Abstract
A convenient method using commercial aqueous concentrated HCl (conc. HCl; 35%, w/w) as an acid catalyst was developed for preparation of fatty acid methyl esters (FAMEs) from sterol esters, triacylglycerols, phospholipids, and FFAs for gas-liquid chromatography (GC). An 8% (w/v) solution of HCl in methanol/water (85:15, v/v) was prepared by diluting 9.7 ml of conc. HCl with 41.5 ml of methanol. Toluene (0.2 ml), methanol (1.5 ml), and the 8% HCl solution (0.3 ml) were added sequentially to the lipid sample. The final HCl concentration was 1.2% (w/v). This solution (2 ml) was incubated at 45°C overnight or heated at 100°C for 1–1.5 h. The amount of FFA formed in the presence of water derived from conc. HCl was estimated to be <1.4%. The yields of FAMEs were >96% for the above lipid classes and were the same as or better than those obtained by saponification/methylation or by acid-catalyzed methanolysis/methylation using commercial anhydrous HCl/methanol. The method developed here could be successfully applied to fatty acid analysis of various lipid samples, including fish oils, vegetable oils, and blood lipids by GC.
Keywords: fatty acid composition, methanolysis, methylation, triacylglycerols, sterol esters, phospholipids, fish oils, vegetable oils, blood lipids
Fatty acids are the major component of lipids, and the physical, chemical, and physiological properties of a lipid class depend primarily on its fatty acid composition. The fatty acid composition is determined as the methyl esters of fatty acids by gas-liquid chromatography (GC) (1–3). Saponification followed by methylation is a classical method for preparation of fatty acid methyl esters (FAMEs) from glycerolipids and sterol esters (SEs). Conventionally, FAMEs are prepared by base- or acid-catalyzed esterification. Base-catalyzed methanolysis proceeds much more rapidly under mild temperature conditions than acid-catalyzed reactions (3, 4), and KOH- or NaOH-catalyzed methanolysis completes within 2 min at room temperature for glycerolipids (5, 6) and within 1 h at 37°C for SE (7). However, bases cannot catalyze the esterification of FFAs. BF3 is a commonly used acid catalyst for methylation and methanolysis (8), but it is harmful, and boron and fluorine are also both restricted by local drainage laws. In addition, the methanolic BF3 reagent has a limited shelf life (9). H2SO4 is also an effective acid catalyst for FAME synthesis, but it is a very corrosive viscous liquid and must be handled with care. When the acid catalysts, BF3 and H2SO4 are used at high concentrations or at high temperatures, artifacts derived from fatty acids can be produced (1–3, 10, 11).
HCl is most widely used as an acid catalyst because it is a relatively mild reagent and gives almost quantitative yields (1–3). Anhydrous methanolic HCl can be prepared from acetyl chloride and methanol (2–4, 9), but the acid chloride is volatile (bp 52°C) and is an extreme irritant to the eyes. It also reacts violently with methanol. Many researchers purchase commercial expensive anhydrous methanolic HCl reagents. However, anhydrous methanolic HCl is unstable, and HCl reacts nucleophilically with methanol to produce chloromethane and water (12); consequently, the concentrations of HCl in commercial anhydrous HCl/methanol reagents decrease during storage. Commercial products of methanolic HCl contain considerable amounts of water that are probably formed during storage or are a contaminant introduced in the process of production.
In this study, we have developed a convenient reagent for preparation of FAMEs from acyl lipids, including SEs, triacylglycerols (TGs), phospholipids (PhLs), and FFAs. The reagent is composed of commercial concentrated HCl (conc. HCl), methanol, and toluene and is superior to other reagents in terms of convenience, safety, and cost. Here, we propose two procedures for derivatization of fatty acyl residues with the reagent: one is for mild reaction, and the other is for rapid reaction. All types of fatty acids with O-ester linkages and FFAs were converted almost quantitatively into the corresponding methyl esters in one-step reactions.
MATERIALS AND METHODS
Reagents
Fatty acids, TG, and cholesterol esters were purchased from Avanti Polar Lipids (Alabaster, AL), Matreya (Pleasant Gap, PA), Nu-Chek-Prep (Elysian, MN), and Sigma-Aldrich (St. Louis, MO). Phosphatidylcholine (PC; dioleoyl) was synthesized according to Ref. 13. Glass-distilled solvents were purchased from Sigma-Aldrich and Wako Pure Chemical Industries (Osaka, Japan). Anhydrous methanolic HCl reagents, HCl (35%, w/w), acetyl chloride, methyl acetate, and 50% BF3 in methanol were of reagent grade.
