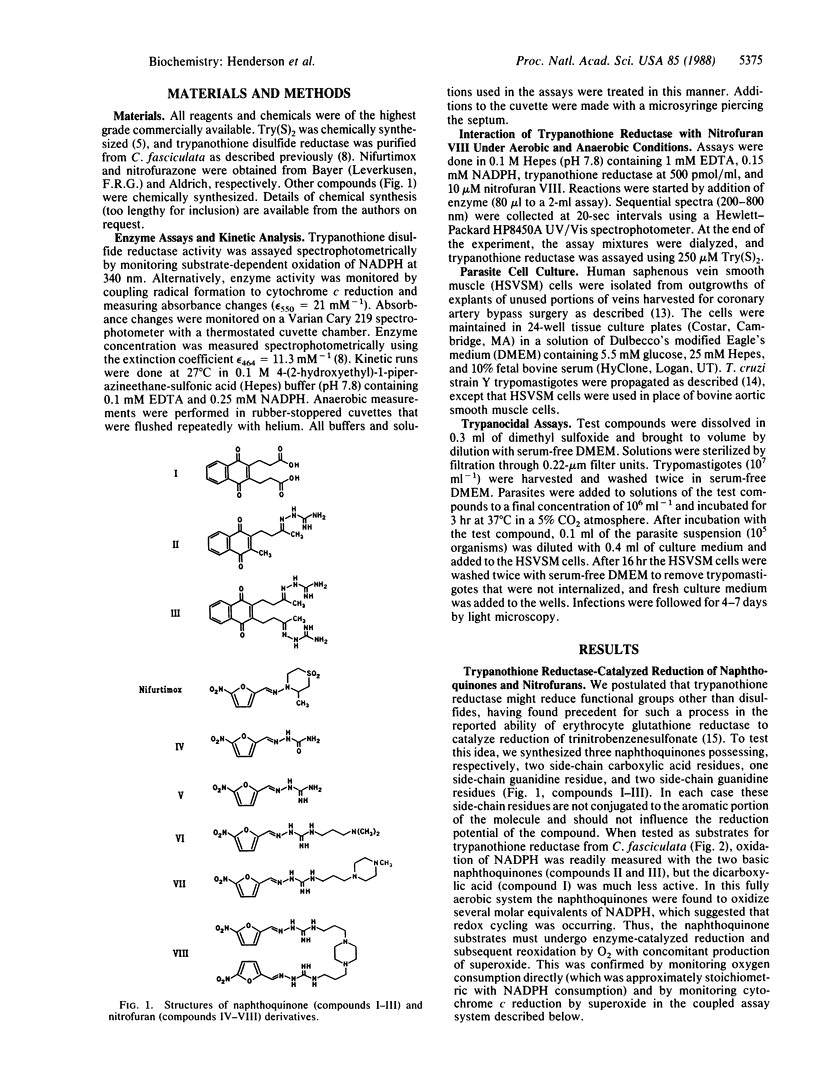
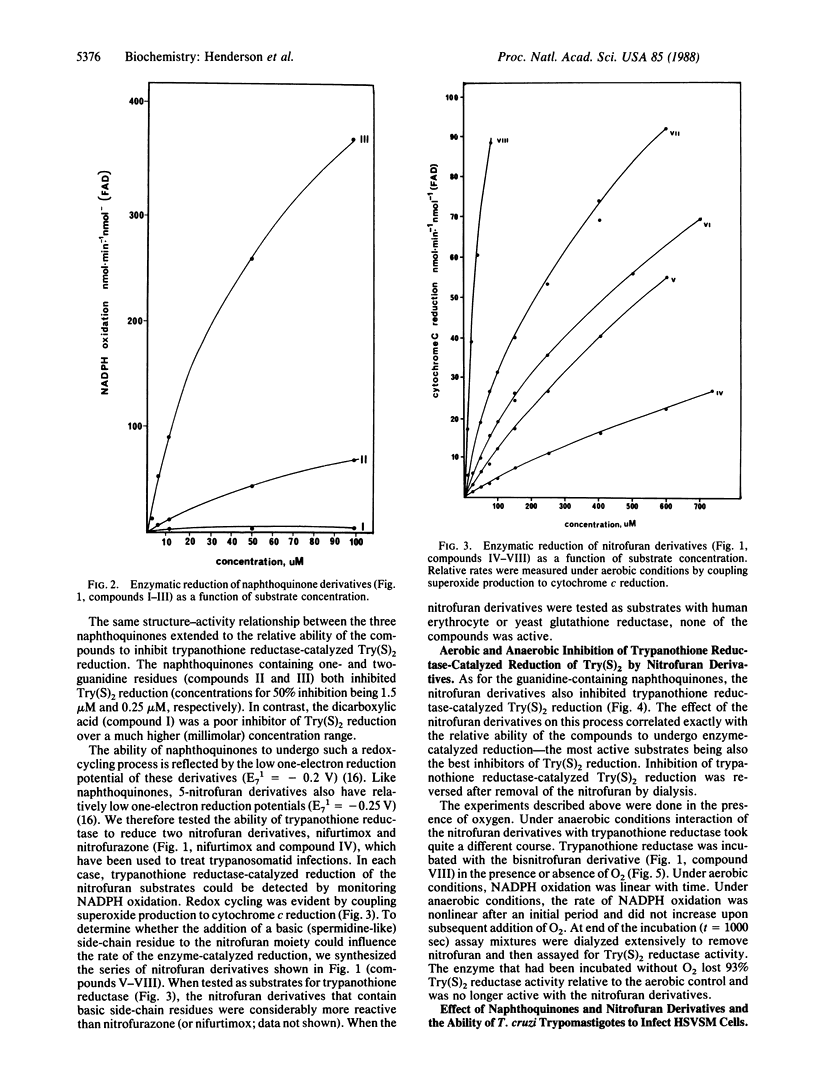
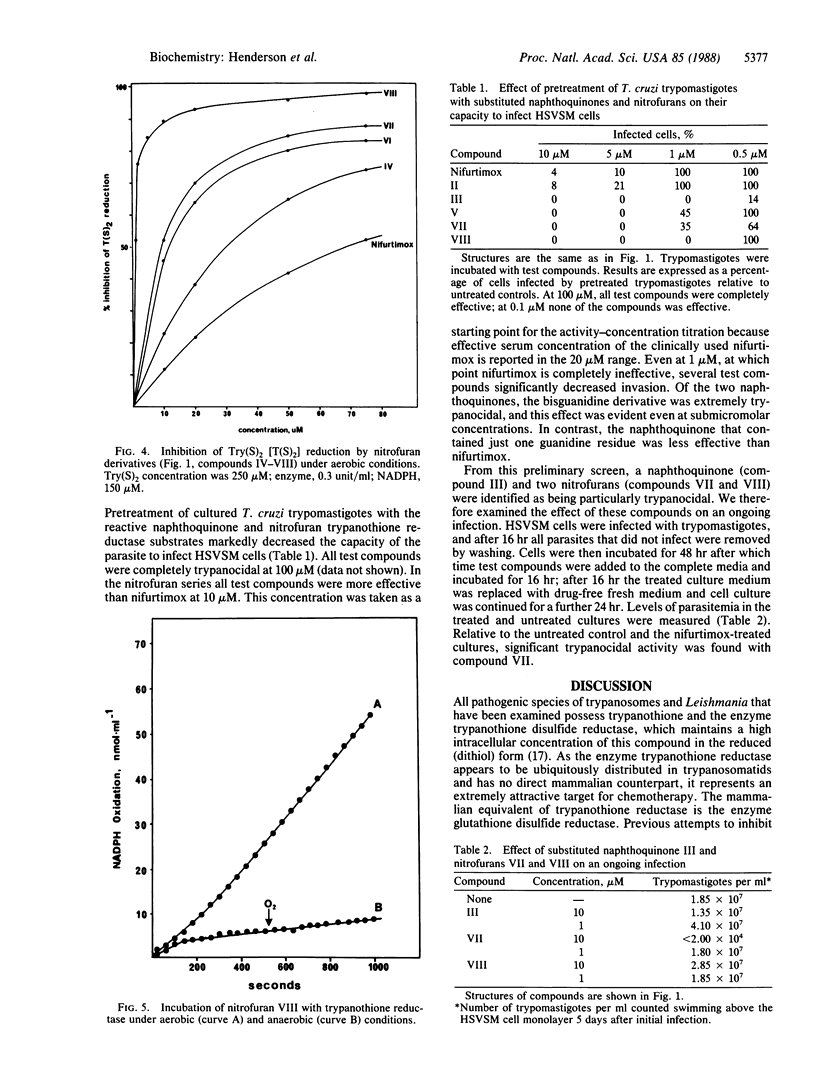
Abstract
The trypanosomatid flavoprotein disulfide reductase, trypanothione reductase, is shown to catalyze one-electron reduction of suitably substituted naphthoquinone and nitrofuran derivatives. A number of such compounds have been chemically synthesized, and a structure-activity relationship has been established; the enzyme is most active with compounds that contain basic functional groups in side-chain residues. The reduced products are readily reoxidized by molecular oxygen and thus undergo classical enzyme-catalyzed redox cycling. In addition to their ability to act as substrates for trypanothione reductase, the compounds are also shown to effectively inhibit enzymatic reduction of the enzyme's physiological substrate, trypanothione disulfide. Under aerobic conditions, trypanothione reductase is not inactivated by these redox-cycling substrates, whereas under anaerobic conditions the nitrofuran compounds cause irreversible inactivation of the enzyme. When tested for biological activity against Trypanosoma cruzi trypomastigotes, many of the test compounds were trypanocidal, and this activity correlated with their relative ability to act as substrates for trypanothione reductase. The activity of the enzyme with these redox-cycling derivatives constitutes a subversion of its normal antioxidant role within the cell. For this reason these compounds