Abstract
Estrogen receptor α (ERα) binds to specific target DNA sequences, estrogen response elements (EREs), to regulate estrogen-responsive gene expression. The progesterone receptor (PR) gene has been used extensively as a marker of estrogen responsiveness. Although we previously identified cis elements within 1 kb of the PR-B transcription start site that are associated with ERα and help to confer estrogen responsiveness, the identification of ERα binding sites far removed from the transcription start site suggested that long-range regulation of this gene may occur. We now show that eight regions of the PR gene from 311 kb upstream to 4 kb downstream of the PR-B transcription start site interact with ERα and that coactivator proteins and acetylated histones are selectively associated with these gene regions. Specific PR gene regions confer estrogen responsiveness to a heterologous reporter plasmid, and mutation of EREs within these regions diminishes estrogen-induced transactivation. Importantly, chromosome conformation capture assays reveal ERα- and ligand-dependent interactions between proximal and distal PR gene regions. Taken together, our studies suggest that distal regions of the PR gene participate in the dynamic regulation of this gene and that the coordinated action of proximal and distal PR gene regions allows cells to respond to changes in hormone levels with extraordinary versatility and sensitivity.
Estrogen receptor alpha and ligands alter interaction of proximal and distal gene regions to regulate progesterone receptor gene expression.
Fine-tuned control of gene expression is required for cells to maintain homeostasis and to respond to various physiological and environmental changes. Altering gene expression requires the coordinated action of an extensive array of cis elements and their associated trans-acting factors. Although the majority of studies to date have examined proximal 5′-flanking regions of target genes to identify cis elements involved in regulating transcription, more recent genome-wide studies have made it clear that transcription factor binding sites can be far removed from a gene's transcription start site (1,2,3,4,5,6,7,8,9,10).
Estrogen receptor α (ERα) is a ligand-induced transcription factor that, upon binding hormone, interacts with estrogen response elements (EREs) to initiate changes in gene expression in target tissues including the reproductive, skeletal, cardiovascular, and nervous systems (11,12,13,14,15). One of the genes regulated by ERα is the progesterone receptor (PR) gene, which encodes two receptors, PR-A and PR-B. PR-B is a 120-kDa protein, which contains an N-terminal region that is not present in 94-kDa PR-A (16). The two PRs are expressed in a tissue-specific manner (17,18) and, as demonstrated in knockout mice, have discrete effects on target tissues. Although PR-A ablation results in infertility in females, PR-B ablation leads to reduced mammary gland morphogenesis (19,20).
In addition to their effects on normal reproductive and mammary tissues, ERα and PR have been extensively used as prognostic indicators in breast cancer patients to predict the responsiveness of mammary tumors to endocrine therapy. Although ERα+/PR+ tumors are more likely to respond positively to endocrine therapy, ERα+/PR− tumors are generally less responsive (21,22,23,24).
Our laboratory has been interested in understanding how the PR gene is regulated, and in earlier studies, we identified four cis elements in the PR-B 5′-flanking region and coding sequence that are involved in conferring estrogen responsiveness (25,26,27,28,29,30). More recently, we and others have used genome-wide screens to identify gene regions associated with ERα (1,2,3,4,5) and, as reported for other transcription factors (6,31,32), many of the sites identified in these studies are far removed from any transcription start site. Interestingly, eight ERα-associated gene regions were identified upstream of the PR gene (1,3,4,5). We have characterized these ERα-associated gene regions, which reside in an extended region from 311 kb upstream to 4 kb downstream of the PR-B transcription start site, to determine whether they might be involved in long-range transcriptional control of PR gene expression.
