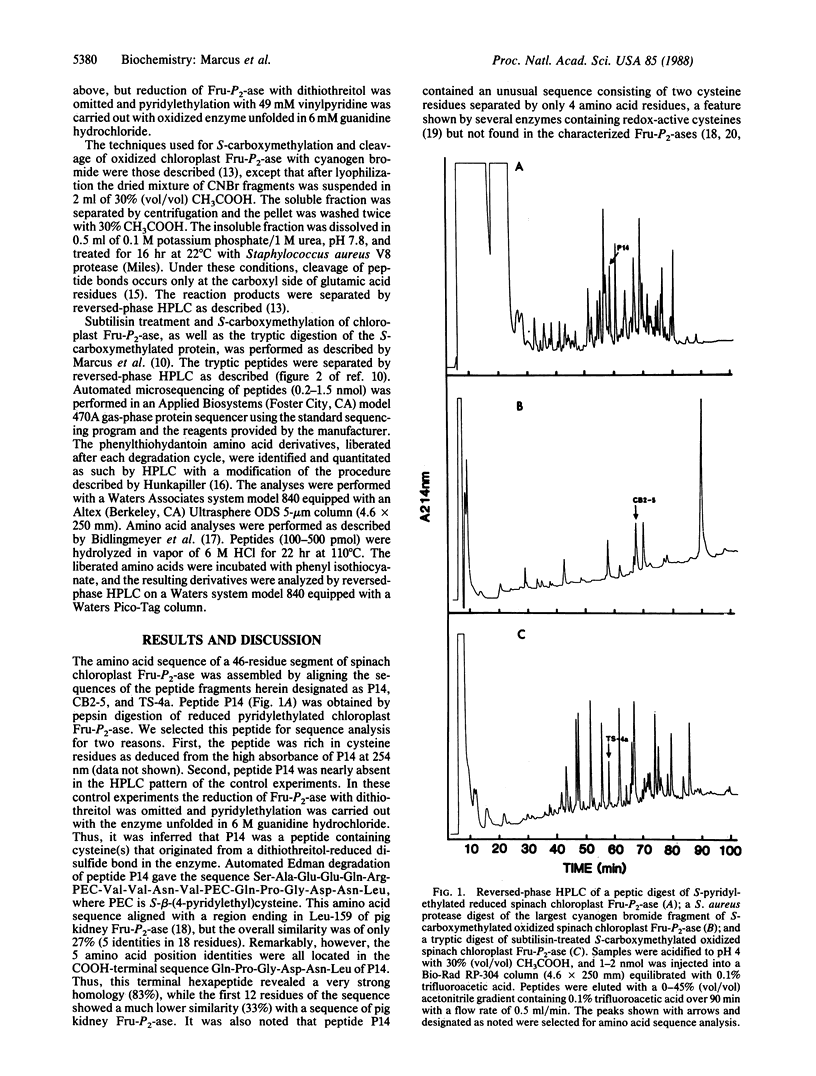
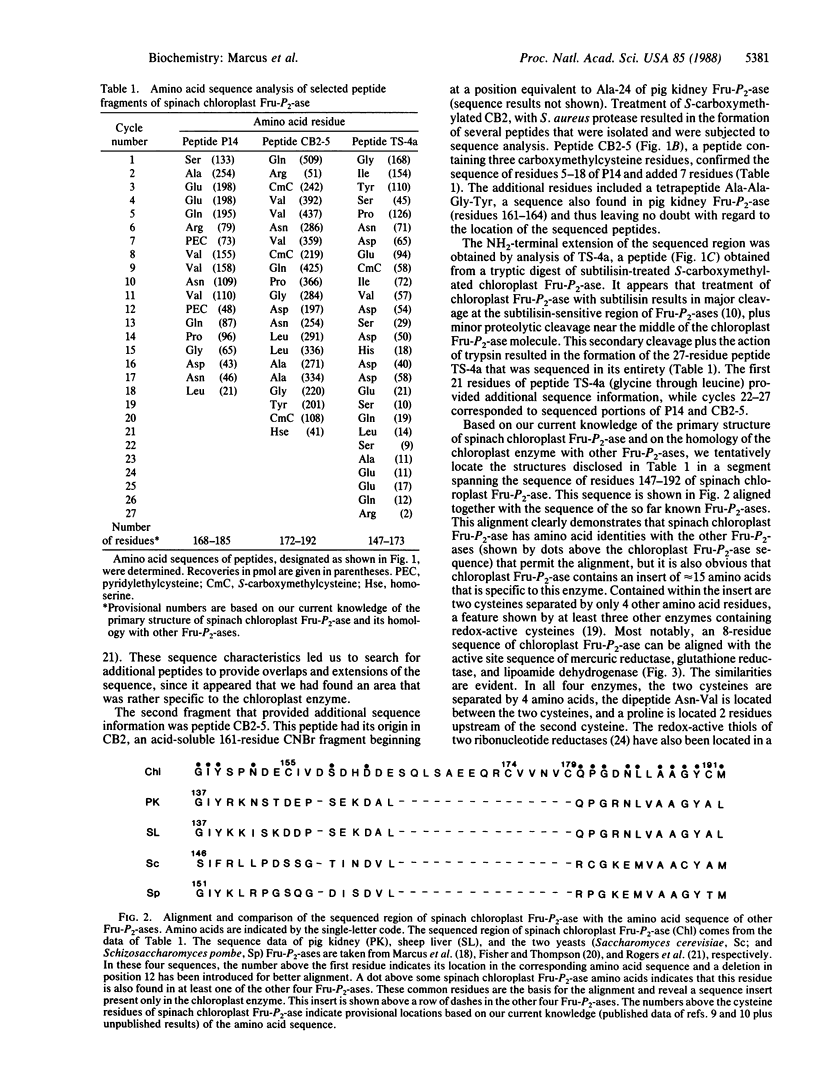
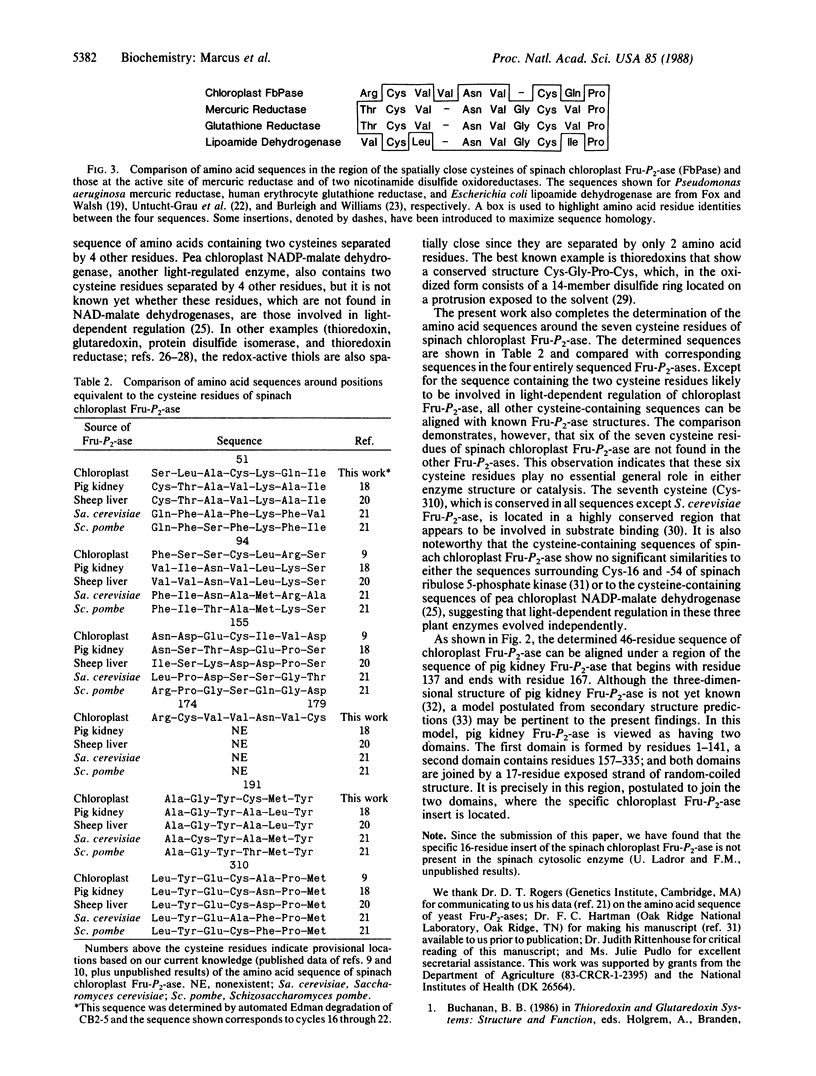
Abstract
Chloroplast fructose-1,6-bisphosphatase (Fru-P2-ase) is an essential enzyme in the photosynthetic pathway of carbon dioxide fixation into sugars. The properties of the chloroplast enzyme are clearly distinct from cytosolic gluconeogenic Fru-P2-ases. Light-dependent activation by way of a ferredoxin/thioredoxin system and insensitivity to AMP inhibition are distinctive characteristics of the chloroplast enzyme. However, the chloroplast enzyme shows a high degree of amino acid sequence similarity to gluconeogenic Fru-P2-ases. Sequence data reported for a total of 285 residues (approximately 75% of the structure) of the spinach chloroplast enzyme reveals a 46% amino acid sequence identity with pig kidney Fru-P2-ase. We now report the amino acid sequence of a region consisting of 46 additional residues. This region is located near the middle of the primary structure of the enzyme and it includes a 16-residue insert not present in other Fru-P2-ases. This sequence insert has two cysteines separated by only 4 amino acid residues (Cys-Val-Val-Asn-Val-Cys), a characteristic feature of at least three other enzymes containing redox-active cysteines. It appears likely that this region of chloroplast Fru-P2-ase is involved in light-dependent activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Burleigh B. D., Jr, Williams C. H., Jr The isolation and primary structure of a paptide containing the oxidation-reduction active cystine of Escherichia coli lipoamide dehydrogenase. J Biol Chem. 1972 Apr 10;247(7):2077–2082. [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Fisher W. K., Thompson E. O. Amino acid sequence studies on sheep liver fructose-bisphosphatase. II. The complete sequence. Aust J Biol Sci. 1983;36(3):235–250. doi: 10.1071/bi9830235. [DOI] [PubMed] [Google Scholar]