may be termed "subversive" substrates for trypanothione reductase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Clarke E. D., Jacobs R. S., Stratford I. J., Wallace R. G., Wardman P., Watts M. E. Mammalian cell toxicity of nitro compounds : dependence upon reduction potential. Biochem Biophys Res Commun. 1976 Oct 4;72(3):824–829. doi: 10.1016/s0006-291x(76)80207-0. [DOI] [PubMed] [Google Scholar]
- Carbone P. P., Zipkin I., Sokoloff L., Frazier P., Cook P., Mullins F. Fluoride effect on bone in plasma cell myeloma. Arch Intern Med. 1968 Feb;121(2):130–140. [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Reduction of 2,4,6-trinitrobenzenesulfonate by glutathione reductase and the effect of NADP+ on the electron transfer. J Biol Chem. 1986 Feb 5;261(4):1629–1635. [PubMed] [Google Scholar]
- Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985 Mar 22;227(4693):1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Cerami A. Identification of a novel, thiol-containing co-factor essential for glutathione reductase enzyme activity in trypanosomatids. Mol Biochem Parasitol. 1985 Feb;14(2):187–198. doi: 10.1016/0166-6851(85)90037-4. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Fairlamb A. H., Cerami A. Trypanothione dependent peroxide metabolism in Crithidia fasciculata and Trypanosoma brucei. Mol Biochem Parasitol. 1987 May;24(1):39–45. doi: 10.1016/0166-6851(87)90113-7. