Abstract
While conventional pharmacogenetic studies have considered single gene effects, we tested if a genetic score of nine LDL- and HDL-associated single nucleotide polymorphisms, previously shown to predict cardiovascular disease, is related to fluvastatin-induced lipid change. In patients with asymptomatic plaque in the right carotid artery, thus candidates for statin therapy, we related score LDL [APOB(rs693), APOE(rs4420638), HMGCR(rs12654264), LDLR(rs1529729), and PCSK9(rs11591147)] and score HDL [ABCA1(rs3890182), CETP(rs1800775), LIPC(rs1800588), and LPL(rs328)] as well as the combined score LDL+HDL to fluvastatin-induced LDL reduction (± metoprolol) (n = 395) and HDL increase (n = 187) following 1 year of fluvastatin treatment. In women, an increasing number of unfavorable alleles (i.e., alleles conferring higher LDL and lower HDL) of score LDL+HDL (P = 0.037) and of score LDL (P = 0.023) was associated with less pronounced fluvastatin-induced LDL reduction. Furthermore, in women, both score LDL+HDL (P = 0.001) and score HDL (P = 0.022) were directly correlated with more pronounced fluvastatin-induced HDL increase, explaining 5.9–11.6% of the variance in treatment response in women. There were no such associations in men. This suggests that a gene score based on variation in nine different LDL- and HDL-associated genes is of importance for the magnitude of fluvastatin HDL increase in women with asymptomatic plaque in the carotid artery.
Keywords: genetic score, statins, pharmacogenetics, atherosclerosis, carotid plaque, lipid levels, treatment response
Cardiovascular disease is a major cause of mortality and morbidity in high- as well as low-income countries (1). Total and LDL-cholesterol is one of the main risk factors for ischemic heart disease in middle aged and older subjects (2) and has been shown to be a predictor of coronary heart disease mortality in a number of different ethnicities (3, 4). Additionally, a high level of HDL-cholesterol has been shown to be protective against ischemic heart disease (2) and to inhibit or even regress atherosclerosis in animal models (5).
Although lifestyle modifications could achieve a more favorable lipid profile with lower LDL and higher HDL (6), drug therapy with statins has been shown to lower LDL and raise HDL in a more prominent way (7, 8) as well as to reduce the risk of cardiovascular morbidity and mortality in both primary (8–10) and secondary (8, 11–13) prevention. Therefore, statins are first line therapy when lifestyle modifications fail to lower LDL to target levels (14). However, only one-third of treated patients do reach their treatment goals (15), and treatment goals for high risk patients are increasingly more strictly set (16). It is possible that one reason for not meeting the desirable lipid levels is the individual genetic differences affecting lipid and/or statin metabolism.
Earlier research suggests a number of genetic polymorphisms influencing blood levels of LDL and HDL (17–22). A recent study conducted in 20,000 individuals suggested common variants at 30 loci contributing to polygenic dyslipidemia (23). Furthermore, pharmacogenetic aspects of statin therapy have been evaluated and discussed (24). Several studies have suggested that genetic polymorphisms of individual genes, known to be involved in lipid metabolism (25–30), general drug metabolism (31–34), and other genes whose relation to lipid metabolism is less clear (35–37), could influence the outcome of statin therapy.
As single gene polymorphisms usually explain only a small proportion of population variance in LDL and HDL (38), each of them is expected to affect cardiovascular outcome only modestly. On the other hand, a combination of gene variants adversely affecting LDL and HDL would be expected to have greater clinical importance. In a middle-aged population based cohort, the Malmö Diet and Cancer-Cardiovascular Cohort (MDC-CC) (39), we recently showed that a genetic score based on a combination of nine common single nucleotide polymorphisms (SNPs), with each individual SNP having prior evidence of association with either LDL [rs693 in apolipoprotein B (APOB), rs4420638 in APOE, rs12654264 in HMG-CoA reductase (HMGCR), rs1529729 in LDL receptor (LDLR), and rs11591147 in PCSK9] or HDL (rs3890182 in ABCA1, rs1800775 in cholesteryl ester transfer protein, rs1800588 in hepatic lipase, and rs328 in LPL) is strongly linearly associated with both increasing LDL and decreasing HDL. Furthermore, we found that the same score is independently related to incident cardiovascular events and that it improves individual cardiovascular risk classification as assessed by using both the Net Reclassification Index and the Integrated Discrimination Index (40).
Given its strong relationship with both LDL and HDL levels as well as with increased cardiovascular risk (40), we hypothesized that this gene score would be related to the magnitude of LDL reduction and HDL increase during statin treatment. We tested this hypothesis in a subset of the MDC-CC population who participated in the β-blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS) (41) taking potentially gender-specific response into account.
METHODS
Study population
The MDC study (39) is an epidemiological cohort study of 28,449 subjects recruited between the years of 1991 and 1996. From this cohort, 6,103 subjects were randomly selected to study the epidemiology of carotid artery disease, including measurement of intima media thickness, occurrence of plaques, and a broad range of cardiovascular risk factors, in the MDC-CC. Using B-mode ultrasound, the right carotid artery bifurcation was scanned within a predefined window comprising 3 cm of the distal common carotid artery, the bifurcation, and 1 cm of the internal and external carotid artery. Intima-media thickness (IMT) was measured in the far wall according to the leading edge principle, using a specially designed computer-assisted image analyzing system. Presence of plaque was defined as focal IMT > 1.2mm. Subjects with asymptomatic plaques in the carotid artery (i.e., subjects with plaques meeting the definition but with no symptoms of carotid artery disease) in the MDC-CC and at the enrollment examination (n = 793) were included in BCAPS. This is a randomized, double blind, placebo-controlled study that tested whether treatment with low dose metoprolol (25 mg) and/or fluvastatin (40 mg) could reduce carotid intima media thickening in comparison with placebo. Exclusion criteria in the BCAPS study were a history of myocardial infarction, angina pectoris, or stroke within the preceding 3 months of the study; history of surgical intervention in the right carotid artery; β-blocker or statin use; blood pressure >160 systolic or >95 diastolic or hyperglycemia suspected to require insulin treatment (41). Since a triglyceride level exceeding 4.5 mmol/l invalidates the use of the Friedewald formula, we additionally excluded one subject with a triglyceride level exceeding this level.
To test our hypothesis, we included all subjects in the BCAPS study who were randomized to receive either 40 mg fluvastatin daily (F group, n = 198) or 40 mg fluvastatin plus 25 metoprolol daily (FM group, n = 197; total n = 395). Clinical characteristics of the study population are shown in Table 1.
TABLE 1.
