70 vs. 897 ± 78 cpm, P < 0.05, Fig. 3D) was significantly lower in SR-BI knockouts whereas apoB100 showed a trend toward a decrease (707 ± 57 vs. 535 ± 44 cpm, P = 0.052, Fig. 3D). These data indicate that SR-BI expression is positively related to hepatic VLDL-triglyceride as well as VLDL apoB production.
Fig. 3.
SR-BI expression is associated with altered hepatic VLDL production. A: Hepatic VLDL-TG production rates and (B) hepatic VLDL-apoB production rates in wild-type mice on day 7 following injection with either AdSR-BI or the control adenovirus AdNull. C: Hepatic VLDL-TG production rates and (D) hepatic VLDL-apoB production rates in SR-BI knockout mice and wild-type controls. VLDL production was measured using P-407 to block VLDL catabolism as described in methods; n = 5–6, data are presented as means ± SEM. * indicates statistically significant differences compared with the respective controls (at least P < 0.05) as assessed by Student's t-test.
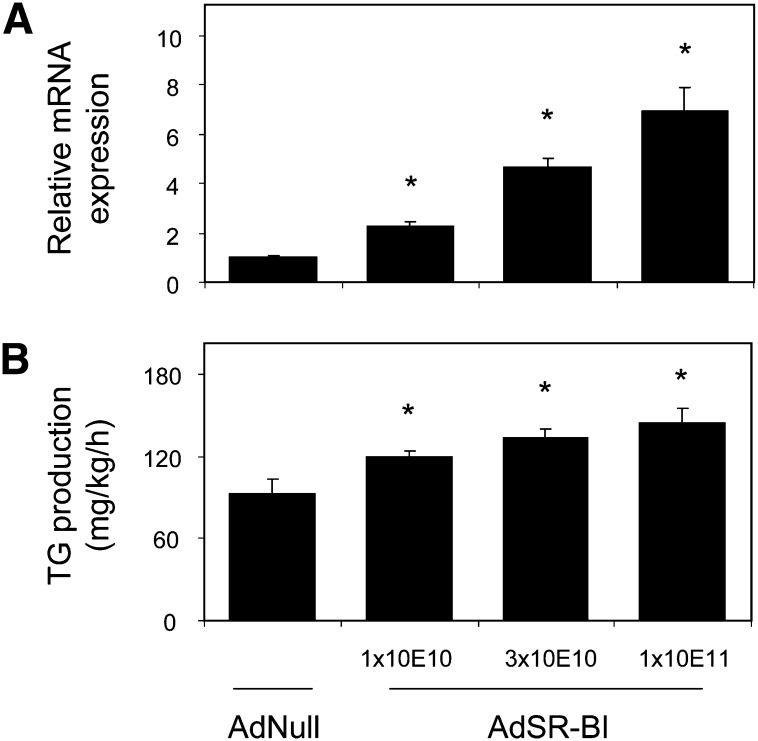
In addition, we performed a dose-response experiment to investigate the metabolic effects of increasing dosages of AdSR-BI. Compared with control adenovirus injected mice, administration of AdSR-BI increased hepatic SR-BI mRNA levels on day 7 after injection significantly in all three groups, namely, mice given 1 × 10E10 (1.00 ± 0.06 vs. 2.27 ± 0.21, P < 0.01, Fig. 4A), 3 × 10E10 (4.68 ± 0.36, P < 0.001, Fig. 4A) and 1 × 10E11 (6.95 ± 0.94, P < 0.001, Fig. 4A) particles/mouse of the adenovirus. On day 3 and day 7 after adenovirus administration, plasma total (day 3: −4%, day 7: +5%) and HDL cholesterol levels (day 3: −3%, day 7: +2%) were unaffected in mice given 1 × 10E10 particles AdSR-BI; however, hepatic VLDL-triglyceride (TG) production rates were already significantly increased compared with AdNull-injected controls (120 ± 4 vs. 93 ± 11 mg/kg/h, P < 0.05, Fig. 4B). In mice receiving 3 × 10E10 particles of AdSR-BI, on day 3, plasma levels of total cholesterol (−31%) and HDL cholesterol (−28%) were decreased, whereas on day 7, total cholesterol was not different from controls and HDL cholesterol was still lower (−11%). In this group, hepatic VLDL-TG production was even higher (134 ± 6 mg/kg/h, P < 0.01 compared with AdNull, Fig. 4B). Mice administered 1 × 10E11 particles of AdSR-BI confirmed our previous findings with decreased plasma total (day 3: −90%, day 7: −31%) as well as HDL cholesterol levels (day 3: −82%, day 7: −68%) on day 3 and day 7. In these mice, hepatic VLDL-TG production rates were highest (144 ± 11 mg/kg/h, P < 0.01 compared with AdNull, Fig. 4B).