Results
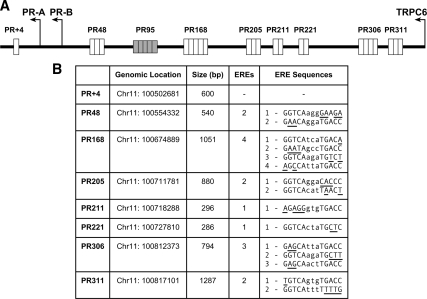
To better understand how estrogen-responsive genes are regulated by 17β-estradiol (E2), we and others have used genome-wide screens in MCF-7 human breast cancer cells to identify endogenous DNA regions associated with ERα (1,3,4,5). Although we had previously identified four proximal PR regions involved in conferring estrogen responsiveness (25,26,27,28,29,30), we were intrigued by the identification of eight regions associated with ERα, which were located 48 kb (PR48), 168 kb (PR168), 205 kb (PR205), 211 kb (PR211), 221 kb (PR221), 306 kb (PR306), and 311 kb (PR311) upstream and 4 kb (PR+4) downstream of the endogenous PR-B transcriptional start site (Fig. 1A). Careful inspection revealed that each of the PR gene regions contained one or more of potential EREs, except for PR+4 (Fig. 1B).
Figure 1.
Map of putative ERα binding sites in the PR gene. A, A schematic drawing shows the gene regions located upstream and downstream of the PR-B transcription start site, each of which was identified in genome-wide screens, except for PR95, which was not identified as an ERα-associated gene region but contains four imperfect EREs and was used as a negative control. B, The chromosomal location (UCSC Genome Browser version 185), size of the region isolated from ChIP assays using an ERα-specific antibody, number of putative EREs in each PR region, and DNA sequence of each putative ERE in each PR region are listed. Nucleotides that differ from the consensus ERE sequence are underlined.
Estrogen responsiveness of the PR and TRPC6 genes
The TRPC6 gene, which encodes a nonselective calcium channel (reviewed in Ref. 33), was closer to a number of the ERE-containing gene regions associated with ERα than the PR gene (Fig. 1A). Although we knew that PR gene expression was substantially increased when MCF-7 cells were exposed to E2 (26,34,35,36,37,38,39), there was no evidence to suggest that TRPC6 might be an estrogen-responsive gene. However, the proximity of the putative ERα binding sites to the TRPC6 gene led us to investigate whether E2 might influence its expression. When MCF-7 cells were treated with E2, PR mRNA levels were significantly increased after 2 and 24 h E2 treatment (Fig. 2A). In contrast, no change was detected in TRPC6 mRNA levels with any E2 treatment. Because mRNA levels do not always reflect the level of protein present, Western analysis was performed. As shown in Fig. 2B, although PR protein levels were increased in response to E2 treatment, neither TRPC6 nor glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein levels were altered. These studies demonstrate that the endogenous PR gene, but not the TRPC6 gene, is regulated by E2 in MCF-7 cells and suggest that the ERα binding sites may be involved in regulating expression of the PR gene, but not the TRPC6 gene.
Figure 2.
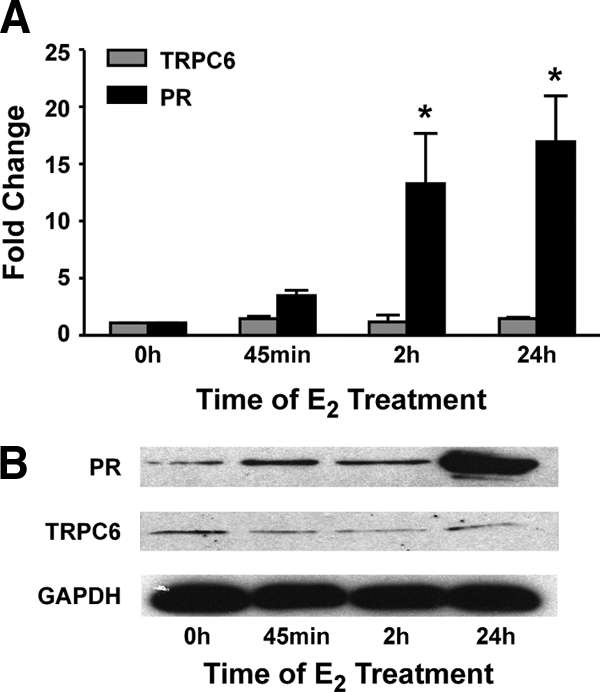
Effect of E2 on PR and TRPC6 mRNA and protein levels. MCF-7 cells were treated with ethanol or 10 nm E2 for the indicated times. A, RNA was isolated, cDNA was synthesized, and qPCR was used to determine the levels of PR, TRPC6, and 36B4 mRNA. Values were calculated using the ΔΔCt method. Data from three independent experiments were normalized and are expressed as the fold increase in mRNA levels ± sem. Statistical differences in the absence and in the presence of E2 were calculated using a one-way ANOVA with a post hoc Student's t test: *, P < 0.05. B, Whole-cell extracts were prepared and subjected to Western blot analysis with a PR-, TRPC6-, or GAPDH-specific antibody. The blots shown are from one of three independent experiments.