Baseline characteristics of the subjects (n = 395)
| Men (n = 180) | Women (n = 215) | |
|---|---|---|
| Mean age, years | 62.3 ± 5.3 | 61.9 ± 5.1 |
| Cholesterol, mmol/l | ||
| Total | 6.03 ± 0.86 | 6.22 ± 1.01a |
| LDL | 4.12 ± 0.80 | 4.20 ± 0.89 |
| HDL | 1.27 ± 0.31 | 1.47 ± 0.35c |
| Mean IMTCCA thickness, mm | 0.90 ± 0.16 | 0.89 ± 0.21 |
| Cholesterol > 5.0 mmol/l, n (%) | 164 (91.1) | 193 (89.8) |
| Triglycerides, mmol/ld | 1.18 (0.86) | 1.05 (0.71) |
| BMI, kg/m2 | 25.8 ± 3.4 | 25.3 ± 3.5 |
| Systolic blood pressure, mm Hg | 139.3 ± 12.8 | 139.4 ± 14.9 |
| Diastolic blood pressure, mm Hg | 86.0 ± 6.2 | 84.0 ± 7.1b |
| Blood glucose, mmol/l | 5.29 ± 0.63 | 4.98 ± 0.73c |
| History of diabetes, n (%) | 4 (2.2) | 4 (1.7) |
| Smokers, n (%) | 60 (33.3) | 64 (29.8) |
| History of CVD, n (%) | 16 (8.9) | 5 (2.3)e |
Data are shown as mean ± SD if not otherwise specified.
For Student's t-test: a P < 0.05, b P < 0.01, and c P < 0.001.
Data shown as median (interquartile range).
P χ2 (Pearson) = 0.004.
The BCAPS and MDCS-CC study protocols were approved by the ethics committee at Lund University, and all subjects gave their written informed consent.
Genotyping and SNP selection
Our study population is a subset of the MDC-CC, and all subjects have thus been genotyped for the nine SNPs included in the gene score that we have previously shown to be related to blood levels of LDL and HDL as well as to risk of cardiovascular events (rs693 in APOB, rs4420638 in APOE, rs12654264 in HMGCR, rs1529729 in LDLR, and rs11591147 in PCSK9 for LDL and rs3890182 in ABCA1, rs1800775 in cholesteryl ester transfer protein, rs1800588 in hepatic lipase, and rs328 in LPL for HDL) (40). Fifteen percent of the samples were run in duplicate without any inconsistencies. All genotypes were called by two different investigators. None of the SNPs included significantly deviates from the Hardy-Weinberg equilibrium in the study population.
Genotype score construction
The concept of genotype score was implemented in our earlier study (40). The genotype score was constructed on the basis of the number of unfavorable alleles of the nine common SNPs (alleles associated with higher LDL or lower HDL levels; score LDL + HDL, range 0–18) (40).
In addition to score LDL + HDL, we evaluated a score based on number of unfavorable alleles of the five SNPs associated specifically with LDL levels (score LDL; range 0–10) and a score based on number of unfavorable alleles of the four SNPs associated specifically with HDL levels (score HDL; range 0–8).
Outcomes
Blood levels of LDL and HDL in the BCAPS study were obtained at randomization and after 12, 24, and 36 months of fluvastatin treatment, as described previously (41). LDL was calculated from the formula of Friedewald. Since the effect of statins on blood cholesterol is usually rapid, and because dropout rate increases and compliance may decrease with time, we used the change of LDL and HDL from baseline to12 months of fluvastatin treatment as the outcome variable.
From the two measurements (baseline and 12 months) we calculated an absolute difference and a percentage (absolute difference divided by baseline value * 100) change of LDL and HDL. As mean LDL levels are expected to decrease and mean HDL levels are expected to increase during statin treatment, we defined mean LDL change as (baseline LDL – 12 months LDL) and mean HDL change as (12 months HDL – baseline HDL).
Statistical analysis
All statistical analyses, except from Power, were conducted using SPSS 16.0. Power was calculated by PS 3.0 from Dupont. Data are given as means ± SD unless otherwise specified. The significance of changes in LDL and HDL during fluvastatin treatment was tested using one-sample t-test. Group-wise differences in clinical characteristics were tested using independent samples t-test for continuous and χ2 test for dichotomous variables. The LDL and HDL change during fluvastatin treatment related to genotype scores was assessed using linear regression analysis in unadjusted and adjusted models. In the adjusted models, residuals of LDL and HDL change, adjusted for age, percentage of body mass index (BMI) reduction during the study period, and baseline blood glucose, were entered as dependent variable and genotype score as the independent variable. We tested for interaction between genotype score and age (genotype score × age, genotype score and age as independent variables) and between genotype score and sex (genotype score × sex, genotype score and sex as independent variables) on the outcome of LDL and HDL change during fluvastatin treatment. Since hormone replacement therapy (HRT) was not an exclusion criteria, we also tested for interaction between genotype score and HRT (genotype score × HRT, genotype score and HRT as independent variables) on the outcome of LDL and HDL in women.
As a secondary analysis, associations between single SNPs and LDL and HDL change during fluvastatin treatment were tested using linear regression, assuming an additive model of inheritance.
A P value of <0.05 was considered significant. The t-tests were two-tailed unless otherwise indicated in the text.
RESULTS
Population characteristics
The baseline characteristics of the 395 subjects treated with fluvastatin are shown in Table 1. Age and BMI were similar between sexes. The mean IMT thickness in the common carotid artery did not differ significantly between sexes. Women had higher total cholesterol and HDL than men, and a history of cardiovascular disease (CVD) was more common in men than in women. There were no significant differences in baseline variables between the F and the FM group (data not shown).
Complete genotype data to construct score LDL + HDL, score LDL, and score HDL were available for 342, 344, and 363 subjects, respectively. Measures of LDL and HDL after 12 months were available for 371 and 375 subjects, respectively. Consequently, gene score analyses on LDL change could be conducted in a total of 319, 321, and 339 subjects for score LDL + HDL, score LDL, and score HDL, respectively. Similarly, gene score analyses for HDL change could be conducted in 323, 325, and 343 subjects for score LDL + HDL, score LDL, and score HDL, respectively.
Change of LDL
At 12 months of treatment, LDL was significantly reduced by an average of 0.909 ± 0.76 mmol/l and 21.11 ± 16.8% (one-tailed t-test, P < 0.001 for both). There was no significant difference in LDL reduction between sexes (data not shown). The magnitude of LDL reduction did not significantly differ between the F (n = 185) and the FM (n = 186) groups (0.905 ± 0.77 mmol/l versus 0.913 ± 0.75; P = 0.92 and 20.90 ± 16.8% versus 21.33 ± 16.8%; P = 0.80). The extent of LDL decrease was positively correlated to age (P < 0.001 for absolute and percentage of reduction) and percentage of BMI reduction during the study period (P = 0.005 for absolute and P = 0.003 for percentage of LDL reduction), with higher age and more BMI reduction resulting in a greater LDL reduction. There was no significant association between baseline blood glucose and the magnitude of LDL reduction (data not shown).