TLC and GC
Reaction products of methanolysis/methylation were analyzed by TLC on silica gel. Lipids separated were visualized by spraying 50% (w/w) sulfuric acid and then heating at 135°C. FAMEs were analyzed with a Shimadzu 2014 gas chromatograph equipped with a column of SUPELCOWAX 10 (0.53 mm × 30 m) at a column temperature of 215°C or 225°C.
Preparation of FAMEs
Lipid substrates and FAME products.
FAMEs were prepared from 1 mg of cholesteryl oleate, 1 mg of glyceryl trioleate, 1 mg of dioleoyl PC, 1 mg of oleic acid, 0.05 mg of cis-9,10-methyleneocatadecanoic acid (a cyclopropane fatty acid), 0.05 mg of conjugated linoleic acids that were mainly composed of cis-9,trans-11 and trans-10,cis-12 isomers, 1 mg of olive oil, 1 mg of soybean oil, 1 mg of linseed oil, 0.5 mg of fish oil (Pacific saury), 0.3 mg of blood lipids that had been extracted by the method of Bligh and Dyer (14) in the presence of 0.05% (w/v) 2,6-di-tert-butyl-p-cresol as an antioxidant, and 0.025 ml of whole blood. The formation of FAMEs was mainly investigated with cholesteryl oleate because SEs are the most resistant to transesterification of lipid classes having ester linkages (7). The internal standard was methyl heptadecanoate or methyl tricosanoate. FAMEs formed from biological materials were purified on cartridge columns packed with 200 mg of silica gel (No.102021; Merck, Darmstadt, Germany) prior to GC. The silica gel cartridge was conditioned with 3 ml of hexane, charged with FAMEs dissolved in 1 ml of hexane, and washed with 3 ml of hexane. FAMEs were eluted with 3 ml of 1.5% (v/v) methyl acetate in hexane.
Conventional method 1: saponification followed by BF3-catalyzed methylation (Sap/BF3).
A lipid sample in a screw-capped glass tube (16.5 × 105 mm) was hydrolyzed with 1 ml of 1 M KOH in 70% ethanol at 90°C for 1 h. The reaction mixture was acidified with 0.2 ml of 6 M HCl, and then 1 ml of water was added. FFAs released were extracted with 1 ml of hexane. After evaporation of the hexane in vacuo, the FFAs were methylated with 1 ml of 10% BF3 in methanol at 37°C for 20 min. Water was added to the solution, and then FAMEs were extracted with 1 ml of hexane.
Conventional method 2: anhydrous methanolic HCl method.
HCl concentrations of commercial anhydrous solutions of HCl/methanol were 5% (w/v) according to the manufacturers' specifications, but the precise concentrations determined by titration were 2.3–3.2%. Karl-Fischer titration indicated that some of these commercial reagents contained 1–4% water. Anhydrous methanolic 5% (w/v) HCl was also prepared by mixing acetyl chloride with methanol (3, 9). To a lipid sample in a screw-capped glass test tube was added 2.0 ml of anhydrous methanolic HCl, and the mixture was heated at 100°C for 1 h in a boiling water bath. After cooling, 1 ml of water was added, and then FAMEs were extracted with 1 ml of hexane.
Mild methanolysis/methylation and rapid methanolysis/methylation using conc. HCl.
Commercial conc. HCl (35%, w/w; 9.7 ml) was diluted with 41.5 ml of methanol to make 50 ml of 8.0% (w/v) HCl. This HCl reagent contained 85% (v/v) methanol and 15% (v/v) water that was derived from conc. HCl and was stored in a refrigerator.
