Abstract
We have previously reported that adenovirus-mediated expression of preprocholecystokin (CCK) stimulates human and mouse islet cell proliferation. In follow-up studies, we became concerned that the CCK adenovirus might have been contaminated with a wild-type E1A-containing adenovirus. Here we show conclusively that the proliferative effects reported in the original paper in mouse and human islets were not due to CCK expression but rather to a contaminating E1A-expressing wild-type adenovirus. We also show, however, that CCK expression does have a proliferative effect in rat islets. We hope that our report of the steps taken to detect the wild-type virus contamination, and purification of the contributing viral stocks, will be helpful to other investigators, and that our experience will serve as a cautionary tale for use of adenovirus vectors, especially for studies on cellular replication.
Wild-type E1A-expressing adenovirus and not cholecystokinin stimulates mouse and human islet cell proliferation. Cholecystokinin and E1A both stimulate rat islet cell proliferation.
In a previous article published in this journal (1), we reported that adenovirus-mediated expression of pre-pro-cholecystokinin (pre-pro-CCK) stimulates proliferation of mouse and human pancreatic islet cells. We now regretfully retract our previous publication based on our discovery that the CCK adenovirus (AdCMV-CCK) preparation was contaminated with wild-type (WT) E1A-expressing adenovirus. We purified pre-pro-CCK and WT viruses from the contaminated preparation and found that our previous findings were replicable but entirely attributable to E1A expression and not pre-pro-CCK expression in mouse and human islets. In contrast, both the purified pre-pro-CCK and WT adenoviruses were able to stimulate rat islet cell proliferation, demonstrating that CCK does have some species-specific replicative activity, but not in mouse or human islets as originally reported.
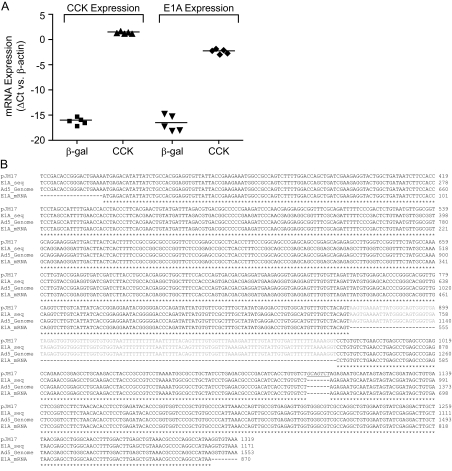
The follow-up studies to our previous publication aimed to determine the bioactive CCK peptide(s) within the pre-pro-CCK sequence responsible for the human and mouse islet cell proliferative effects and their mechanism of action. We constructed multiple adenoviruses that expressed truncated CCK peptides. None of these mutant pre-pro-CCK constructs replicated the effects of AdCMV-CCK. These results made us question the purity of our original AdCMV-CCK. We therefore decided to measure E1A mRNA expression by quantitative RT-PCR (qRT-PCR) in human islets treated with AdCMV-CCK. We found a large increase in E1A mRNA expression in AdCMV-CCK-treated islets compared with control islets treated with an AdCMV-βGal virus (Fig. 1A; P < 0.001). In contrast, none of the mutant pre-pro-CCK adenoviruses increased E1A mRNA expression in islets (data not shown).
Figure 1.
Full-length E1A from HAd5 is expressed in human islets treated with AdCMV-CCK. A, qRT-PCR analysis of human islets treated with AdCMV-β-Gal or AdCMV-CCK. Data are normalized to β-actin mRNA expression. Comparisons were made by Student's paired t test. B, Consensus sequence data from pJM17 vector, sequenced E1A from AdCMV-CCK (E1A_seq), human adenoviral 5 genome (HAd5, GenBank accession no. AY339865), and E1A mRNA 13S sequence. Intronic E1A sequence is represented in gray, and several representative nucleotides denoting the position of pBR322 plasmid inserted into pJM17 are underlined and italicized.