Change of LDL and score models
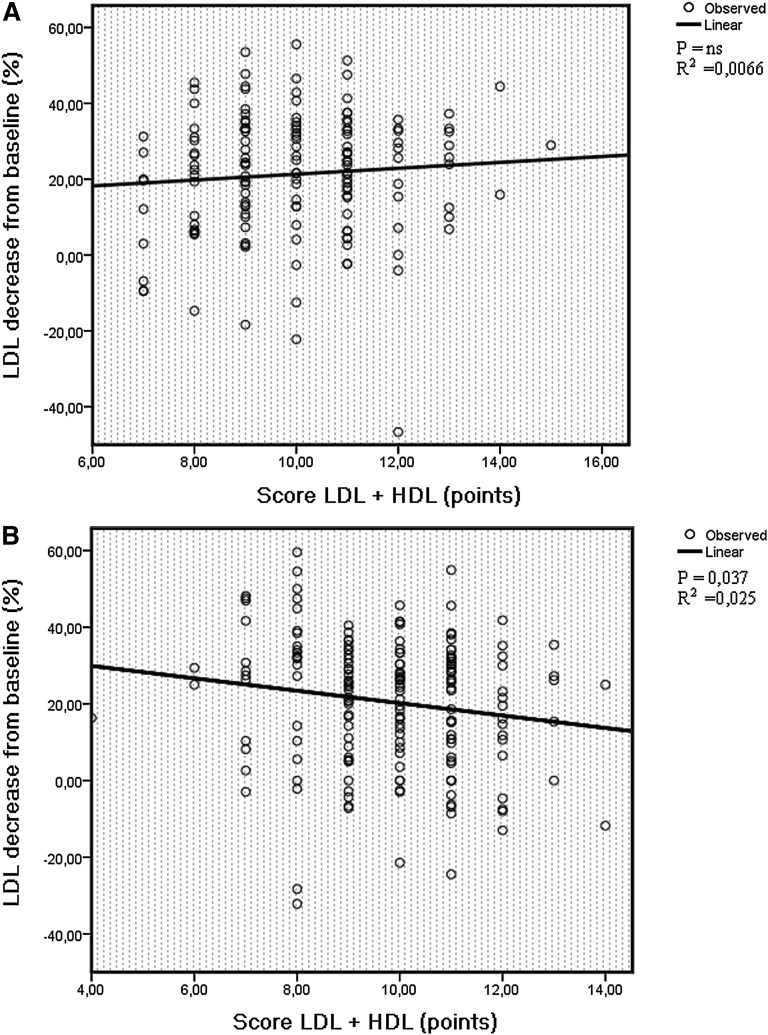
There was no significant association between genotype scores and LDL change in the group including both sexes (Table 2); however, interaction analyses revealed a significant interaction between sex and score LDL + HDL on fluvastatin-induced LDL change (P = 0.012 for absolute and P = 0.033 for percentage). In line with this finding, there was no significant association between score LDL + HDL and LDL change or between score LDL and LDL change among men (Table 3, Fig. 1A), whereas among women, score LDL + HDL and score LDL were significantly associated with absolute as well as percentage of LDL change, with a higher score resulting in a smaller LDL reduction (Table 4, Fig. 1B). The significance remained after adjustment for age, percentage of BMI reduction, and baseline blood glucose for score LDL + HDL, whereas for score LDL, the significance was slightly attenuated. Score HDL was not associated with fluvastatin-induced change of LDL. We found no evidence of interaction between age and genotype scores or between HRT and genotype scores on the outcome of LDL.
TABLE 2.
Association between score and LDL decrease (all subjects)
| Min-Max |
Unadjusted Data |
Adjusted Dataa |
||||||
|---|---|---|---|---|---|---|---|---|
| Score Name (Possible Points) | β-Coefficientmmol/l per point (% per point) | Variance Explained % | P | β-Coefficient | Variance Explained % | P | ||
| Score LDL + HDL | 4-15 | Absolute | −0.0200 | 0.185 | 0.444 | −0.026 | 0.336 | 0.306 |
| (0–18) | Percentage | −0.500 | 0.250 | 0.371 | −0.676 | 0.490 | 0.219 | |
| Score LDL | 2-8 | Absolute | −0.0540 | 0.884 | 0.094 | −0.043 | 0.593 | 0.176 |
| (0–10) | Percentage | −1.18 | 0.884 | 0.093 | −0.968 | 0.640 | 0.158 | |
| Score HDL | 0-8 | Absolute | 0.0380 | 0.292 | 0.318 | 0.004 | 0.0036 | 0.913 |
| (0–8) | Percentage | 0.721 | 0.221 | 0.383 | −0.059 | 0.0016 | 0.941 | |
For residuals adjusted for age and blood glucose at randomization and percentage of BMI change during the study period.
TABLE 3.
Association between score and LDL decrease (men)
| Min-Max |
Unadjusted Data |
Adjusted Dataa |
||||||
|---|---|---|---|---|---|---|---|---|
| Score Name (Possible Points) | β-Coefficientmmol/l per point (% per point) | Variance Explained % | P | β-Coefficient | Variance Explained % | P | ||
| Score LDL + HDL | 7-15 | Absolute | 0.0500 | 1.44 | 0.154 | 0.026 | 0.410 | 0.449 |
| (0–18) | Percentage | 0.774 | 0.656 | 0.337 | 0.206 | 0.0529 | 0.790 | |
| Score LDL | 3-8 | Absolute | 0.0140 | 0.0576 | 0.776 | 0.002 | 0.0016 | 0.966 |
| (0–10) | Percentage | 0.111 | 0.00810 | 0.919 | −0.160 | 0.0169 | 0.879 | |
| Score HDL | 2-7 | Absolute | 0.100 | 2.34 | 0.058 | 0.061 | 1.000 | 0.226 |
| (0–8) | Percentage | 1.75 | 1.37 | 0.150 | 0.832 | .348 | 0.473 | |
For residuals adjusted for age and blood glucose at randomization and percentage of BMI change during the study period.
Fig. 1.
The relationship between score LDL + HDL and percentage of LDL decrease among men (A) and women (B) after 12 months of fluvastatin therapy. A higher score corresponds to a higher number of unfavorable alleles in lipid-regulating genes (resulting in higher baseline LDL or lower HDL levels). In women, a higher score LDL + HDL confers a less prominent response to statin treatment.
TABLE 4.
Association between dcore and LDL decrease (women)
| Min-Max |
Unadjusted Data |
Adjusted Dataa |
||||||
|---|---|---|---|---|---|---|---|---|
| Score Name (Possible Points) | β-Coefficientmmol/l per point (% per point) | Variance Explained % | P | β-Coefficient | Variance Explained % | P | ||
| Score LDL + HDL | 4-14 | Absolute | −0.0800 | 2.66 | 0.031 | −0.073 | 2.37 | 0.046 |
| (0–18) | Percentage | −1.62 | 2.50 | 0.037 | −1.55 | 2.43 | 0.043 | |
| Score LDL | 2-8 | Absolute | −0.101 | 2.92 | 0.023 | −0.074 | 1.69 | 0.089 |
| (0–10) | Percentage | −2.11 | 2.92 | 0.023 | −1.61 | 1.82 | 0.078 | |
| Score HDL | 0-8 | Absolute | −0.014 | 0.0400 | 0.789 | −0.049 | 0.49 | 0.349 |
| (0–8) | Percentage | −0.132 | 0.00810 | 0.908 | −0.909 | 0.38 | 0.411 | |
For residuals adjusted for age and blood glucose at randomization and percentage of BMI-change during the study period.Bold indicates significant P-value as described in the Method section.