Fig. 4.
Increasing hepatic SR-BI expression promotes hepatic VLDL-TG production in a dose-dependent fashion. Mice were investigated on day 7 after administration of either AdNull (1 × 10E11 particles/mouse) or increasing dosages of AdSR-BI as indicated. Please note that in the case of AdSR-BI injected mice, the total dose of virus given to each mouse was kept constant at 1 × 10E11 particles/mouse by the addition of control adenovirus AdNull. A: Hepatic SR-BI mRNA expression levels determined by quantitative real-time PCR. B: Hepatic VLDL-TG production rates assessed with P-407 to block VLDL catabolism as detailed in Experimental Procedures; n = 5–6 mice/group, data are presented as means ± SEM. * indicates statistically significant differences compared with AdNull injected controls (at least P < 0.05) as assessed by Student's t-test.
Next, we determined the composition of nascent VLDL particles secreted by the liver within the different experimental groups. As summarized in Table 1, VLDL composition did not change in response to SR-BI overexpression (1 × 10E11 particles/mouse) indicating that under these conditions more VLDL particles of the same size are secreted by the liver. In VLDL from SR-BI knockout mice, the relative contents of free cholesterol (P < 0.05, Table 1) as well as cholesterol ester (P < 0.05, Table 1) were significantly increased compared with wild-type controls. However, the contents of phospholipids and triglycerides were not significantly different pointing toward unchanged size of VLDL secreted by the liver also in SR-BI knockout animals.
TABLE 1.
Composition of nascent VLDL particles secreted by the liver in mice on the C57BL/6 genetic background with different hepatic SR-BI expression
| Increased Expression |
Decreased Expression |
|||
|---|---|---|---|---|
| AdNull | AdSR-BI | C57BL/6 | SR-BI ko | |
| Triglycerides (%) | 62.4 ± 2.4 | 62.7 ± 1.4 | 63.7 ± 2.1 | 61.4 ± 1.9 |
| Free cholesterol (%) | 3.6 ± 0.7 | 2.9 ± 0.8 | 3.5 ± 0.4 | 6.3 ± 0.9§ |
| Cholesteryl ester (%) | 4.9 ± 0.8 | 5.1 ± 1.0 | 4.5 ± 0.6 | 7.9 ± 1.0§ |
| Phospholipids (%) | 18.5 ± 1.3 | 19.8 ± 1.9 | 18.8 ± 1.7 | 15.7 ± 0.9 |
| Protein (%) | 10.6 ± 0.9 | 9.6 ± 0.6 | 9.6 ± 0.5 | 8.8 ± 0.5 |
Values are means ± SEM.
Significantly different from the respective control group: § at least P < 0.05.
Hepatic SR-BI expression influences liver cholesterol content
Relative liver weight increased significantly in response to hepatic SR-BI overexpression (P < 0.001, Table 2), whereas SR-BI knockout mice exhibited a tendency toward lower liver weight. SR-BI overexpression resulted in a significant increase in hepatic total as well as free cholesterol content (each P < 0.001). Phospholipids remained unchanged, but hepatic triglyceride content was higher in AdSR-BI injected mice (P < 0.05). However, in SR-BI knockout mice hepatic total and free cholesterol contents were also significantly increased (P < 0.05), in agreement with previously published data (19). In the SR-BI knockout model, liver phospholipids and triglycerides did not change compared with wild-type controls.
TABLE 2.
Liver weight and liver lipid composition in mice on the C57BL/6 genetic background with different hepatic SR-BI expression
| Increased Expression |
Decreased Expression |
|||
|---|---|---|---|---|
| AdNull | AdSR-BI | C57BL/6 | SR-BI ko | |
| Liver weight (% of bw) | 4.48 ± 0.21 | 6.27 ± 0.13§ | 4.42 ± 0.19 | 4.15 ± 0.38 |
| Total cholesterol (µmol/g) | 6.26 ± 0.18 | 8.07 ± 0.61§ | 5.90 ± 0.18 | 6.49 ± 0.19§ |
| Free cholesterol (µmol/g) | 4.48 ± 0.33 | 6.02 ± 0.56§ | 4.26 ± 0.10 | 4.65 ± 0.14§ |
| Phospholipids (µmol/g) | 34.2 ± 2.4 | 35.4 ± 5.3 | 32.2 ± 1.2 | 33.7 ± 0.8 |
| Triglycerides (µmol/g) | 12.0 ± 8.0 | 27.2 ± 3.8§ | 8.3 ± 1.5 | 9.2 ± 2.1 |
Values are means ± SEM.
In the experiments involving the use of recombinant adenovirus livers were analyzed on day 7 following virus administration. Significantly different from the respective control group: § at least P < 0.05.