A lipid sample was placed in a screw-capped glass test tube (16.5 × 105 mm) and dissolved in 0.20 ml of toluene. To the lipid solution, 1.50 ml of methanol and 0.30 ml of the 8.0% HCl solution were added in this order. The final HCl concentration was 1.2% (w/v) or 0.39 M, which corresponded to 0.06 ml of conc. HCl in a total volume of 2 ml. Addition of mixed 1.2% HCl/methanol/toluene solution to SE or TG samples should be avoided because of the low solubilities of these lipids in water-containing methanol. The tube was vortexed and then incubated at 45°C overnight (14 h or longer) for mild methanolysis/methylation or heated at 100°C for 1 h for rapid reaction. The reaction time at 100°C was extended up to 1.5 h for samples containing SE. After cooling to room temperature, 1 ml of hexane and 1 ml of water were added for extraction of FAMEs. The tube was vortexed, and then the hexane layer was analyzed by GC directly or after purification through a silica gel column.
Preparation of FAMEs from blood on a micro scale.
FAMEs were also prepared from one drop of whole blood spotted on filter paper with 1.2% HCl/methanol/toluene under the mild conditions. The procedure was a modification of a KOH/methanol method (6), which had been developed for fatty acid analysis of blood glycerolipids. Blood (0.025 ml) was spotted onto a small piece of filter paper (1.5 × 1.5 cm; Whatman 3MM) that had been washed with acetone containing 0.05% 2,6-di-tert-butyl-p-cresol. Each piece, once it had dried, was put in a screw-capped test tube, to which 0.2 ml of toluene, 1.5 ml of methanol, and 0.3 ml of the 8% HCl reagent were added sequentially and then incubated at 45°C overnight. To the reaction mixture were added 1 ml of hexane and 0.2 ml of water, and the tube was vortexed. FAMEs in the hexane layer were purified through a silica gel cartridge column.
RESULTS AND DISCUSSION
Mild methanolysis/methylation at 45degC
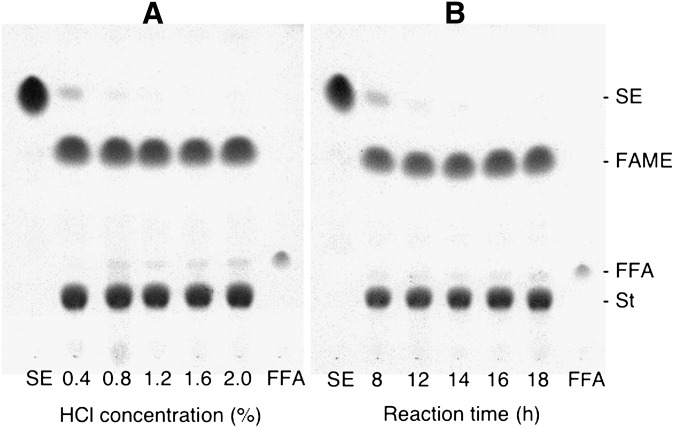
Reaction conditions were investigated for mild methanolysis and methylation. Figure 1A shows that the methanolysis of cholesteryl oleate at 45°C for 16 h proceeded almost quantitatively in the presence of 1.2–1.6% HCl, which corresponded to 0.06–0.08 ml of conc. HCl in a total volume of 2 ml. The HCl concentration of 2.0% increased the amount of cholesteryl oleate that did not undergo methanolysis, presumably by lowering the solubility of the substrate. The formation of FFA was stimulated as the concentration of HCl increased. This would be due to an increase in water content. In the presence of 1.2% HCl, cholesteryl oleate required reaction times longer than 14 h for completion of methanolysis at 45°C (Fig. 1B).
Fig. 1.
A: Effects of the concentration of HCl on methanolysis of cholesteryl oleate. The reaction mixtures were incubated at 45°C for 16 h. B: Dependence of methanolysis of cholesteryl oleate on reaction time. Reaction mixtures containing 1.2% (w/v) HCl were incubated at 45°C. Reaction products were analyzed by double development of TLC. The silica gel plate was developed to 2.5 cm from the origin with hexane/tert-butyl methyl ether/acetic acid (50:50:0.5, v/v/v), dried in vacuo, and redeveloped to 8 cm from the origin with hexane/tert-butyl methyl ether/acetic acid (97:3:0.5, v/v/v). St, sterol.