Change of LDL and single SNPs
None of the individual SNPs were associated with LDL change in the entire study population (see supplementary Table IA). Among men, there was an association between absolute LDL change and the APOB polymorphism (rs693) and absolute and percentage of LDL change and the HMGCR polymorphism (rs12654264) (see supplementary Table IB). The association for the APOB polymorphism was positively correlated; thus, the more unfavorable alleles, the greater LDL reduction. The HMGCR polymorphism showed an inverse relationship, with more unfavorable alleles resulting in a smaller magnitude of LDL reduction (see supplementary Table IB). Among women, there was no significant association between any single SNP and LDL change (see supplementary Table IC).
Change of HDL
In the F group (n = 187), there was a significant percentage of increase of HDL of 2.58 ± 13.8% (one-tailed t-test, P = 0.006) and a significant absolute increase of 0.0258 ± 0.20 mmol/l (one-tailed t-test, P = 0,037) after 12 months fluvastatin treatment. By contrast, in the FM group (n = 188), there was a nonsignificant percentage of HDL decrease by an average of 0.909 ± 11.9% (one-tailed t-test, P = 0.149) and a significant absolute decrease of 0.0262 ± 0.18 mmol/l (one-tailed t-test, P = 0.021) after 12 months. There was a significant difference in statin-induced HDL change between subjects with and without simultaneous metoprolol treatment (P = 0.007 for absolute and P = 0.009 for percentage of change). Thus, in order to exclude the confounding effect of metoprolol on HDL levels, which is well known from previous trials of β-blockers (42), all the analyses of HDL changes were performed exclusively on the F group (complete data for score LDL + HDL, score LDL, and score HDL for 160, 162, and 169 subjects, respectively).
There was no difference of HDL change between sexes, and age, baseline blood glucose, and percentage of change of BMI during the study period did not affect HDL change (data not shown).
Change of HDL and score models
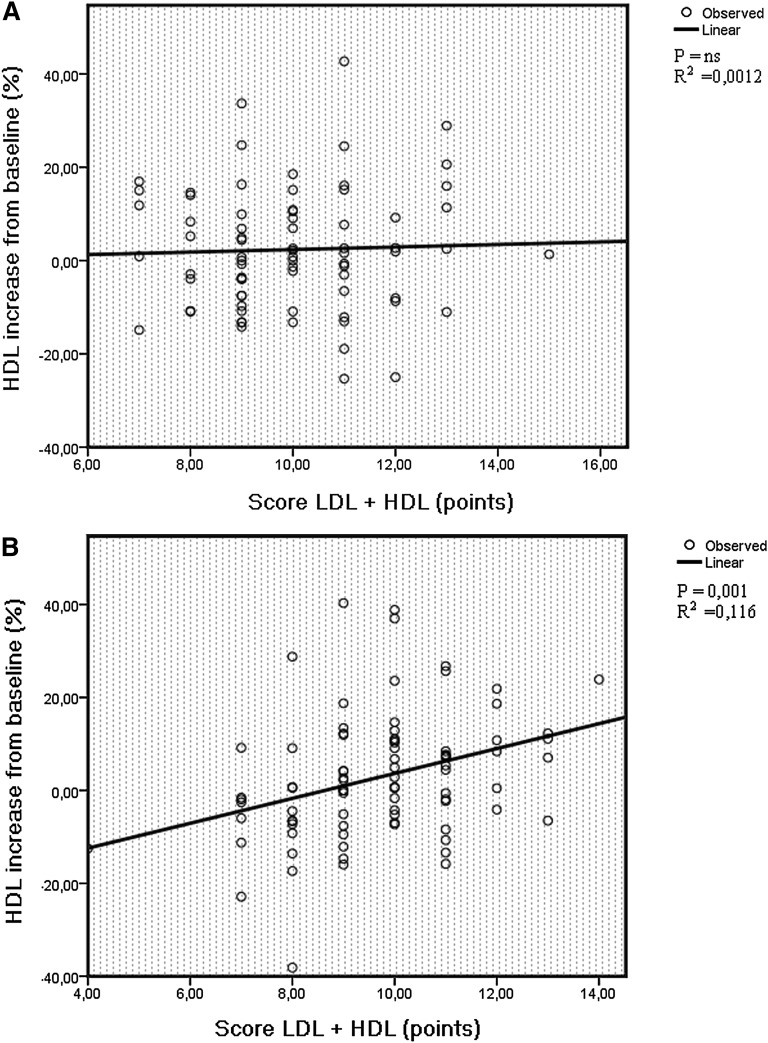
In the F group, there was a significant association between absolute and percentage of HDL increase and score LDL + HDL and score HDL (Table 5). The relationship remained significant after adjustment for age, percentage of BMI reduction, and baseline blood glucose. A higher score generated a more prominent HDL increase after fluvastatin treatment (Table 5). As was the case for fluvastatin-induced LDL change, there was a significant interaction between sex and score LDL + HDL on HDL change during fluvastatin treatment (P = 0.015 for absolute P = 0.047 for percent). In men, there was no significant relationship between score LDL + HDL or score HDL and HDL change (Table 6, Fig. 2A), while in women, there was a strong direct relationship between fluvastatin-induced HDL increase and score LDL + HDL and between fluvastatin-induced HDL increase and score HDL (Table 7, Fig. 2B), implying that a higher score was associated with a more prominent HDL increase following 12 months of fluvastatin treatment. The significance remained after adjustments (Table 7). Score LDL was not significantly associated with fluvastatin-induced HDL change (Tables 5–7). There was no evidence of interaction between genotype scores and age, or between genotype scores and HRT on the outcome of HDL levels.
TABLE 5.
Association between score and HDL increase (all subjects)
| Min-Max |
Unadjusted Data |
Adjusted Dataa |
||||||
|---|---|---|---|---|---|---|---|---|
| Score Name (Possible Points) | β-Coefficientmmol/l per point (% per point) | Variance Explained % | P | β-Coefficient | Variance Explained % | P | ||
| Score LDL + HDL | 4-15 | Absolute | 0.0250 | 4.80 | 0.005 | 0.027 | 5.76 | 0.003 |
| (0–18) | Percentage | 1.54 | 4.00 | 0.011 | 1.61 | 4.37 | 0.009 | |
| Score LDL | 2-8 | Absolute | 0.0170 | 1.25 | 0.155 | 0.020 | 1.90 | 0.085 |
| (0–10) | Percentage | 1.12 | 1.23 | 0.161 | 1.24 | 1.49 | 0.127 | |
| Score HDL | 0-7 | Absolute | 0.0310 | 3.53 | 0.014 | 0.032 | 3.61 | 0.014 |
| (0–8) | Percentage | 1.85 | 2.66 | 0.035 | 1.90 | 2.76 | 0.033 | |
For residuals adjusted for age and blood glucose at randomization and percentage of BMI change during the study period.Bold indicates significant P-value as described in the Method section.