SR-BI expression alters hepatic gene expression and MTP activity
Hepatic gene expression levels in the different models investigated are given in Table 3. The expression of MTP and apoE did not change in response to altered SR-BI expression. Hepatic expression of SREBP1c as well as FAS was unchanged in mice injected with AdSR-BI; however, both genes were significantly increased in SR-BI knockout mice compared with controls (P < 0.01, Table 3). Interestingly, there was a consistent change in the expression of SREBP2 and its target genes HMG-CoA reductase and LDLR. The mRNA expression of all three genes was decreased in response to SR-BI overexpression (P < 0.01, Table 3), as was the level of nuclear SREBP2 (Fig. 5), indicating alterations in endoplasmic reticulum (ER) cholesterol content dependent on SR-BI expression. On the other hand, expression of SREBP2, HMG-CoA reductase, and LDLR was significantly increased in SR-BI knockout mice (P < 0.01, Table 3).
TABLE 3.
Hepatic gene expression levels in mice on the C57BL/6 genetic background with different hepatic SR-BI expression
| Increased Expression |
Decreased Expression |
|||
|---|---|---|---|---|
| AdNull | AdSR-BI | C57BL/6 | SR-BI ko | |
| MTP | 1.00 ± 0.13 | 0.97 ± 0.08 | 1.00 ± 0.09 | 1.09 ± 0.10 |
| apoE | 1.00 ± 0.12 | 0.73 ± 0.05 | 1.00 ± 0.02 | 0.92 ± 0.03 |
| FAS | 1.00 ± 0.22 | 0.75 ± 0.08 | 1.00 ± 0.24 | 1.64 ± 0.22§ |
| SREBP2 | 1.00 ± 0.12 | 0.62 ± 0.10§ | 1.00 ± 0.08 | 1.39 ± 0.09§ |
| LDLR | 1.00 ± 0.07 | 0.59 ± 0.05§ | 1.00 ± 0.06 | 1.59 ± 0.16§ |
| HMG-CoAR | 1.00 ± 0.16 | 0.51 ± 0.09§ | 1.00 ± 0.14 | 1.83 ± 0.08§ |
| SREBP1c | 1.00 ± 0.18 | 1.08 ± 0.12 | 1.00 ± 0.21 | 2.54 ± 0.32§ |
Values are means ± SEM.
Significantly different from the respective control group: § at least P < 0.05.
Fig. 5.
Nuclear levels of SREBP2 protein are decreased in response to SR-BI overexpression. Western blots for nuclear SREBP2 were performed as detailed in Experimental Procedures using livers from mice injected with either AdNull or AdSR-BI as indicated (day 7 after adenovirus administration).
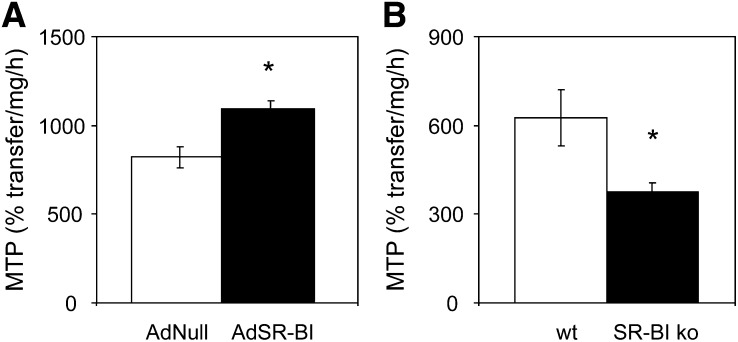
Because MTP plays a central role in VLDL production, we also assessed hepatic MTP activity in the different groups of mice in addition to measuring mRNA expression. SR-BI overexpression resulted in increased MTP activity (1094 ± 45 vs. 821 ± 59%transfer/mg/h, P < 0.01, Fig. 6A), whereas MTP activity was decreased in the SR-BI knockout mice (376 ± 30 vs. 626 ± 94%transfer/mg/h, P < 0.05, Fig. 6B) each compared with the respective control groups.
Fig. 6.
Hepatic MTP activity depending on the hepatic SR-BI expression level. A: Livers from wild-type mice analyzed on day 7 following injection with either AdSR-BI or the control adenovirus AdNull. B: Livers from SR-BI knockout mice and wild-type controls; n = 6–7, data are presented as means ± SEM. * indicates statistically significant differences compared with the respective controls (at least P < 0.05) as assessed by Student's t-test.