Association of ERα with the distal PR gene regions
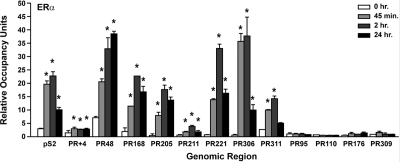
Because ERα associates with the estrogen-responsive pS2 and cathepsin D genes in a cyclic manner (40,41), the association of ERα with the distal PR gene regions was examined using chromatin immunoprecipitation (ChIP) assays after MCF-7 cells had been treated with ethanol or E2 for various times. Significantly more ERα was associated with PR168, PR205, PR221, PR306, and PR311 after 45 min and 2 h E2 treatment, but the association of ERα with these regions declined after 24 h (Fig. 3). E2 increased the association of ERα with PR48 over each of the time points examined. Although the association of ERα with PR+4 and PR211 was increased when MCF-7 cells were treated with E2, the increases were modest when compared with the other distal PR gene regions. In contrast, no changes were observed in the association of ERα with PR95, which contains four putative imperfect EREs, or three other intervening gene regions, PR110, PR176, and PR309. None of these regions were identified as an ERα binding site in any of the genome-wide studies (1,3,4,5). As expected, ERα was associated with the pS2 promoter region, which is a well-characterized estrogen-responsive gene that contains an imperfect ERE (40,41).
Figure 3.
ERα association with the PR gene regions. ChIP assays, which were performed in MCF-7 cells that had been treated with ethanol or 10 nm E2 for the indicated times, used an ERα-specific antibody to determine the occupancy level at each gene region. qPCR was used to detect the number of genomic copies for each gene region and corrected to the number of 36B4 genomic copies to obtain the relative occupancy units. Data reported are the average relative occupancy units of three replicates ± sem in a single experiment and are representative of four to eight independent experiments. Statistical differences in the absence and in the presence of E2 were calculated using a one-way ANOVA with a post hoc Student's t test: *, P < 0.05.
Coregulatory protein recruitment and acetylation at the distal PR regions
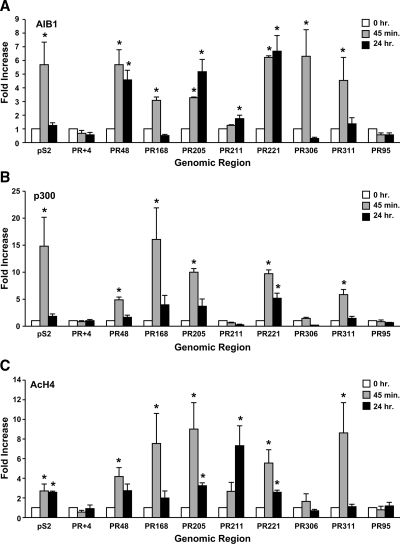
ERα interacts with numerous coactivator proteins including the p160 family member amplified in breast cancer 1 (AIB1) and the histone acetyl transferase p300 (42,43). The participation of these coactivators is required for effective estrogen responsiveness (44,45). The association of coregulatory proteins with the pS2 and cathepsin D genes has been examined in detail (40,41), and yet little is known about the regulation of the PR gene. Thus, we examined the association of AIB1 and p300 with the distal PR gene regions using ChIP assays in MCF-7 cells that had been treated with ethanol or E2. Although the association of AIB1 increased at PR168, PR306, and PR311 after cells had been treated with E2 for 45 min, the association of AIB1 with PR48, PR205, and PR221 was increased after both 45 min and 24 h E2 treatment (Fig. 4A). E2 also enhanced the association of AIB1 with PR211 after 24 h, but the increase was very modest when compared with the other distal PR gene regions. No change was observed in the association of AIB1 with PR+4 or the negative control, PR95. Although the association of p300 was increased at PR48, PR168, PR205, and PR311 after 45 min E2 treatment, the association of p300 was increased at PR221 after 45 min and 24 h E2 treatment (Fig. 4B). PR+4, PR211, PR306, and the negative control PR95 was not associated with p300. The association of AIB1 and p300 with the pS2 gene was increased at 45 min as described previously (40,41).