Under conditions of 45°C/14 h, methanolysis of TG proceeded at the lower HCl concentration of 0.6% (see supplementary Fig. IA). The lower concentration of HCl means a lower water content of the reaction medium and resulted in a decrease in FFA formed by hydrolysis. TG and PhL were converted into FAME within 8 h at 45°C in 1.2% HCl (see supplementary Figs. IB and IIA)
In contrast to methanolysis of esters, methylation of FFA occurs very rapidly. The formation of FAME from FFA was completed within 20 min in 1.2% HCl and continued at much lower concentrations of HCl (see supplementary Figs. IIB and III). The best reaction conditions for methylation of FFA were a reaction temperature of 45°C, reaction time of 60 min, and HCl concentration of 0.2%. The alternative conditions 45°C/20 min/0.5% HCl also gave good efficiency of methylation. The reactions of glycerolipids and FFA were thus more rapid than that of SE, and the order of reactivities of acyl lipids with HCl/methanol was FFA >> PhL = TG > SE.
The reaction conditions 0.6% HCl/45°C/14 h or 1.2% HCl/45°C/8 h gave good yields of FAME for lipid samples composed of only glycerolipids and FFA, while the reaction conditions 1.2% HCl/45°C/16 h are recommended for lipid samples containing SE.
Cyclopropane fatty acids are heat- and acid-labile components of bacteria and cottonseed oil; hence, their derivatization to methyl esters had been carried out by base-catalyzed methanolysis or Sap/BF3 (15). A cyclopropane fatty acid, cis-9,10-methyleneoctadecanoic acid, was recovered almost quantitatively as the methyl ester with trace amounts of artifacts under mild temperature conditions of 45°C (1.2% HCl/14 h), under which glycerolipids can be converted into FAME (see supplementary Fig. IV). Conjugated linoleic acids are unstable under acidic conditions, and synthesis of their methyl esters resulted in the formation of 5% artifacts even under the mild conditions of 1.2% HCl/45°C/14 h. However, no artifacts were detected in FAME prepared by the combination of alkali-catalyzed methanolysis at room temperature or at 37°C (5, 7) and acid-catalyzed methylation for 20 min (0.5% HCl/45°C) as suggested in a previous work (16).
Rapid methanolysis/methylation at 100degC
Methyl oleate was formed from cholesteryl oleate when SE was heated at 100°C for 90 min with 1.2% HCl in methanol and toluene, but two major artifacts were produced under elevated temperature conditions (see supplementary Fig. V). These artifacts were probably due to 3,5-cholestadiene and cholesteryl methyl ether (17, 18), and the latter artifact could not be separated from FAME by pretreatment with a silica gel column for GC. Taking into account the lifespan of GC columns, an overnight reaction at 45°C is recommended for blood lipids that contain cholesterol and its esters.
Methanolysis of TG at 100°C produced no artifacts except FFA (see supplementary Fig. VI). The reaction time required for methanolysis of TG was 30 min with 1.2% HCl or 90 min with 0.6% HCl, in which the amount of FFA released was smaller than that for 1.2% HCl. The reaction times required for methanolysis of PC and methylation of FFA were shorter than 15 min in the presence of 1.2% HCl, and methylation of oleic acid with 0.2% HCl was completed within 5 min at 100°C.
Reaction conditions of 1.2% HCl/100°C/90 min are thus required for lipid samples containing SE, while 30 min is a sufficient reaction time to give good yields of FAME for lipid samples not containing SE.
Water contents and solubilities of lipids as factors affecting FAME formation
Because the reagent contains water derived from aqueous conc. HCl as a component and the acid-catalyzed methanolysis is a reversible reaction, the formation of FFA is inevitable, as seen in Fig. 1. To assess the rate of hydrolysis of FAME formed, methyl oleate was incubated in methanol containing 1.0% HCl and water at 100°C for 1 h. Hydrolysis of FAME was a function of water content, and the percentage of remaining methyl oleate decreased with increasing water content (see supplementary Fig. VII). The 2 ml reaction mixture of 1.2% HCl contains 0.044 ml of water, i.e., 2.2% water derived from conc. HCl, and it was estimated that not more than 1.4% of FAME produced can be hydrolyzed during methanolysis/methylation. These findings indicate that the presence of water inevitably promotes the hydrolysis of FAME formed but does not significantly affect the yield of FAME. Ulberth and Henninger (19) reported that the formation of FAME was not hindered by addition of up to 2% water in a reaction mixture, and a similar observation that 5% water was acceptable for methanolysis was also reported by Lepage and Roy (20). Some of commercial methanolic 4.6–5% HCl reagents contained more than 1% water, and their HCl concentrations were 2.3–3.2% at the time of purchase. It must be kept in mind that HCl reacts with methanol to form chloromethane and water. Half the HCl in methanol is lost within 1.5 months at room temperature, and a considerable amount of HCl is lost during methanolysis at 100°C (12).