SR-BI facilitates the hepatic resecretion of HDL-derived cholesterol within VLDL
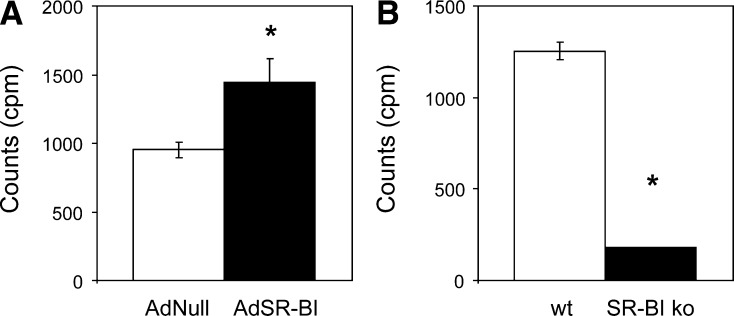
Because SR-BI mediates the selective uptake of HDL cholesteryl ester, and liver cholesterol content as well as hepatic VLDL production were increased in response to SR-BI overexpression, we hypothesized that under these circumstances HDL-derived cholesterol might be resecreted within VLDL. To test this hypothesis, HDL labeled with 3H-cholesteryl ester was injected into mice in which VLDL catabolism was blocked with P-407. P-407 has been previously demonstrated to not affect the integrity of the HDL particle in contrast to tyloxapol (10). Using this approach, we were able to recover HDL-derived cholesteryl ester within VLDL of wild-type mice, and hepatic overexpression of SR-BI resulted in significantly increased recovery (954 ± 67 vs. 1441 ± 174 cpm/200 µl, P < 0.05, Fig. 7A). In contrast, HDL-derived cholesteryl ester was almost absent within VLDL of SR-BI knockout mice compared with controls (1253 ± 49 vs. 148 ± 13 cpm/200 µl, P < 0.001, Fig. 7B).
Fig. 7.
HDL-derived cholesterol is resecreted by the liver within VLDL particles. A: Wild-type mice investigated on day 7 following injection with either AdSR-BI or the control adenovirus AdNull. B: SR-BI knockout mice and wild-type controls. Mice were injected with 3 H-cholesteryl ester labeled HDL under conditions of blocking VLDL catabolism with P-407 as outlined in Experimental Procedures. Recovered counts (cpm) 4 h after injection with labeled HDL are given; n = 5–6. Data are presented as means ± SEM. * indicates statistically significant differences compared with the respective controls (at least P < 0.05) as assessed by Student's t -test.
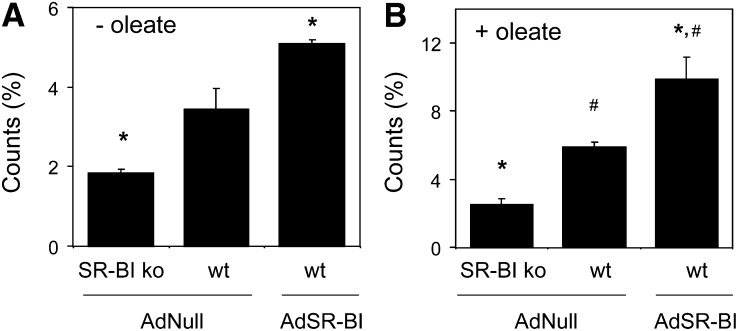
To further substantiate these observations, pulse-chase experiments were carried out in primary hepatocytes isolated from wild-type and SR-BI knockout mice each injected with the control adenovirus AdNull as well as from wild-type mice following injection with AdSR-BI. Because the SR-BI expression status impacts on the cellular uptake of HDL cholesteryl ester, counts within VLDL were normalized for the total counts recovered from the respective well. Under basal conditions without oleate added, hepatocytes from SR-BI knockout mice secreted significantly less labeled cholesterol within VLDL compared with wild-type control hepatocytes (1.8 ± 0.1 vs. 3.4 ± 0.5%, P < 0.05, Fig. 8A). On the other hand, significantly higher counts were recovered within VLDL from hepatocytes isolated from wild-type mice injected with AdSR-BI compared with wild-type control cells (5.1 ± 0.1%, P < 0.05, Fig. 8A). When the experiment was performed with oleate present, HDL-derived labeled cholesterol within VLDL did not increase significantly in SR-BI knockout hepatocytes (2.5 ± 0.4%, Fig. 8B), whereas recovered counts within VLDL were increased by 72% in wild-type hepatocytes (5.9 ± 0.3%, P < 0.05 compared with SR-BI knockout hepatocytes as well as to conditions without oleate, Fig. 8B). SR-BI overexpression resulted, under these conditions, in an even further increase in HDL-derived counts within VLDL by 94% (9.9 ± 1.3%, P < 0.05 compared with wild-type hepatocytes as well as to conditions without oleate, Fig. 8B). Taken together, these data indicate that in liver, a metabolic shunt might exist between the HDL catabolic and the VLDL secretion pathways and suggest that SR-BI might represent a central link within this process.
Fig. 8.