Figure 4.
Coregulatory protein recruitment and acetylation at the PR gene regions. ChIP assays were performed in MCF-7 cells that had been treated with ethanol or 10 nm E2 for the indicated times using antibody specific for AIB1- (A), p300- (B), or AcH4 (C). qPCR was used to detect the number of genomic copies for each gene region and corrected to the number of 36B4 genomic copies to obtain the relative occupancy units. Data are expressed as fold increase of the relative occupancy units at the indicated gene regions. Data reported are the average of three replicates ± sem in a single experiment and are representative of three to six independent experiments. Statistical differences in the absence and in the presence of E2 were calculated using a Student's t test: *, P < 0.05.
Increased acetylation of histones produces a more open chromatin structure and has been associated with enhanced transcriptional activity (46). Both p300 and AIB1 possess histone acetyltransferase activity and have been linked to enhanced estrogen-responsive gene expression (42,47). It has been suggested that increased acetylated histone H4 (AcH4) plays a critical role in regulating chromatin structure (48). Moreover, increased histone H4 acetylation has been observed at the native, estrogen-responsive pS2 promoter in E2-treated MCF-7 cells (49). Thus, we investigated whether E2 treatment altered AcH4 levels at each of the distal PR gene regions. ChIP assays were performed in MCF-7 cells that had been treated with ethanol or E2. Increased acetylation was observed at PR48, PR168, and PR311 after 45 min E2 treatment; at PR211 after 24 h E2; and at PR205 and PR221 after 45 min and 24 h E2 treatment (Fig. 4C). No significant changes in histone acetylation were observed at PR+4, PR306, or PR95. Thus, E2 not only induces the differential association of coregulatory proteins with distal PR gene regions but also selectively enhances acetylation of chromatin at these sites.
Functional capacity of putative EREs
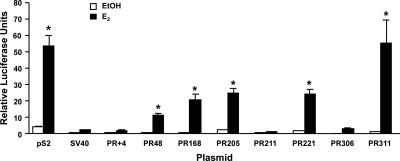
The increased association of ERα, AIB1, p300, and AcH4 with the distal PR gene regions in the presence of E2 suggested that the putative EREs residing in these gene regions could be involved in mediating estrogen responsiveness. Thus, MCF-7 cells were transfected with reporter plasmids containing a simian virus 40 (SV40) promoter alone or combined with one of the distal PR gene regions and then treated with ethanol or E2. These experiments relied solely on the endogenously expressed ERα and coregulatory proteins normally present in MCF-7 cells. As seen in Fig. 5, the most potent increases in estrogen responsiveness were observed when PR311 (50-fold) and PR168 (40-fold) were included in the SV40 reporter plasmid. Similar but less potent increases were observed when PR221 (14-fold), PR48 (12-fold), and PR205 (11-fold) were included, and although the reporter plasmid containing PR306 responded modestly to hormone (7-fold), no changes were detected with PR+4, PR211, or the parent reporter plasmid containing only the SV40 promoter in the presence of E2. A reporter plasmid containing pS2 DNA sequence was also included for comparison.