The presence of water also gives rise to hydrolysis of ester bonds in SE, TG, and PhL, and it is presumed that FAMEs are synthesized through either transesterification pathway, i.e., methanolysis (reaction A) or hydrolysis/methylation (reaction B), in methanolic HCl solutions that are not absolutely anhydrous. FFA liberated by hydrolysis should be immediately methylated, as acid-catalyzed methylation of FFA occurs rapidly. Progress of reaction from PC to FAME in 1.2% HCl/methanol was accompanied by a change in the amount of FFA liberated, which suggests that the hydrolysis/methylation pathway operates to some extent. Trace amounts of water may therefore stimulate the formation of FAME and may not necessarily be a negative factor with respect to the rate of FAME production.
Methanol is a poor solvent for SE and TG, and the solubilities of these nonpolar lipids in methanol further decreased with increases in the concentration of water (see supplementary Fig. VIII). Glyceryl trioleate dissolved at a concentration of 3.3 mg in 2 ml of methanol at 25°C, but in the presence of 2.2% water, the solubility was 1.3 mg/ 2 ml. Addition of 10% toluene to water-containing methanol doubled the solubility of TG in the solution. The solubility of glyceryl trioleate in 2 ml of methanol solution containing 0.044 ml of water and 0.2 ml of toluene is equivalent to that of methanol containing 0.008 ml of water. Methanol containing both 2.0% water and 10% toluene is almost equivalent to anhydrous methanol with respect to the solubility of glyceryl trioleate. In the absence of toluene, methanolysis of SE and TG was slow, and considerable amounts of these lipids remained unchanged. The adverse effect of water derived from conc. HCl on the solubilities of hydrophobic lipids was thus appreciably diminished by addition of 10% toluene. The reaction of PhL or FFA did not require the addition of toluene.
Yields of FAME and limits of lipid amounts to be treated
Reaction conditions were thoroughly investigated for methanolysis/methylation of four lipid classes, i.e., SE, TG, PhL, and FFA. Table 1 shows the most appropriate conditions for mild reaction at 45°C and rapid reaction at 100°C, and yields of FAME. FAMEs were synthesized in yields >96% for these four lipid classes by the mild and rapid reactions. Table 1 indicates that lipid samples containing SE, such as blood lipids, should be treated at 45°C for 16 h or at 100°C for 90 min in 1.2% HCl/methanol/toluene and that lipid samples not containing SE, such as vegetable oils, can be derivatized at 1.2% or lower concentrations of HCl and with shorter reaction times.
TABLE 1.
Optimized conditions for preparation of FAMEs from individual lipid classes with conc. HCl/methanol/toluene
| Lipid Class | HCl Conc. | Time | Yield of FAMEa |
|---|---|---|---|
| Mild methanolysis/methylation at 45°C | |||
| % | % | ||
| SE | 1.2 | 16 h | 98.2 ± 0.7 |
| TG | 1.2 | 8 h | 98.8 ± 0.7 |
| 0.6 | 14 h | 97.8 ± 0.2 | |
| PhL | 1.2 | 8 h | 96.7 ± 0.2b |
| FFA | 0.5 | 20 min | 98.4 ± 0.1c |
| 0.2 | 60 min | 99.1 ± 0.2 | |
| Rapid methanolysis/methylation at 100°C | |||
| % | % | ||
| SE | 1.2 | 90 min | 96.8 ± 0.3d,e |
| TG | 1.2 | 30 min | 97.3 ± 0.3e |
| PhL | 1.2 | 30 min | 96.5 ± 0.5 |
| FFA | 0.2 | 5 min | 98.4 ± 0.3 |
Molecular species of lipid classes analyzed were cholesteryl oleate as SE, glyceryl trioleate as TG, dioleoyl PC as PhL, and oleic acid as FFA.