HDL-derived cholesterol is resecreted within VLDL particles by isolated primary hepatocytes. Isolated primary hepatocytes from SR-BI knockout and wild-type mice receiving the respective adenoviruses as indicated were incubated for 1 h with 3H-cholesteryl ester labeled HDL followed by a chase for 3 h either in the presence of (A) 0.25 mM albumin or (B) 1 mM oleate complexed to 0.25 mM albumin. Counts recovered within secreted apoB-containing lipoproteins were determined following precipitation as detailed in Experimental Procedures. Recovered counts from the precipitate are expressed as percentage of total counts present in each well at the end of the experiment. n = 4, data are presented as means ± SEM. * indicates statistically significant differences compared with AdNull infected wild-type hepatocytes (P < 0.05) and # indicates statistically significant differences compared with the respective groups from experiments performed without the addition of oleate.
DISCUSSION
The results of this study demonstrate that SR-BI expression affects hepatic VLDL production with decreased VLDL-TG as well as VLDL-apoB production in SR-BI knockout mice and increased VLDL production in response to adenoviral SR-BI overexpression. Our data further indicate that HDL-derived cholesterol taken up via SR-BI can be packaged into VLDL particles and secreted by the liver, thereby pointing toward a potential intrahepatic metabolic shunt between HDL and apoB-containing lipoproteins.
SR-BI is well characterized as a receptor mediating the selective uptake of cholesteryl ester from HDL into cells (1, 2). It has also been established by in vitro as well as in vivo studies that SR-BI and its human ortholog Cla-1 can bind apoB-containing lipoproteins and mediate their internalization (3–6, 20). This property of SR-BI might depend on the physiological context as hepatic overexpression of SR-BI in hapoB transgenic mice (21) or attenuated expression in LDLR knockout (22) mice did not affect the LDL catabolic rate. As suggested by one study using apoE-deficient mice, apoE is required for the cellular uptake of apoB-containing lipoproteins by SR-BI (23). In contrast, hepatic SR-BI overexpression in fibrate-treated apoE knockout mice decreased plasma apoB levels significantly by more than 40% and reversed fibrate-induced hypercholesterolemia (24).
To our knowledge, this is the first report implicating SR-BI in hepatic VLDL production. However, circumstantial evidence has been provided by previous studies. In the important first demonstration that SR-BI increases the catabolic rate of HDL and enhances cholesterol uptake in vivo in mice, FPLC profiles indicated that, after a lag phase, on days 7, 10, and 14 following injection of AdSR-BI, levels of apoB-containing lipoproteins increased substantially (7). This observation would argue against a sole effect of SR-BI on the catabolism of apoB-containing lipoproteins. Therefore, we directly measured hepatic VLDL production rates in SR-BI knockout as well as SR-BI overexpressing mice and found these to be positively related to the SR-BI expression level. In the previous study, hepatic LDLR mRNA expression was reported to be unchanged in response to SR-BI overexpression (7). This is in contrast to our results that SREBP2 as well as its target gene LDLR are significantly decreased following injection of AdSR-BI, in line with increased hepatic cholesterol content. Interestingly, hepatic VLDL production rates in the context of altered hepatic SR-BI expression were measured in only one other earlier study using SR-BI knockout mice (6). This report revealed a clear trend toward a 30% decrease in VLDL-TG production in SR-BI knockout mice (6) that, however, did not reach statistical significance. These studies used tyloxapol as a means of blocking endogenous VLDL catabolism, whereas we employed P-407 in our present study. In our hands, the experimental variability is considerably lower with the use of P-407 than with tyloxapol, which might provide an explanation for the previously reported findings not turning out significant.
In general, the effect of SR-BI on VLDL production should not be regarded as an exclusive denominator of steady-state plasma VLDL cholesterol levels as these depend on factors influencing the production rate as well as the catabolic rate. However, as we observed significant and congruent results on SR-BI facilitating hepatic VLDL production, both in the knockout mouse and in a dose-dependent fashion under conditions of hepatic SR-BI overexpression, we believe that this property of SR-BI has physiological relevance. Although in chow-fed SR-BI transgenic mice with life-long metabolic adaptations, plasma levels of cholesterol within apoB-containing lipoproteins are lower, indicating the catabolism effect of SR-BI overweighing the effect on production rate (25–27), altering the metabolic context in SR-BI transgenic models reveals effects of SR-BI overexpression that are consistent with increased production of apoB-containing lipoproteins. Feeding a Western-type diet to SR-BI transgenic mice was associated with increased plasma levels of apoB-containing lipoproteins (25, 27). In addition, SR-BI transgenic mice on the human apoB-transgenic background fed an atherogenic diet also displayed increased levels of apoB-containing lipoproteins, which, interestingly, translated in this model into increased atherogenesis (28). Other available studies in the literature that achieved SR-BI overexpression using a recombinant adenovirus all investigated earlier time points, namely day 3 or day 5 after adenovirus injection, and observed on these days very similar steady-state plasma lipoprotein effects as seen in our present report (4, 21, 29, 30), mostly no or minor effects on VLDL cholesterol. The only other study looking at time points later than day 5, however, revealed plasma lipid and lipoprotein data similar to our study, namely increased apoB-containing lipoproteins on day 7 and later (7). Of note, however, none of the previous studies discussed in this paragraph measured hepatic VLDL production rates.