TABLE 6.
Association between score and HDL increase (men)
| Min-Max |
Unadjusted Data |
Adjusted Dataa |
||||||
|---|---|---|---|---|---|---|---|---|
| Score Name (Possible Points) | β-Coefficientmmol/l per point (% per point) | Variance Explained % | P | β-Coefficient | Variance Explained % | P | ||
| Score LDL + HDL | 7-15 | Absolute | 0.00200 | 0.0361 | 0.868 | 0.003 | 0.116 | 0.774 |
| (0–18) | Percentage | 0.271 | 0.123 | 0.763 | 0.260 | 0.123 | 0.769 | |
| Score LDL | 3-8 | Absolute | −0.006 | 0.212 | 0.692 | −0.003 | 0.0441 | 0.860 |
| (0–10) | Percentage | −0.187 | 0.0289 | 0.883 | −0.147 | 0.0196 | 0.907 | |
| Score HDL | 2-7 | Absolute | 0.012 | 0.656 | 0.473 | 0.011 | 0.593 | 0.499 |
| (0–8) | Percentage | 0.841 | 0.548 | 0.517 | 0.818 | 0.533 | 0.521 | |
For residuals adjusted for age and blood glucose at randomization and percentage of BMI change during the study period.
Fig. 2.
The relationship between score LDL + HDL and percentage of HDL increase among men (A) and women (B) after 12 months of fluvastatin therapy. A higher score corresponds to a higher number of unfavorable alleles in lipid regulating genes (resulting in higher baseline LDL or lower HDL levels). As a group, the treatment response in HDL was small; however, the figure shows high interindividual variation in HDL treatment response depending on score LDL + HDL in women.
TABLE 7.
Association between score and HDL increase (women)
| Min-Max |
Unadjusted Data |
Adjusted Dataa |
||||||
|---|---|---|---|---|---|---|---|---|
| Score Name (Possible Points) | β-Coefficient mmol/l per point (% per point) | Variance Explained % | P | β-Coefficient | Variance Explained % | P | ||
| Score LDL + HDL | 4-14 | Absolute | 0.045 | 12.9 | 0.001 | 0.045 | 12.6 | 0.001 |
| (0–18) | Percentage | 2.68 | 11.6 | 0.001 | 2.64 | 11.2 | 0.002 | |
| Score LDL | 2-8 | Absolute | 0.032 | 4.20 | 0.056 | 0.032 | 4.28 | 0.059 |
| (0–10) | Percentage | 1.98 | 4.20 | 0.057 | 1.96 | 4.04 | 0.067 | |
| Score HDL | 0-7 | Absolute | 0.048 | 7.08 | 0.012 | 0.050 | 7.13 | 0.013 |
| (0–8) | Percentage | 2.75 | 5.90 | 0.022 | 2.84 | 6.05 | 0.022 | |
For residuals adjusted for age and blood glucose at randomization and percentage of BMI change during the study period.Bold indicates significant P-value as described in the Method section.
Change of HDL and single SNPs
In the F group, the APOB polymorphism (rs693) showed an association with percentage of HDL increase, with more unfavorable alleles resulting in a more prominent HDL increase (see supplementary Table IIA). Furthermore, there was a significant association between the LPL polymorphism (rs328) and absolute HDL increase, with more unfavorable alleles resulting in a larger HDL increase response to treatment. No single SNP showed any association among men (see supplementary Table IIB). Among women, LPL (rs328) showed an association to absolute and percentage of HDL increase, with more unfavorable alleles resulting in a higher HDL level after statin treatment (see supplementary Table IIC).
Power calculations
As we detected a significant association between HDL change and LDL + HDL score in women, we tested whether we were powered enough to detect similar effects in the smaller group of males. At α 0.05, we had 89% power to detect such an effect in males. As we also detected a significant association between LDL change and score LDL + HDL in women, we performed the same test here. At α 0.05, we had 48% power to detect such an effect in males.
DISCUSSION
The key findings of our study are that a genotype score, recently shown to influence blood levels of LDL, HDL, and CVD risk at the population level (40), is associated with variation in fluvastatin treatment response in women with asymptomatic carotid plaques. The associations between the genotype score and fluvastatin-induced LDL and HDL changes were dependent on gender, as demonstrated by significant interactions between genotype score and gender. Consequently, there was no association between genotype score and fluvastatin response in males.
The strongest association of genotype scores was observed with fluvastatin-induced HDL response, where score LDL + HDL explained up to 12% of the variance of fluvastatin-induced HDL change in women. The proportion of variance of LDL response explained by score LDL + HDL was ∼2% but marginally significant in women only. Although statin treatment primarily affects LDL, the proportion of variance of HDL change explained by score LDL + HDL in women was large. Thus, the genetic association with both HDL and LDL change may be of some clinical importance.
As we hypothesized, introducing the gene score concept in this pharmacogenetic setting seems to be more informative as compared with the study of single gene effects. Although the unfavorable allele of all individual HDL and LDL SNPs had positive point estimates of the β-coefficient in relation to the statin-induced percentage of HDL increase in women, only one of the individual HDL SNPs was significant (LPL rs328 for HDL response). Similarly, all individual LDL SNPs were negatively but nonsignificantly related to percentage of statin-induced LDL decrease among women. Thus, score LDL + HDL seems to be more informative than its individual SNP components regarding the effect of statin treatment, at least in women. However, considering the skew distribution of many of the individual SNPs in this cohort, we were not adequately powered to detect significant associations in many of these analyses. Thus, our results involving single SNPs should be interpreted with great caution.
The association between score LDL + HDL and LDL response in women was clearly driven by the combined effect of the five LDL SNPs (i.e., score LDL), and the directionality of the association showed that the LDL lowering effect of fluvastatin was gradually attenuated with increasing number of LDL elevating alleles, suggesting resistance to fluvastatin treatment in subjects with a high score LDL and score LDL + HDL. However, the P values were modest and did not remain significant after Bonferroni corrections for multiple testing. This weakens the conclusions that can be drawn from the LDL + HDL score on LDL change in women, although the trend is an interesting finding.
The association between score LDL + HDL and HDL response in women seemed to be driven by both the five LDL SNPs and the four HDL SNPs, although more strongly by the four HDL SNPs (score HDL). Here, an increasing number of unfavorable score LDL + HDL and score HDL alleles was associated with a more pronounced statin-induced HDL elevation. The significance remained after Bonferroni corrections for multiple testing were made. The elevation of HDL with more unfavorable alleles (a high score LDL+HDL) could be contrasted to the trend of attenuation of LDL lowering in those subjects. Thus, from a strict clinical point of view, it can be questioned whether the fluvastatin resistance in LDL response or the more beneficial effect of fluvastatin on HDL response, in subjects with a high score LDL + HDL, is the more important and whether the net effect on cardiovascular outcome would be similar as in the population as a whole. As significance after Bonferroni correction for multiple testing remained for the results of HDL only, our study mainly highlights the genetic susceptibility for HDL-elevating properties of fluvastatin in women and suggest this might be the most important finding. However, from a mechanistic point of view, we also find it informative that LDL response is primarily influenced by score LDL, whereas HDL response is mostly influenced by score HDL.