E1A is a potent stimulator of cellular proliferation. E1A contains four conserved regions (CR1–CR4) of homology among the primate adenoviruses. CR2 has been extensively studied because it binds tightly to retinoblastoma protein (pRb) via an LxCxE motif (2,3,4). After CR2 sequesters pRb, CR1 inhibits the binding between pRb and E2F (2,3,4). This results in derepression of E2F transcription factors, transcription of cyclin E, cyclin A, and cdk2, and S-phase progression. CR1 can also globally regulate transcription. CR1 interacts with the histone acetyl transferases, cAMP response element-binding protein-binding protein and p300, to stimulate cell cycle progression (5). Additionally, CR1 can interact with p400 to create an E1A-p400-Myc complex that will prevent Myc degradation and stimulate Myc-dependent transcription (6).
The original AdCMV-CCK was constructed by cotransfecting FF001-CCK and pJM17 into human embryonic kidney (HEK)293 cells. FF001 is an adenoviral shuttle vector that contains an expression cassette flanked by homologous regions to the adenoviral genome. The pJM17 plasmid contains the entire human adenoviral serotype 5 (HAd5) genome with a bacterial plasmid (pBR322) inserted into the E1A gene. This insertion allows pJM17 to be amplified in bacteria, disrupts the E1A gene, and increases the size of pJM17 so that it cannot be packaged into an adenovirus. Cotransfection of an FF001-derived clone and pJM17 into HEK293 cells, however, results in a recombination event that produces an E1A-free HAd5 genome and the relevant expression cassette, which is now small enough to be packaged into an adenovirus. The E1A that is required for completion of the lytic life cycle is provided in trans in HEK293 cells, allowing the recombinant E1-deleted genome to be packaged into a recombinant virus.
We sequenced the AdCMV-CCK adenovirus to determine how the contamination with E1A occurred and the identity of the E1A contaminant. We found that full-length E1A from HAd5 was present in AdCMV-CCK, including its intron (shown in gray, Fig. 1B). We also determined that no pRB322 sequence remained (italicized and underlined, Fig. 1B). Therefore, full-length genomic HAd5-E1A is present in AdCMV-CCK. We postulate that pJM17 recombined with both FF001-CCK and HEK293-derived E1A sequence to generate a CCK and a WT adenovirus, respectively. At the time that AdCMV-CCK virus was prepared, we confirmed the presence of the pre-pro-CCK cDNA insert by PCR and sequencing but did not test for E1A.
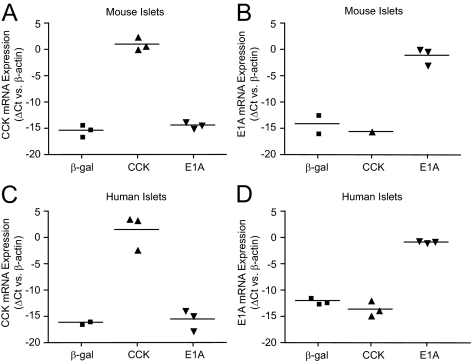
After our recent discovery of the E1A contamination, we purified WT and recombinant CCK viruses from the contaminated AdCMV-CCK stock and tested each on isolated mouse and human islets. This was achieved by dilution of the original AdCMV-CCK stock to obtain individual viral plaques, followed by amplification and screening of multiple plaque isolates. All individual plaques were found to be CCK positive and E1A negative, or CCK negative and E1A-positive by RT-PCR analysis. Individual plaques were used to purify CCK (AdCMV-pureCCK) and WT E1A-expressing (HAd5-E1A) adenoviruses. Treatment of mouse and human islets with AdCMV-pureCCK resulted in significantly increased CCK mRNA expression (Fig. 2, A and C) and unchanged E1A mRNA levels (Fig. 2, B and D). Treatment with HAd5-E1A significantly increased E1A mRNA expression (Fig. 2, B and D) with no change in CCK mRNA expression (Fig. 2, A and C). These data demonstrate successful purification of the contaminated AdCMV-CCK stock into AdCMV-pureCCK and HAd5-E1A.
Figure 2.