After reaction of each lipid class, a hexane solution of methyl heptadecanoate as an internal standard and water were added to the reaction mixture. The yield of FAME in the hexane layer was determined by GC. Each value of yield is the average of three determinations.
Methanolysis of PhL with 0.6% HCl for 14 h at 45°C also gave a similar yield.
A similar yield, 97.9%, was obtained with 1.2% HCl.
Artifacts were produced from cholesterol. A shorter reaction time, 60 min, gave a yield of 96.5%.
For comparison, anhydrous methanolic 5% HCl was prepared prior to use by mixing 2 ml of acetyl chloride and 20 ml of chilled methanol. SE and TG were each dissolved in toluene and heated at 100°C for 90 and 60 min, respectively, with the anhydrous reagent. The HCl concentration of the reaction mixture was 4.5%. The yields of FAME were 99.0 ± 0.8% for SE and 99.6 ± 0.1% for TG.
In comparison to base-catalyzed methanolysis (5–7), acid-catalyzed methanolysis has two major intrinsic disadvantages, i.e., low capacity for amounts of lipids to be treated and slow reaction rate. For single lipid classes, 1 mg of SE or 2 mg of TG could be thoroughly transesterified in 2 ml of the 1.2% HCl reagent used in the present method, and these amounts, 1 and 2 mg, were the limits for complete methanolysis of SE and TG, respectively. In methylation of FFA, at least 20 mg could be converted into FAME.
Application for blood lipids, vegetable oils, and fish oils
The accuracy and validity of the present methanolysis/methylation method using conc. HCl were confirmed with a variety of lipid samples. The mild 45°C/16 h conditions were used for blood lipids that contain considerable amounts of SE, and FAMEs were prepared from one drop of blood spotted onto a small piece of filter paper. SE, TG, FFA, PC, and phosphatidylethanolamine were all efficiently converted to FAMEs, which were purified to a single spot on TLC through a silica gel cartridge column for GC (Fig. 2). For vegetable oils rich in TG, FAMEs were prepared by the rapid methanolysis at 100°C. Fish oils have many polyunsaturated fatty acid species in TG, and they are subject to autoxidation. FAMEs were prepared by both the mild and rapid procedures. No obvious differences in fatty acid composition were found between the present method using conc. HCl and conventional methods, and also between 45°C/16 h and 100°C/90 min of the present method for the lipid samples tested. Table 2 shows fish oil fatty acid compositions obtained by different methods (for blood fatty acid compositions, see supplementary Table I; for GC of FAMEs prepared from fish oil under the mild conditions, see supplementary Fig. IX).
Fig. 2.
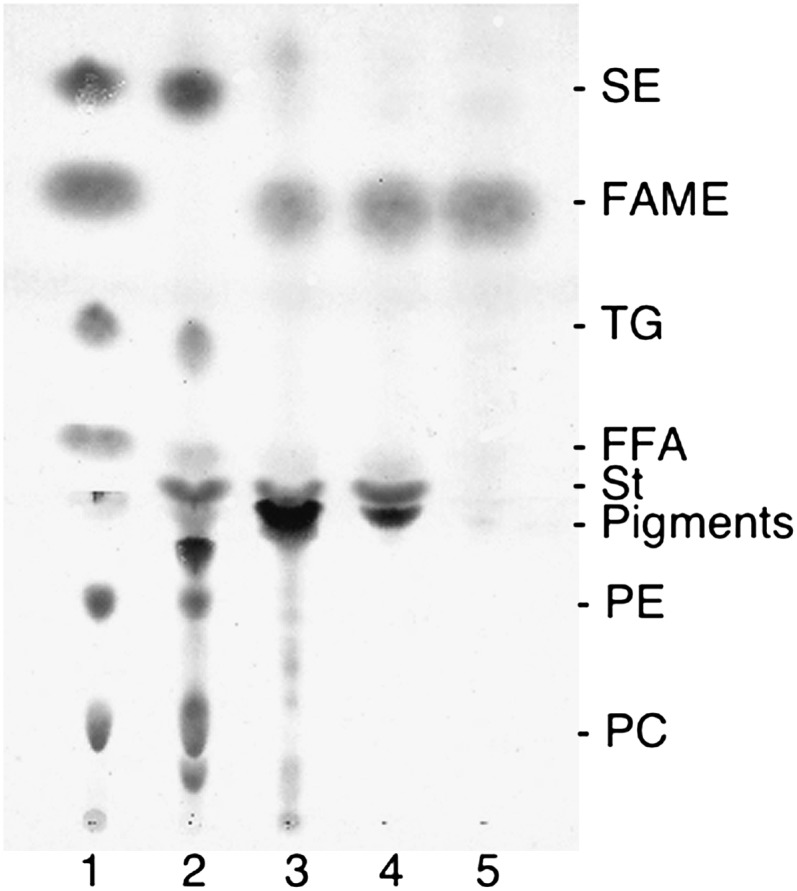