Gene expression analysis in SR-BI knockout as well as in SR-BI overexpressing mice demonstrated that the SR-BI expression level is inversely related to the expression of SREBP2 and its target genes LDLR and HMG-CoA reductase. These results are in agreement with previous in vitro findings that SR-BI delivers HDL cholesterol to an intracellular regulatory pool (31). Because SREBP2 has been established as a sensitive sensor for the ER cholesterol content (32), these results indicate that SR-BI-mediated cholesterol uptake results in increased ER cholesterol content, whereas the absence of SR-BI leads to a depletion in ER cholesterol. Because this relative depletion in the SR-BI knockout mice results in increased HMG-CoA reductase-driven hepatic cholesterol synthesis, such an adaptive metabolic response would also explain the slight but significant increase in hepatic cholesterol content that we and others previously (19) observed. Cholesterol used for VLDL secretion is also thought to be derived from the ER (33), which then conceivably might represent the point where the two pathways, HDL-derived cholesterol taken up and cholesterol secreted within VLDL, merge. Therefore, in our view, increased VLDL production in response to SR-BI overexpression might not be a direct intrinsic effect of SR-BI but might rather represent a cholesterol-driven process.
HDL-based therapies for atherosclerotic cardiovascular disease are emerging and SR-BI is among the prime candidates as a therapeutic target (34–37). Studies have suggested that especially hepatic SR-BI expression is anti-atherogenic, increases reverse cholesterol transport (38), and results in reduced atherosclerotic lesion formation in most models (25, 28, 39, 40). Hepatic SR-BI expression is also associated with increased biliary cholesterol content (7) as well as secretion rates (17). However, the results of our present study add a cautionary note suggesting that there might be limits to metabolic pathways and also that a beneficial player might exert potential negative effects such as increased hepatic VLDL production under certain metabolic conditions.
In summary, our results demonstrate that in addition to its established role in increasing the catabolism of apoB-containing lipoproteins, hepatic SR-BI expression is also linked to increased VLDL production. Our data further indicate that within the liver, a metabolic shunt might exist with HDL cholesterol entering, at least in part, the same pool from which cholesterol is recruited for VLDL production. However, the relative balance between catabolism and production determines the net effect of SR-BI on plasma levels of triglycerides and cholesterol within apoB-containing lipoproteins. These results might have implications when considering HDL-based therapies against atherosclerotic cardiovascular disease, especially with SR-BI as target.
Acknowledgments
We are grateful to Dr. Karen Kozarsky (GlaxoSmithKline, King of Prussia, PA) for providing the SR-BI expressing adenovirus used in this study.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- ER
- endoplasmic reticulum
- FPLC
- fast protein liquid chromatography
- LDLR
- LDL receptor
- MTP
- microsomal triglyceride transfer protein
- PC
- phosphatidylcholine
- SR-BI
- scavenger receptor BI
- SREBP
- sterol-regulatory element binding protein
- TG
- triglyceride
This work was supported by the Netherlands Organization for Scientific Research (VIDI Grant 917-56-358 to U.J.F.T.) and DK46900 (to M.M.H.).