Recently, after score LDL + HDL was originally defined (40), the knowledge of LDL and HDL genetics have greatly advanced with several novel gene discoveries (38). Studies incorporating such novel cholesterol regulating SNPs into extended score models, in order to test if a greater proportion of the variance in statin-induced LDL and HDL response can be explained, are warranted.
There were significant interactions between score LDL + HDL and gender on both LDL response and HDL response, and significant associations between genetic scores and LDL and HDL responses were found in women only. Earlier studies of gender differences in statin-induced changes of lipoproteins are not very abundant. Sakabe et al. (43) found that 3 months atorvastatin treatment lowered small dense LDL more in women than in men. Nakajima (44) noted a greater LDL reduction in women than in men with hypercholesterolemia after 12 months of simvastatin treatment. Fluvastatin pharmacokinetics has not been shown to be different among sexes (45). However, Leitersdorf (46) found a significantly greater HDL increase in women compared with men treated with fluvastatin because of familial hypercholesterolemia.
In our study, there was no significant sex difference in total LDL or HDL hange during the study period; however, the association with score LDL + HDL was clearly dependent on gender. Previous studies on single gene pharmacogenetic associations have suggested that women, but not men, respond with greater HDL increase after statin therapy depending on polymorphisms in genes coding for Estrogen Receptor Alpha (ESR1) and APOA-1 (35), which is in line with our findings that women are more genetically sensitive to HDL response during statin treatment. Pedro-Botet et al. (47), on the other hand, reported a greater magnitude of LDL reduction in men, but not women, depending on the epsilon2 allele of APOE. The lack of evidence regarding effect on clinical outcome following primary prevention with statins among women (10, 48, 49) and the fact that some studies show greater HDL elevating and LDL lowering effects in women compared with men suggests that pharmacogenetic gender differences may be important to take into account in outcome studies of statin therapy involving both sexes. However, we were not adequately powered to detect an association between the magnitude of LDL change and score in men. Thus, these results have to be interpreted with caution. Importantly, the clinical role of the gender-specific response to statins deserves further evaluation. However, although the HDL elevating effect of statins is marginal on the average, statins may be beneficial for improvement of HDL in a subset of women with high score LDL + HDL. Such an effect could be important, considering that a higher HDL level after 3 months of statin treatment has been shown be associated with protection from major cardiovascular events (50) and since novel therapies developed to increase HDL did not lead to an overall benefit on endpoints (51–54).
Our study population consisted of middle-aged and older subjects with asymptomatic carotid plaques. Thus, the population has significant atherosclerosis and is thus relevant to study in this respect as such patients would commonly be subject to statin therapy in clinical practice. Furthermore, considering the generally high prevalence of carotid plaque at these ages (55), the results could be generalized to a large proportion of the population aged over 50 years.
We do acknowledge that our study included a small number of subjects and that our results need to be replicated in a larger cohort before any clinical conclusions can be drawn. Furthermore, from a clinical point of view, the debate on how much the protective effect of statins could be attributed to factors other than their cholesterol-modifying effects (56–60) makes pharmacogenetic studies with hard endpoints as the outcome warranted. In our study, we did not have power to study whether the association between gene score and LDL and/or HDL response is of any relevance for outcome, such as differences in IMT progression or cardiovascular endpoints; however, our data encourage such studies to be performed.
In conclusion, a genotype score based on nine common lipid-linked SNPs was associated with the magnitude of HDL increase achieved after 12 months of fluvastatin treatment in middle-aged women with asymptomatic carotid plaque. The score was also associated with the magnitude of LDL decrease among the same subgroup, but with a marginal significance that did not withstand multiple testing corrections. No associations were seen in men.
This suggests gender differences in genetic susceptibility to statin therapy and implies the possibility of genotyping being used for predicting subjects having the greatest benefit of statin therapy, mainly considering HDL increase. Further studies involving larger cohorts are warranted in order to investigate whether this could have a role in clinical practice.
Footnotes
Abbreviations:
- APO
- apolipoprotein
- BCAPS
- β-blocker Cholesterol-Lowering Asymptomatic Plaque Study
- BMI
- body mass index
- CVD
- cardiovascular disease
- hmgcr
- HMG-CoA reductase
- HRT
- hormone replacement therapy
- IMT
- intima-media thickness
- LDLR
- low density lipoprotein receptor
- MDC-CC
- Malmö Diet and Cancer-Cardiovascular Cohort
- SNP
- single nucleotide polymorphism
This study was supported by grants from the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, the Medical Faculty of Lund University, Malmö University Hospital, the Albert Påhlsson Research Foundation, the Crafoord Foundation, the Ernhold Lundströms Research Foundation, the Region Skane, the Hulda and Conrad Mossfelt Foundation, the King Gustaf V and Queen Victoria Foundation, the Lennart Hanssons Memorial Fund, the Wallenberg Foundation, and National Institutes of Health Grant U01HL-069757. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Lopez A. D., Mathers C. D., Ezzati M., Jamison D. T., Murray C. J. 2006. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 367: 1747–1757. [DOI] [PubMed] [Google Scholar]
- 2.Castelli W. P., Anderson K., Wilson P. W., Levy D. 1992. Lipids and risk of coronary heart disease: the Framingham Study. Ann. Epidemiol. 2: 23–28. [DOI] [PubMed] [Google Scholar]
- 3.Verschuren W. M., Jacobs D. R., Bloemberg B. P., Kromhout D., Menotti A., Aravanis C., Blackburn H., Buzina R., Dontas A. S., Fidanza F. 1995. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 274: 131–136. [PubMed] [Google Scholar]
- 4.Law M. R., Wald N. J. 1994. An ecological study of serum cholesterol and ischaemic heart disease between 1950 and 1990. Eur. J. Clin. Nutr. 48: 305–325. [PubMed] [Google Scholar]
- 5.Rader D. J. 2002. High-density lipoproteins and atherosclerosis. Am. J. Cardiol. 90: 62i–70i. [DOI] [PubMed] [Google Scholar]
- 6.Brunner E. J., Rees K., Ward K., Burke M., Thorogood M. 2007. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 4: CD002128. [DOI] [PubMed] [Google Scholar]
- 7.Edwards J. E., Moore R. A. 2003. Statins in hypercholesterolaemia: a dose-specific meta-analysis of lipid changes in randomised, double blind trials. BMC Fam. Pract. 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law M. R., Wald N. J., Rudnicka A. R. 2003. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 326: 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford I., Murray H., Packard C. J., Shepherd J., Macfarlane P. W., Cobbe S. M.; West of Scotland Coronary Prevention Study Group. 2007. Long-term follow-up of the West of Scotland Coronary Prevention Study. N. Engl. J. Med. 357: 1477–1486. [DOI] [PubMed] [Google Scholar]
- 10.Sever P. S., Dahlöf B., Poulter N. R., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S. E., Kristinsson A., McInnes G. T., et al. ; ASCOT investigators. 2003. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 361: 1149–1158. [DOI] [PubMed] [Google Scholar]
- 11.The Scandinavian Simvastatin Survival Study Group. 1994. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 344: 1383–1389. [PubMed] [Google Scholar]
- 12.The Heart Protection Study Collaborative Group. 2002. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 360: 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studer M., Briel M., Leimenstoll B., Glass T. R., Bucher H. C. 2005. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch. Intern. Med. 165: 725–730. [DOI] [PubMed] [Google Scholar]
- 14.National Heart Lung and Blood Institute. 2001. Detection, evaluation and treatment of high blood cholesterol in adults. (Adult Treatment Panel III). Executive Summary. Accessed August 2, 2008, at http://www.nhlbi.nih.gov/guidelines/cholesterol/atp_iii.htm.