Formation of FAMEs from one drop of whole blood on filter paper and purification of FAMEs formed. Lane 1, a standard mixture of SE, FAME, TG, FFA, phosphatidylethanolamine, and PC; lane 2, blood lipids; lane 3, chloroform extract after reaction; lane 4, hexane extract after reaction; lane 5, eluate of 1.5% methyl acetate/hexane from a cartridge column of silica gel that adsorbed the hexane extract. The TLC plate was first developed with chloroform/methanol/water/acetic acid (65:35:4:1, v/v/v/v) to 3.5 cm from the origin, dried in vacuo, and then redeveloped with hexane/tert-butyl methyl ether/acetic acid (90:10:0.5, v/v/v) to the top of the plate. St, sterol.
TABLE 2.
Comparison of fish oil fatty acid compositions determined by a conventional Sap/BF3 method, by a conventional anhydrous methanolic HCl method, and by the conc. HCl/methanol/toluene method
| Conventional Method |
Present Method (1.2% HCl) |
|||
|---|---|---|---|---|
| Fatty Acida | Sap/BF3 | Anhyd. HCl | 45°C/16 h | 100°C/1 h |
| % | % | |||
| 14:0 | 7.9 ± 0.0 | 7.8 ± 0.2 | 7.6 ± 0.1 | 7.5 ± 0.1 |
| 16:0 | 12.5 ± 0.0 | 12.2 ± 0.2 | 11.8 ± 0.1 | 12.0 ± 0.0 |
| 16:1 | 6.6 ± 0.0 | 6.6 ± 0.1 | 6.3 ± 0.1 | 6.5 ± 0.0 |
| 18:0 | 2.0 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 |
| 18:1 | 6.0 ± 0.0 | 5.8 ± 0.1 | 5.8 ± 0.1 | 5.8 ± 0.0 |
| 18:2 n-6 | 1.5 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 |
| 18:3 n-3 | 1.4 ± 0.1 | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.3 ± 0.0 |
| 18:4 n-3 | 4.5 ± 0.0 | 4.8 ± 0.1 | 4.7 ± 0.1 | 4.8 ± 0.0 |
| 20:1 | 15.1 ± 0.1 | 14.6 ± 0.0 | 14.7 ± 0.1 | 14.7 ± 0.1 |
| 20:4 n-6 | 0.6 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 20:4 n-3 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.0 | 1.1 ± 0.0 |
| 20:5 n-3 | 9.2 ± 0.1 | 10.1 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.1 |
| 22:1 | 20.3 ± 0.3 | 19.5 ± 0.4 | 20.4 ± 0.5 | 19.8 ± 0.1 |
| 21:5 n-3 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.1 |
| 22:5 n-3 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.0 |
| 22:6 n-3 | 9.4 ± 0.1 | 10.4 ± 0.2 | 10.4 ± 0.2 | 10.5 ± 0.2 |
Each value is the average of three determinations.
Fatty acids are designated by number of carbon atoms:number of double bonds.
Concluding remarks
The mixed solution of conc. HCl, methanol, and toluene is a convenient reagent for methanolysis and methylation of acyl lipids. The reagent can be readily prepared in laboratories from common chemicals. Yields of FAME prepared and fatty acid compositions obtained using this reagent are almost identical to those determined with other conventional reagents of acid or base catalysts. Anhydrous HCl/methanol reagents for methanolysis and methylation of acyl lipids can thus be replaced by conc. HCl/methanol/toluene, which can also replace alkaline reagents such as sodium methoxide/methanol or KOH/methanol for lipid samples of milligram order.
Supplementary Material
Acknowledgments
The authors thank Dr. Kouhei Yamamoto (Osaka Prefecture University) for his practical advice on GC analysis.