Figure 5.
Effect of PR gene regions on E2-mediated transcription. MCF-7 cells were transfected with a luciferase reporter plasmid containing pS2 DNA sequence or an SV40 promoter alone (SV40) or combined with the indicated PR gene region and treated with ethanol (EtOH) or 10 nm E2 for 24 h. Data from four independent experiments were normalized and are expressed as the relative luciferase units ± sem. Statistical differences in the absence and in the presence of E2 were calculated using a one-way ANOVA with a post hoc Student's t test: *, P < 0.05.
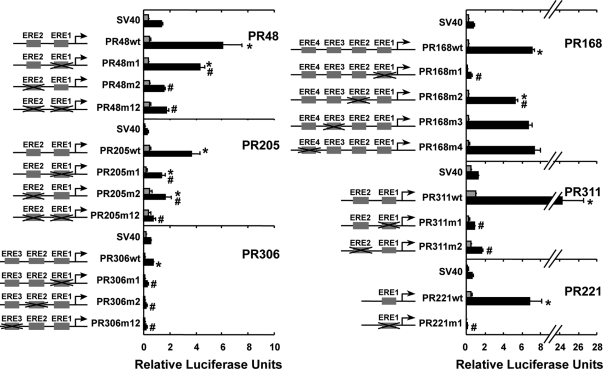
To define whether the putative EREs residing in the distal PR gene regions (Fig. 1B) were functional, the EREs were mutated individually and in combination. MCF-7 cells were transfected with reporter plasmids containing each of the distal PR gene regions with wild-type or mutant ERE sequence (Fig. 6). Although PR48 and PR168 contain two and four putative EREs, respectively, only one ERE contributed significantly (PR48/ERE2 and PR168/ERE1), and another ERE contributed modestly to estrogen responsiveness (PR48/ ERE1 and PR168/ERE2). In contrast, each of the putative EREs within PR205, PR311, and PR306 and the sole ERE residing in pPR221 played critical roles in mediating the effects of E2. These studies combined with our ChIP analyses suggested that regulation of the PR gene is not restricted to regions in close proximity to the PR-B transcription start site but that it was dispersed over an extended region and that multiple, widely distributed EREs are functional and may be involved in mediating estrogen responsiveness.
Figure 6.
Role of putative EREs in conferring estrogen responsiveness. MCF-7 cells were transfected with a luciferase reporter plasmid containing an SV40 promoter alone (SV40) or combined with a PR gene region with wild-type or mutant ERE sequences and treated with ethanol or 10 nm E2 for 24 h. Data from four independent experiments were normalized and are expressed as the relative luciferase units ± sem. Statistical differences were calculated using a one-way ANOVA with a post hoc Student's t test (P < 0.05). Significant differences in the absence and in the presence of E2 (*) or in the transcriptional activity of the plasmids with wild-type and mutant ERE sequences (#) are indicated.
Physical interaction of the proximal and distal PR gene regions
If the distal PR regions are involved in regulating PR gene expression, one would anticipate that they would physically associate with the proximal PR region. It is possible to assess the interaction of proximal and distal gene regions using chromosome conformation capture (3C) assays (50). In these assays, formaldehyde is used to cross-link chromatin, and the DNA is digested with a restriction enzyme and then ligated under dilute conditions to produce specific and unique ligation products when the DNA is affiliated. Thus, if two DNA regions are in close proximity in native chromatin, the number of amplicons produced in subsequent PCR amplification will be significantly enhanced compared with more widely separated regions.