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Fairlamb A. H., Ulrich P., Cerami A. Substrate specificity of the flavoprotein trypanothione disulfide reductase from Crithidia fasciculata. Biochemistry. 1987 Jun 2;26(11):3023–3027. doi: 10.1021/bi00385a011. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel R. L., Enders B., Henderson G. B., Fairlamb A. H., Schirmer R. H. Trypanothione reductase from Trypanosoma cruzi. Purification and characterization of the crystalline enzyme. Eur J Biochem. 1987 Apr 1;164(1):123–128. doi: 10.1111/j.1432-1033.1987.tb11002.x. [DOI] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr J. J., Docampo R. Chemotherapy for Chagas' disease: a perspective of current therapy and considerations for future research. Rev Infect Dis. 1986 Nov-Dec;8(6):884–903. doi: 10.1093/clinids/8.6.884. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meshnick S. R., Blobstein S. H., Grady R. W., Cerami A. An approach to the development of new drugs for African trypanosomiasis. J Exp Med. 1978 Aug 1;148(2):569–579. doi: 10.1084/jem.148.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh P. G., Klein R. A. Hydrogen peroxide metabolism in Trypanosoma brucei. Mol Biochem Parasitol. 1986 Aug;20(2):111–121. doi: 10.1016/0166-6851(86)90023-x. [DOI] [PubMed] [Google Scholar]
- Shames S. L., Fairlamb A. H., Cerami A., Walsh C. T. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulfide-containing flavoprotein reductases. Biochemistry. 1986 Jun 17;25(12):3519–3526. doi: 10.1021/bi00360a007. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]