Footnotes
Abbreviations:
- FAME
- fatty acid methyl ester
- GC
- gas-liquid chromatography
- PC
- phosphatidylcholine
- PhL
- phospholipid
- Sap/BF3
- saponification followed by BF3-catalyzed methylation
- SE
- sterol ester
- TG
- triacylglycerol
The online version of this article (available at http:www.jlr.org) contains supplementary data in the form of one table and nine figures.
REFERENCES
- 1.Sheppard A. J., Iverson J. L. 1975. Esterification of fatty acids for gas-liquid chromatographic analysis. J. Chromatogr. Sci. 13: 448–452. [DOI] [PubMed] [Google Scholar]
- 2.Liu K-S. 1994. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. J. Am. Oil Chem. Soc. 71: 1179–1187. [Google Scholar]
- 3.Christie W. W. 2003. Lipid Analysis. 3rd edition The Oily Press, Bridgwater, UK. [Google Scholar]
- 4.Carrapiso A. I., Garcia C. 2000. Development in lipid analysis: some new extraction techniques and in situ transesterification. Lipids. 35: 1167–1177. [DOI] [PubMed] [Google Scholar]
- 5.Ichihara K., Shibahara A., Yamamoto K., Nakayama T. 1996. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 31: 535–539. [Erratum. 1996. Lipids31: 889]. [DOI] [PubMed] [Google Scholar]
- 6.Ichihara K., Waku K., Yamaguchi C., Saito K., Shibahara A., Miyatani S., Yamamoto K. 2002. A convenient method for determination of the C20–22 PUFA composition of glycerolipids in blood and breast milk. Lipids. 37: 523–526. [DOI] [PubMed] [Google Scholar]
- 7.Ichihara K., Yamaguchi C., Nishijima H., Saito K. 2003. Preparation of FAME from sterol esters. J. Am. Oil Chem. Soc. 80: 833–834. [Google Scholar]
- 8.Ackman R. G. 1998. Remarks on official methods employing boron trifluoride in the preparation of methyl esters of the fatty acids of fish oils. J. Am. Oil Chem. Soc. 75: 541–545. [Google Scholar]
- 9.Christie W. W. 1992. Preparation of fatty acid methyl esters. Inform. 3: 1031–1034. [Google Scholar]
- 10.Hansen R. P., Smith J. F. 1966. The occurrence of methyl methoxystearate isomers in the methyl esters prepared from sheep perinephric fat. Lipids. 1: 316–321. [DOI] [PubMed] [Google Scholar]
- 11.Klopfenstein W. E. 1971. On methylation of unsaturated acids using boron trihalide-methanol reagents. J. Lipid Res. 12: 773–776. [PubMed] [Google Scholar]
- 12.Kishimoto Y., Radin N. S. 1965. A reaction tube for methanolysis; instability of hydrogen chloride in methanol. J. Lipid Res. 6: 435–436. [PubMed] [Google Scholar]
- 13.Ichihara K., Iwasaki H., Ueda K., Takizawa R., Naito H., Tomosugi M. 2005. Synthesis of phosphatidylcholine: an improved method without using the cadmium chloride complex of sn-glycero-3-phosphocholine. Chem. Phys. Lipids. 137: 94–99. [DOI] [PubMed] [Google Scholar]
- 14.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 15.Grogan D. W., Cronan J. E., Jr 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61: 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer J. K. G., Fellner V., Dugan M. E. R., Sauer F. D., Mossoba M. M., Yurawecz M. P. 1997. Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids. 32: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro I. L., Kritchevsky D. 1965. Degradation of cholesterol during transesterification of cholesterol stearate. J. Chromatogr. 18: 599–601. [DOI] [PubMed] [Google Scholar]
- 18.Kramer J. K. G., Hulan H. W. 1976. Artifacts produced during acid-catalyzed methanolysis of sterol esters. J. Lipid Res. 17: 674–676. [PubMed] [Google Scholar]
- 19.Ulberth F., Henninger M. 1992. One-step extraction/methylation method for determining the fatty acid composition of processed foods. J. Am. Oil Chem. Soc. 69: 174–177. [Google Scholar]
- 20.Lepage G., Roy C. C. 1986. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 27: 114–120. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.