REFERENCES
- 1.Trigatti B. L., Krieger M., Rigotti A. 2003. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23: 1732–1738. [DOI] [PubMed] [Google Scholar]
- 2.Van Eck M., Pennings M., Hoekstra M., Out R., Van Berkel T. J. 2005. Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr. Opin. Lipidol. 16: 307–315. [DOI] [PubMed] [Google Scholar]
- 3.Calvo D., Gomez-Coronado D., Lasuncion M. A., Vega M. A. 1997. CLA-1 is an 85-kD plasma membrane glycoprotein that acts as a high-affinity receptor for both native (HDL, LDL, and VLDL) and modified (OxLDL and AcLDL) lipoproteins. Arterioscler. Thromb. Vasc. Biol. 17: 2341–2349. [DOI] [PubMed] [Google Scholar]
- 4.Out R., Hoekstra M., de Jager S. C., de Vos P., van der Westhuyzen D. R., Webb N. R., Van Eck M., Biessen E. A., Van Berkel T. J. 2005. Adenovirus-mediated hepatic overexpression of scavenger receptor class B type I accelerates chylomicron metabolism in C57BL/6J mice. J. Lipid Res. 46: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 5.Out R., Kruijt J. K., Rensen P. C., Hildebrand R. B., de Vos P., Van Eck M., Van Berkel T. J. 2004. Scavenger receptor BI plays a role in facilitating chylomicron metabolism. J. Biol. Chem. 279: 18401–18406. [DOI] [PubMed] [Google Scholar]
- 6.Van Eck M., Hoekstra M., Out R., Bos I. S., Kruijt J. K., Hildebrand R. B., Van Berkel T. J. 2008. Scavenger receptor BI facilitates the metabolism of VLDL lipoproteins in vivo. J. Lipid Res. 49: 136–146. [DOI] [PubMed] [Google Scholar]
- 7.Kozarsky K. F., Donahee M. H., Rigotti A., Iqbal S. N., Edelman E. R., Krieger M. 1997. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 387: 414–417. [DOI] [PubMed] [Google Scholar]
- 8.Tietge U. J. F., Maugeais C., Lund-Katz S., Grass D., deBeer F. C., Rader D. J. 2002. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and apoA-I in response to inflammation in human apoA-I transgenic mice. Arterioscler. Thromb. Vasc. Biol. 22: 1213–1218. [DOI] [PubMed] [Google Scholar]
- 9.Tietge U. J. F., Nijstad N., Havinga R., Baller J. F., van der Sluijs F. H., Bloks V. W., Gautier T., Kuipers F. 2008. Secretory phospholipase A2 increases SR-BI-mediated selective uptake from HDL but not biliary cholesterol secretion. J. Lipid Res. 49: 563–571. [DOI] [PubMed] [Google Scholar]
- 10.Millar J. S., Cromley D. A., McCoy M. G., Rader D. J., Billheimer J. T. 2005. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 46: 2023–2028. [DOI] [PubMed] [Google Scholar]
- 11.Gautier T., Tietge U. J. F., Boverhof R., Perton F. G., Le Guern N., Masson D., Rensen P. C., Havekes L. M., Lagrost L., Kuipers F. 2007. Hepatic lipid accumulation in apolipoprotein C–I-deficient mice is potentiated by cholesteryl ester transfer protein. J. Lipid Res. 48: 30–40. [DOI] [PubMed] [Google Scholar]
- 12.Tietge U. J. F., Bakillah A., Maugeais C., Tsukamoto K., Hussain M., Rader D. J. 1999. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res. 40: 2134–2139. [PubMed] [Google Scholar]
- 13.Tietge U. J. F., Maugeais C., Cain W., Rader D. J. 2003. Acute inflammation increases selective uptake of HDL cholesteryl esters into adrenals of mice overexpressing human sPLA2. Am. J. Physiol. Endocrinol. Metab. 285: E403–E411. [DOI] [PubMed] [Google Scholar]
- 14.Maugeais C., Tietge U. J. F., Tsukamoto K., Glick J. M., Rader D. J. 2000. Hepatic apolipoprotein E expression promotes very low density lipoprotein-apolipoprotein B production in vivo in mice. J. Lipid Res. 41: 1673–1679. [PubMed] [Google Scholar]
- 15.Athar H., Iqbal J., Jiang X. C., Hussain M. M. 2004. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45: 764–772. [DOI] [PubMed] [Google Scholar]
- 16.Rava P., Athar H., Johnson C., Hussain M. M. 2005. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46: 1779–1785. [DOI] [PubMed] [Google Scholar]
- 17.Wiersma H., Gatti A., Nijstad N., Oude Elferink R. P., Kuipers F., Tietge U. J. F. 2009. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology. 50: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 18.Wiersma H., Nijstad N., de Boer J. F., Out R., Hogewerf W., Van Berkel T. J., Kuipers F., Tietge U. J. F. 2009. Lack of Abcg1 results in decreased plasma HDL cholesterol levels and increased biliary cholesterol secretion in mice fed a high cholesterol diet. Atherosclerosis. 206: 141–147. [DOI] [PubMed] [Google Scholar]
- 19.Mardones P., Quinones V., Amigo L., Moreno M., Miquel J. F., Schwarz M., Miettinen H. E., Trigatti B., Krieger M., VanPatten S., et al. 2001. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J. Lipid Res. 42: 170–180. [PubMed] [Google Scholar]