- 15.Rosamond W., Flegal K., Friday G., Furie K., Go A., Greenlund K., Haase N., Ho M., Howard V., Kissela B., et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. 2007. Heart disease and stroke statistics 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 115: e69–e171. [DOI] [PubMed] [Google Scholar]
- 16.Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B., Jr., Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr., Stone N. J.; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 110: 227–239. [DOI] [PubMed] [Google Scholar]
- 17.Benn M., Nordestgaard B. G., Jensen J. S., Grande P., Sillesen H., Tybjaerg-Hansen A. 2005. Polymorphism in APOB associated with increased low-density lipoprotein levels in both genders in the general population. J. Clin. Endocrinol. Metab. 90: 5797–5803. [DOI] [PubMed] [Google Scholar]
- 18.Boright A. P., Connelly P. W., Brunt J. H., Morgan K., Hegele R. A. 1998. Association and linkage of LDLR gene variation with variation in plasma low density lipoprotein cholesterol. J. Hum. Genet. 43: 153–159. [DOI] [PubMed] [Google Scholar]
- 19.Kotowski I. K., Pertsemlidis A., Luke A., Cooper R. S., Vega G. L., Cohen J. C., Hobbs H. H. 2006. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am. J. Hum. Genet. 78: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frikke-Schmidt R., Nordestgaard B. G., Jensen G. B., Tybjaerg-Hansen A. 2004. Genetic variation in ABC transporter A1 contributes to HDL cholesterol in the general population. J. Clin. Invest. 114: 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boekholdt S. M., Sacks F. M., Jukema J. W., Shepherd J., Freeman D. J., McMahon A. D., Cambien F., Nicaud V., de Grooth G. J., Talmud P. J., et al. 2005. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: individual patient meta-analysis of 13,677 subjects. Circulation. 111: 278–287. [DOI] [PubMed] [Google Scholar]
- 22.Wittrup H. H., Andersen R. V., Tybjaerg-Hansen A., Jensen G. B., Nordestgaard B. G. 2006. Combined analysis of six lipoprotein lipase genetic variants on triglycerides, high-density lipoprotein, and ischemic heart disease: cross-sectional, prospective, and case-control studies from the Copenhagen City Heart Study. J. Clin. Endocrinol. Metab. 91: 1438–1445. [DOI] [PubMed] [Google Scholar]
- 23.Kathiresan S., Willer C. J., Peloso G. M., Demissie S., Musunuru K., Schadt E. E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. 2009. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangravite L. M., Wilke R. A., Zhang J., Krauss R. M. 2008. Pharmacogenomics of statin response. Curr. Opin. Mol. Ther. 10: 555–561. [PubMed] [Google Scholar]
- 25.Chasman D. I., Posada D., Subrahmanyan L., Cook N. R., Stanton V. P., Jr., Ridker P. M. 2004. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA. 291: 2821–2827. [DOI] [PubMed] [Google Scholar]
- 26.Kajinami K., Takekoshi N., Brousseau M. E., Schaefer E. J. 2004. Pharmacogenetics of HMG-CoA reductase inhibitors: exploring the potential for genotype-based individualization of coronary heart disease management. Atherosclerosis. 177: 219–234. [DOI] [PubMed] [Google Scholar]
- 27.Ojala J. P., Helve E., Ehnholm C., Aalto-Setälä K., Kontula K. K., Tikkanen M. J. 1991. Effect of apolipoprotein E polymorphism and XbaI polymorphism of apolipoprotein B on response to lovastatin treatment in familial and non-familial hypercholesterolaemia. J. Intern. Med. 230: 397–405. [DOI] [PubMed] [Google Scholar]
- 28.Guzmán E. C., Hirata M. H., Quintão E. C., Hirata R. D. 2000. Association of the apolipoprotein B gene polymorphisms with cholesterol levels and response to fluvastatin in Brazilian individuals with high risk for coronary heart disease. Clin. Chem. Lab. Med. 38: 731–736. [DOI] [PubMed] [Google Scholar]
- 29.Carmena R., Roederer G., Mailloux H., Lussier-Cacan S., Davignon J. 1993. The response to lovastatin treatment in patients with heterozygous familial hypercholesterolemia is modulated by apolipoprotein E polymorphism. Metabolism. 42: 895–901. [DOI] [PubMed] [Google Scholar]
- 30.Lahoz C., Peña R., Mostaza J. M., Jiménez J., Subirats E., Pintó X., Taboada M., López-Pastor A. 2003. Apo A-I promoter polymorphism influences basal HDL-cholesterol and its response to pravastatin therapy. Atherosclerosis. 168: 289–295. [DOI] [PubMed] [Google Scholar]
- 31.Kajinami K., Brousseau M. E., Ordovas J. M., Schaefer E. J. 2004. CYP3A4 genotypes and plasma lipoprotein levels before and after treatment with atorvastatin in primary hypercholesterolemia. Am. J. Cardiol. 93: 104–107. [DOI] [PubMed] [Google Scholar]
- 32.Kivistö K. T., Niemi M., Schaeffeler E., Pitkälä K., Tilvis R., Fromm M. F., Schwab M., Eichelbaum M., Strandberg T. 2004. Lipid-lowering response to statins is affected by CYP3A5 polymorphism. Pharmacogenetics. 14: 523–525. [DOI] [PubMed] [Google Scholar]
- 33.Mulder A. B., van Lijf H. J., Bon M. A., van den Bergh F. A., Touw D. J., Neef C., Vermes I. 2001. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin. Pharmacol. Ther. 70: 546–551. [DOI] [PubMed] [Google Scholar]
- 34.Kajinami K., Brousseau M. E., Ordovas J. M., Schaefer E. J. 2004. Polymorphisms in the multidrug resistance-1 (MDR1) gene influence the response to atorvastatin treatment in a gender-specific manner. Am. J. Cardiol. 93: 1046–1050. [DOI] [PubMed] [Google Scholar]
- 35.Kajinami K., Brousseau M. E., Lamon-Fava S., Ordovas J. M., Schaefer E. J. 2005. Gender-specific effects of estrogen receptor alpha gene haplotype on high-density lipoprotein cholesterol response to atorvastatin: interaction with apolipoprotein AI gene polymorphism. Atherosclerosis. 178: 331–338. [DOI] [PubMed] [Google Scholar]