Purification of AdCMV-pureCCK and HAd5-E1A adenoviruses from the contaminated AdCMV-CCK stock. qRT-PCR analysis of mouse (A and B) and human (C and D) islets treated with AdCMV-β-Gal (β-gal), AdCMV-pureCCK (CCK), or HAd5-E1A (E1A) for CCK (A and C) and E1A (B and D) gene expression. Data are normalized to β-actin mRNA levels.
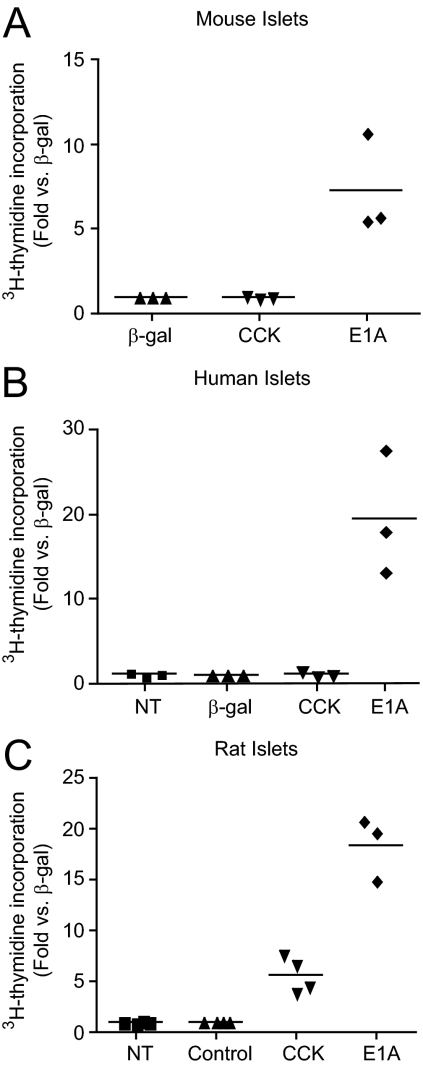
We measured [3H]thymidine incorporation into DNA to determine the effects of AdCMV-pureCCK and HAd5-E1A on islet cell proliferation. In mouse and human islets, HAd5-E1A treatment caused 7.3-fold and 19.6-fold increases in [3H]thymidine incorporation into DNA, respectively (P < 0.01; Fig. 3, A and B). AdCMV-pureCCK did not increase islet cell proliferation in mouse or human islets (Fig. 3, A and B). In contrast, in rat islets, HAd5-E1A and AdCMV-pureCCK caused 18.4- (P = 0.01) and 5.7-fold (P < 0.02) increases in [3H]thymidine incorporation into DNA, respectively (Fig. 3C). Thus, CCK overexpression stimulated rat, but not mouse or human, islet cell DNA replication. We have previously observed patterns of species specificity for other β-cell mitogens, and future studies will aim to understand this phenomenon.
Figure 3.
HAd5-E1A and AdCMV-pureCCK effects on mouse, human, and rat islet cell proliferation. [3H]Thymidine incorporation assays in mouse (A), human (B), and rat (C) islets. Islets were untreated or treated with control adenovirus (β-gal or green fluorescent protein), AdCMV-pureCCK (CCK), or HAd5-E1A (E1A). In mouse islets (A), only HAd5-E1A stimulated islet cell proliferation (7.3-fold; P < 0.01). In human islets (B), again only HAd5-E1A stimulated islet cell replication (19.6-fold, P < 0.01). In rat islets (C), HAd5 and AdCMV-pureCCK stimulated islet cell proliferation by 18.4-fold (P = 0.01) and 5.7-fold (P < 0.02), respectively. Comparisons were made by Student's paired t test.
We next investigated the effects of E1A or CCK expression on cyclin and cdk expression. We performed qRT-PCR on AdCMV-β-Gal-, AdCMV-pureCCK-, and HAd5-E1A-treated mouse and human islets. We found that HAd5-E1A treatment of mouse and human islets stimulated increases in mRNAs encoding cyclins A2, B1, B2, E1, E2, F, cdk1, and cdk2 vs. AdCMV-βGal-treated islets (Figs. S1 and S2, published as supplemental data on The Endocrine Society's Journals Online web site at http://mend.endojournals.org; P < 0.05 for all). AdCMV-pureCCK treatment of mouse and human islets did not significantly increase any cyclin or cdk mRNAs (supplemental Figs. S1 and S2). In short, all of our previously reported changes in cyclin and cdk abundance in mouse and human islets (1) were due to E1A and not CCK overexpression.