- Fox B. S., Walsh C. T. Mercuric reductase: homology to glutathione reductase and lipoamide dehydrogenase. Iodoacetamide alkylation and sequence of the active site peptide. Biochemistry. 1983 Aug 16;22(17):4082–4088. doi: 10.1021/bi00286a014. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S. Identification of cysteine-containing peptides in protein digests by high-performance liquid chromatography. Anal Biochem. 1984 Nov 1;142(2):336–339. doi: 10.1016/0003-2697(84)90473-1. [DOI] [PubMed] [Google Scholar]
- Harrsch P. B., Kim Y., Fox J. L., Marcus F. Amino acid sequence similarity between spinach chloroplast and mammalian gluconeogenic fructose-1,6-bisphosphatase. Biochem Biophys Res Commun. 1985 Dec 17;133(2):520–526. doi: 10.1016/0006-291x(85)90937-4. [DOI] [PubMed] [Google Scholar]
- Hög J. O., Jörnvall H., Holmgren A., Carlquist M., Persson M. The primary structure of Escherichia coli glutaredoxin. Distant homology with thioredoxins in a superfamily of small proteins with a redox-active cystine disulfide/cysteine dithiol. Eur J Biochem. 1983 Oct 17;136(1):223–232. doi: 10.1111/j.1432-1033.1983.tb07730.x. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Fructose-bisphosphatase from spinach leaf chloroplast and cytoplasm. Methods Enzymol. 1982;90(Pt E):371–378. doi: 10.1016/s0076-6879(82)90158-6. [DOI] [PubMed] [Google Scholar]