- 36.Marian A. J., Safavi F., Ferlic L., Dunn J. K., Gotto A. M., Ballantyne C. M. 2000. Interactions between angiotensin-I converting enzyme insertion/deletion polymorphism and response of plasma lipids and coronary atherosclerosis to treatment with fluvastatin: the Lipoprotein and Coronary Atherosclerosis Study. J. Am. Coll. Cardiol. 35: 89–95. [DOI] [PubMed] [Google Scholar]
- 37.Basso F., Lowe G. D., Rumley A., McMahon A. D., Humphries S. E. 2002. Interleukin-6-174G>C polymorphism and risk of coronary heart disease in West of Scotland coronary prevention study (WOSCOPS). Arterioscler. Thromb. Vasc. Biol. 22: 599–604. [DOI] [PubMed] [Google Scholar]
- 38.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berglund G., Elmstähl S., Janzon L., Larsson S. A. 1993. The Malmo Diet and Cancer Study: design and feasibility. J. Intern. Med. 233: 45–51. [DOI] [PubMed] [Google Scholar]
- 40.Kathiresan S., Melander O., Anevski D., Guiducci C., Burtt N. P., Roos C., Hirschhorn J. N., Berglund G., Hedblad B., Groop L., et al. 2008. Polymorphisms associated with cholesterol and risk of cardiovascular events. N. Engl. J. Med. 358: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 41.Hedblad B., Wikstrand J., Janzon L., Wedel H., Berglund G. 2001. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: Main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS). Circulation. 103: 1721–1726. [DOI] [PubMed] [Google Scholar]
- 42.Lehtonen A. 1985. Effect of beta blockers on blood lipid profile. Am. Heart J. 109: 1192–1196. [DOI] [PubMed] [Google Scholar]
- 43.Sakabe K., Fukuda N., Fukuda Y., Wakayama K., Nada T., Morishita S., Shinohara H., Tamura Y. 2008. Gender differences in short-term effects of atorvastatin on lipid profile, fibrinolytic parameters, and endothelial function. Nutr. Metab. Cardiovasc. Dis. 18: 182–188. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima K. 1999. Sex-related differences in response of plasma lipids to simvastatin: the Saitama Postmenopausal Lipid Intervention Study. S-POLIS Group. Clin. Ther. 21: 2047–2057. [DOI] [PubMed] [Google Scholar]
- 45.Scripture C. D., Pieper J. A. 2001. Clinical pharmacokinetics of fluvastatin. Clin. Pharmacokinet. 40: 263–281. [DOI] [PubMed] [Google Scholar]
- 46.Leitersdorf E. 1994. Gender-related response to fluvastatin in patients with heterozygous familial hypercholesterolaemia. Drugs. 47(Suppl 2): 54–58. [DOI] [PubMed] [Google Scholar]
- 47.Pedro-Botet J., Schaefer E. J., Bakker-Arkema R. G., Black D. M., Stein E. M., Corella D., Ordovas J. M. 2001. Apolipoprotein E genotype affects plasma lipid response to atorvastatin in a gender specific manner. Atherosclerosis. 158: 183–193. [DOI] [PubMed] [Google Scholar]
- 48.Downs J. R., Clearfield M., Weis S., Whitney E., Shapiro D. R., Beere P. A., Langendorfer A., Stein E. A., Kruyer W., Gotto A. M., Jr 1998. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 279: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 49.Petretta M., Costanzo P., Perrone-Filardi P., Chiariello M. 2010. Impact of gender in primary prevention of coronary heart disease with statin therapy: a meta-analysis. Int. J. Cardiol. 138: 25–31. [DOI] [PubMed] [Google Scholar]
- 50.Barter P., Gotto A. M., LaRosa J. C., Maroni J., Szarek M., Grundy S. M., Kastelein J. J., Bittner V., Fruchart J. C.; Treating to New Targets Investigators. 2007. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357: 1301–1310. [DOI] [PubMed] [Google Scholar]
- 51.de Haan W., de Vries-van der Weij J., van der Hoorn J. W., Gautier T., van der Hoogt C. C., Westerterp M., Romijn J. A., Jukema J. W., Havekes L. M., Princen H. M., et al. 2008. Torcetrapib does not reduce atherosclerosis beyond atorvastatin and induces more proinflammatory lesions than atorvastatin. Circulation. 117: 2515–2522. [DOI] [PubMed] [Google Scholar]
- 52.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. ; ILLUMINATE Investigators. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 53.Nissen S. E., Tardif J. C., Nicholls S. J., Revkin J. H., Shear C. L., Duggan W. T., Ruzyllo W., Bachinsky W. B., Lasala G. P., Tuzcu E. M.; ILLUSTRATE Investigators. 2007. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356: 1304–1316. [DOI] [PubMed] [Google Scholar]
- 54.Kastelein J. J., van Leuven S. I., Burgess L., Evans G. W., Kuivenhoven J. A., Barter P. J., Revkin J. H., Grobbee D. E., Riley W. A., Shear C. L., W, et al. ; RADIANCE 1 Investigators. 2007. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N. Engl. J. Med. 356: 1620–1630. [DOI] [PubMed] [Google Scholar]
- 55.Rosvall M., Janzon L., Berglund G., Engström G., Hedblad B. 2005. Incident coronary events and case fatality in relation to common carotid intima- media thickness. J. Intern. Med. 257: 430–437. [DOI] [PubMed] [Google Scholar]
- 56.Howard-Alpe G., Foëx P., Biccard B. 2008. Cardiovascular protection by anti-inflammatory statin therapy. Best Pract. Res. Clin. Anaesthesiol. 22: 111–133. [DOI] [PubMed] [Google Scholar]
- 57.Schönbeck U., Libby P. 2004. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 109 (21 Suppl. 1): 18–26. [DOI] [PubMed] [Google Scholar]
- 58.Ray K. K., Cannon C. P., McCabe C. H., Cairns R., Tonkin A. M., Sacks F. M., Jackson G., Braunwald E. 2005. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: results from the PROVE ITTIMI 22 trial. J. Am. Coll. Cardiol. 46: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 59.Albert M. A., Danielson E., Rifai N., Ridker P. M. 2001. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 286: 64–70. [DOI] [PubMed] [Google Scholar]
- 60.Horvath B., Marton Z., Alexy T., Kesmarky G., Toth K., Szapary L. 2004. Short-term effects of atorvastatin on haemorheologic parameters, platelet aggregation and endothelium dysfunction in patients with hypercholesterolaemia. Eur. Heart J. 25: 96. [DOI] [PubMed] [Google Scholar]