Since the initial observation that adenoviruses can transduce primary islets with high efficiency (7,8), these vectors have been used extensively for islet research. In light of the events outlined in this article, we have now developed a qRT-PCR SYBR green-based assay to rapidly detect WT virus contamination. This assay readily detected the contamination of AdCMV-CCK with WT virus. We have previously used adenovirus-mediated gene knockdown and overexpression to discover β-cell-proliferative effects of two other genes (Nkx6.1 and trefoil factor-3) in human and rat islets (9,10). Using the qRT-PCR assay, the viruses used in these prior studies (AdCMV-Nkx6.1, Ad-siNkx6.1, AdCMV-TFF3, and Ad-siTFF3) were all found to be devoid of WT virus contamination. For researchers who may be interested in a rapid and direct assay for WT virus contamination via measurement of E1A levels in adenoviral stocks or transduced cells, the oligonucleotides used in our qRT-PCR E1A assay are forward primer TB964 (TATGCCAAACCTTGTACCGGAGGT: temperature, 59.5 C) and reverse primer TB1038 (CCGGGGTGCTCCACATAATCT; temperature, 57.4 C). The 120-bp TB964/TB1038 amplicon corresponds to bases 892–1011 of adenovirus type 5 (GenBank accession no. AY339865).
Supplementary Material
Footnotes
This work was supported by the following grants: Juvenile Diabetes Research Foundation Grant 17-2007-1026; Grants DK 66539 and DK 58037 from the National Institutes of Health; National Human Genome Research Institute Training Grant 5T32HG002760; and National Institute on Aging Training Grant T32 AG20013.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 15, 2010
Abbreviations: AdCMV-CCK, CCK adenovirus; CCK, cholecystokin; HAd5, human adenoviral serotype 5; HEK, human embryonic kidney; pRb, retinoblastoma protein; qRT-PCR, quantitative RT-PCR; WT, wild type.
References
- Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, Beinfeld MC, Kopin AS, Newgard CB, Attie AD 2008 Overexpression of pre-pro-cholecystokinin stimulates β-cell proliferation in mouse and human islets with retention of islet function. Mol Endocrinol 22:2716–2728 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Berk AJ 2005 Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 24:7673–7685 [DOI] [PubMed] [Google Scholar]
- Pelka P, Ablack JN, Fonseca GJ, Yousef AF, Mymryk JS 2008 Intrinsic structural disorder in adenovirus E1A: a viral molecular hub linking multiple diverse processes. J Virol 82:7252–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio JA 2009 How the Rb tumor suppressor structure and function was revealed by the study of adenovirus and SV40. Virology 384:274–284 [DOI] [PubMed] [Google Scholar]
- Turnell AS, Mymryk JS 2006 Roles for the coactivators CBP and p300 and the APC/C E3 ubiquitin ligase in E1A-dependent cell transformation. Br J Cancer 95:555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty AA, Tansey WP 2009 Adenoviral E1A function through Myc. Cancer Res 69:6–9 [DOI] [PubMed] [Google Scholar]
- Becker TC, BeltrandelRio H, Noel RJ, Johnson JH, Newgard CB 1994 Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J Biol Chem 269:21234–21238 [PubMed] [Google Scholar]
- Becker TC, Noel RJ, Coats WS, Gómez-Foix AM, Alam T, Gerard RD, Newgard CB 1994 Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol 43:161–189 [DOI] [PubMed] [Google Scholar]
- Fueger PT, Schisler JC, Lu D, Babu DA, Mirmira RG, Newgard CB, Hohmeier HE 2008 Trefoil factor 3 stimulates human and rodent pancreatic islet β-cell replication with retention of function. Mol Endocrinol 22:1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG, Newgard CB 2008 Stimulation of human and rat islet β-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol 28:3465–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.