- Lin A. N., Ashley G. W., Stubbe J. Location of the redox-active thiols of ribonucleotide reductase: sequence similarity between the Escherichia coli and Lactobacillus leichmannii enzymes. Biochemistry. 1987 Nov 3;26(22):6905–6909. doi: 10.1021/bi00396a006. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Adkins S., La Du B. N. Location of disulfide bonds within the sequence of human serum cholinesterase. J Biol Chem. 1987 Sep 25;262(27):12945–12952. [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Reardon I., Heinrikson R. L. Complete amino acid sequence of pig kidney fructose-1,6-bisphosphatase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7161–7165. doi: 10.1073/pnas.79.23.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Gontero B., Harrsch P. B., Rittenhouse J. Amino acid sequence homology among fructose-1,6-bisphosphatases. Biochem Biophys Res Commun. 1986 Mar 13;135(2):374–381. doi: 10.1016/0006-291x(86)90005-7. [DOI] [PubMed] [Google Scholar]
- Marcus F., Harrsch P. B., Moberly L., Edelstein I., Latshaw S. P. Spinach chloroplast fructose-1,6-bisphosphatase: identification of the subtilisin-sensitive region and of conserved histidines. Biochemistry. 1987 Nov 3;26(22):7029–7035. doi: 10.1021/bi00396a026. [DOI] [PubMed] [Google Scholar]
- Martínez J., Cid H. A model for the structure of fructose-1,6-bisphosphatase from pig kidney. Arch Biol Med Exp (Santiago) 1986 Jan;19(1):77–83. [PubMed] [Google Scholar]
- Porter M. A., Stringer C. D., Hartman F. C. Characterization of the regulatory thioredoxin site of phosphoribulokinase. J Biol Chem. 1988 Jan 5;263(1):123–129. [PubMed] [Google Scholar]
- Pradel J., Soulié J. M., Buc J., Meunier J. C., Ricard J. On the activation of fructose-1,6-bisphosphatase of spinach chloroplasts and the regulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- Preiss J., Biggs M. L., Greenberg E. The effect of magnesium ion concentration on the pH optimum of the spinach leaf alkaline fructose diphosphatase. J Biol Chem. 1967 May 10;242(9):2292–2294. [PubMed] [Google Scholar]