- 20.Hu L., van der Hoogt C. C., Espirito Santo S. M., Out R., Kypreos K. E., van Vlijmen B. J., Van Berkel T. J., Romijn J. A., Havekes L. M., van Dijk K. W., et al. 2008. The hepatic uptake of VLDL in lrp-ldlr−/−vldlr−/− mice is regulated by LPL activity and involves proteoglycans and SR-BI. J. Lipid Res. 49: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 21.Webb N. R., de Beer M. C., Yu J., Kindy M. S., Daugherty A., van der Westhuyzen D. R., de Beer F. C. 2002. Overexpression of SR-BI by adenoviral vector promotes clearance of apoA-I, but not apoB, in human apoB transgenic mice. J. Lipid Res. 43: 1421–1428. [DOI] [PubMed] [Google Scholar]
- 22.Huszar D., Varban M. L., Rinninger F., Feeley R., Arai T., Fairchild-Huntress V., Donovan M. J., Tall A. R. 2000. Increased LDL cholesterol and atherosclerosis in LDL receptor-deficient mice with attenuated expression of scavenger receptor B1. Arterioscler. Thromb. Vasc. Biol. 20: 1068–1073. [DOI] [PubMed] [Google Scholar]
- 23.Webb N. R., de Beer M. C., de Beer F. C., van der Westhuyzen D. R. 2004. ApoB-containing lipoproteins in apoE-deficient mice are not metabolized by the class B scavenger receptor BI. J. Lipid Res. 45: 272–280. [DOI] [PubMed] [Google Scholar]
- 24.Fu T., Kozarsky K. F., Borensztajn J. 2003. Overexpression of SR-BI by adenoviral vector reverses the fibrateinduced hypercholesterolemia of apolipoprotein E-deficient mice. J. Biol. Chem. 278: 52559–52563. [DOI] [PubMed] [Google Scholar]
- 25.Arai T., Wang N., Bezouevski M., Welch C., Tall A. R. 1999. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J. Biol. Chem. 274: 2366–2371. [DOI] [PubMed] [Google Scholar]
- 26.Ueda Y., Royer L., Gong E., Zhang J., Cooper P. N., Francone O., Rubin E. M. 1999. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J. Biol. Chem. 274: 7165–7171. [DOI] [PubMed] [Google Scholar]
- 27.Wang N., Arai T., Ji Y., Rinninger F., Tall A. R. 1998. Liver-specific overexpression of scavenger receptor BI decreases levels of very low density lipoprotein ApoB, low density lipoprotein ApoB, and high density lipoprotein in transgenic mice. J. Biol. Chem. 273: 32920–32926. [DOI] [PubMed] [Google Scholar]
- 28.Ueda Y., Gong E., Royer L., Cooper P. N., Francone O. L., Rubin E. M. 2000. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J. Biol. Chem. 275: 20368–20373. [DOI] [PubMed] [Google Scholar]
- 29.Webb N. R., Connell P. M., Graf G. A., Smart E. J., de Villiers W. J., de Beer F. C., van der Westhuyzen D. R. 1998. SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J. Biol. Chem. 273: 15241–15248. [DOI] [PubMed] [Google Scholar]
- 30.Yesilaltay A., Morales M. G., Amigo L., Zanlungo S., Rigotti A., Karackattu S. L., Donahee M. H., Kozarsky K. F., Krieger M. 2006. Effects of hepatic expression of the high-density lipoprotein receptor SR-BI on lipoprotein metabolism and female fertility. Endocrinology. 147: 1577–1588. [DOI] [PubMed] [Google Scholar]
- 31.Stangl H., Hyatt M., Hobbs H. H. 1999. Transport of lipids from high and low density lipoproteins via scavenger receptor-BI. J. Biol. Chem. 274: 32692–32698. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein J. L., DeBose-Boyd R. A., Brown M. S. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 33.Shelness G. S., Sellers J. A. 2001. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 12: 151–157. [DOI] [PubMed] [Google Scholar]
- 34.Brewer H. B., Jr. 2007. HDL metabolism and the role of HDL in the treatment of high-risk patients with cardiovascular disease. Curr. Cardiol. Rep. 9: 486–492. [DOI] [PubMed] [Google Scholar]
- 35.Trigatti B. L. 2005. Hepatic high-density lipoprotein receptors: roles in lipoprotein metabolism and potential for therapeutic modulation. Curr. Atheroscler. Rep. 7: 344–350. [DOI] [PubMed] [Google Scholar]
- 36.Rader D. J. 2007. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat. Clin. Pract. Cardiovasc. Med. 4: 102–109. [DOI] [PubMed] [Google Scholar]
- 37.Linsel-Nitschke P., Tall A. R. 2005. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4: 193–205. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Da Silva J. R., Reilly M., Billheimer J. T., Rothblat G. H., Rader D. J. 2005. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 115: 2870–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozarsky K. F., Donahee M. H., Glick J. M., Krieger M., Rader D. J. 2000. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 20: 721–727. [DOI] [PubMed] [Google Scholar]
- 40.Huby T., Doucet C., Dachet C., Ouzilleau B., Ueda Y., Afzal V., Rubin E., Chapman M. J., Lesnik P. 2006. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J. Clin. Invest. 116: 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]