- Rogers D. T., Hiller E., Mitsock L., Orr E. Characterization of the gene for fructose-1,6-bisphosphatase from Saccharomyces cerevisiae and Schizosaccharomyces pombe. Sequence, protein homology, and expression during growth on glucose. J Biol Chem. 1988 May 5;263(13):6051–6057. [PubMed] [Google Scholar]
- Ronchi S., Williams C. H., Jr The isolation and primary structure of a peptide containing the oxidation-reduction active cystine of Escherichia coli thioredoxin reductase. J Biol Chem. 1972 Apr 10;247(7):2083–2086. [PubMed] [Google Scholar]
- Seaton B. A., Campbell R. L., Petsko G. A., Rose D. R., Edelstein I., Marcus F. Preliminary X-ray crystallographic studies of pig kidney fructose-1,6-bisphosphatase. J Biol Chem. 1984 Jul 25;259(14):8915–8916. [PubMed] [Google Scholar]
- Tejwani G. A. Regulation of fructose-bisphosphatase activity. Adv Enzymol Relat Areas Mol Biol. 1983;54:121–194. doi: 10.1002/9780470122990.ch3. [DOI] [PubMed] [Google Scholar]
- Untucht-Grau R., Schirmer R. H., Schirmer I., Krauth-Siegel R. L. Glutathione reductase from human erythrocytes: amino-acid sequence of the structurally known FAD-binding domain. Eur J Biochem. 1981 Nov;120(2):407–419. doi: 10.1111/j.1432-1033.1981.tb05718.x. [DOI] [PubMed] [Google Scholar]
- Xu G. J., Datta A. G., Singh V. N., Suda H., Pontremoli S., Horecker B. L. Rabbit liver fructose 1,6-bisphosphatase: labeling of the active and allosteric sites with pyridoxal 5-phosphate and sequence of a nonapeptide from the active site. Arch Biochem Biophys. 1981 Aug;210(1):98–103. doi: 10.1016/0003-9861(81)90168-5. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]