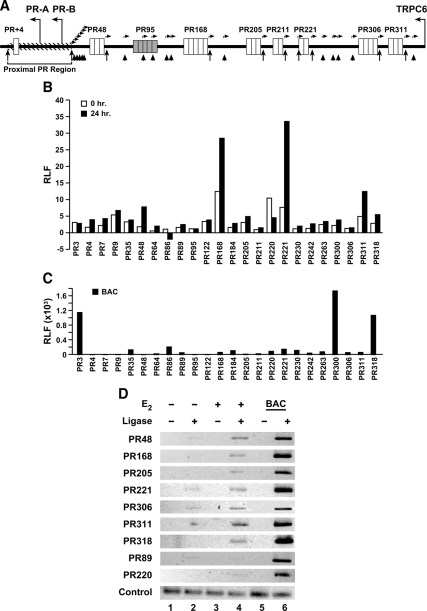
To determine whether the proximal and distal PR regions could interact and participate in sustaining PR gene expression, 3C assays were performed in MCF-7 cells that had been treated with ethanol or E2 for 24 h, a time when PR mRNA and protein levels are significantly increased (Fig. 2, A and B). After digestion of chromatin with EcoR1, a series of 24 primer sets were used to scan 318 kb upstream of the PR-B transcription start site (Fig. 7A). As shown in Fig. 7B, the affiliation of PR48, PR168, PR221, and PR311 with the proximal PR region was greater in the presence of E2 than in its absence. Interestingly, in the absence of E2, PR168, PR220, PR221, and PR311 were affiliated with the proximal PR region suggesting that, even in the absence of hormone, specific distal PR regions interact with the proximal PR region.
Figure 7.
Physical interaction of proximal and distal PR gene regions. A, Schematic diagram showing the position of Eco R1 sites in the distal PR regions ( ), intervening control regions (
), intervening control regions ( ), and primer sets (
), and primer sets ( ) used to examine the PR gene. The association of distal and intervening PR regions with the proximal PR region (hatched portion) was monitored. B and C, 3C assays were performed with chromatin from MCF-7 cells that had been treated with ethanol or 10 nm E2 (B) or purified BAC clone DNA (C). DNA was digested with EcoRI and ligated, and qPCR was used to detect the number of ligation products obtained with each individual primer set. The number of ligation products obtained was corrected to a no-ligation control for each of the PR gene regions (relative ligation frequency, RLF). Data reported are the average relative number of ligation products of three replicates in a single experiment and are representative of three independent experiments. The location of each gene region is reported relative to the PR-B transcription start site. D, 3C-derived amplicons from MCF-7 chromatin were examined on ethidium-stained agarose gels.
) used to examine the PR gene. The association of distal and intervening PR regions with the proximal PR region (hatched portion) was monitored. B and C, 3C assays were performed with chromatin from MCF-7 cells that had been treated with ethanol or 10 nm E2 (B) or purified BAC clone DNA (C). DNA was digested with EcoRI and ligated, and qPCR was used to detect the number of ligation products obtained with each individual primer set. The number of ligation products obtained was corrected to a no-ligation control for each of the PR gene regions (relative ligation frequency, RLF). Data reported are the average relative number of ligation products of three replicates in a single experiment and are representative of three independent experiments. The location of each gene region is reported relative to the PR-B transcription start site. D, 3C-derived amplicons from MCF-7 chromatin were examined on ethidium-stained agarose gels.
Bacterial artificial clone (BAC) DNA, which contained the entire region of the PR gene examined, was used as a control template to produce all possible ligation products. Surprisingly, the generated profile demonstrated that not all ligation products were equally formed (Fig. 7C). The ability of some of the most distal regions (PR300 and PR318) to affiliate with the proximal PR region was striking. The interaction of the proximal PR region with PR3 was also significantly increased. These preferential associations were not unique to the EcoR1-digested DNA or the primer sets used. A similar profile was observed when HindIII was used to create a BAC DNA library (data not shown). These combined findings suggest that the PR gene has an intrinsic propensity to assume a preferred structure driven by the physical properties of the DNA itself.
To ensure that an appropriately sized, single product was produced with individual primer sets, 3C amplicons were generated from MCF-7 cells that had been treated with ethanol or E2 and separated on an agarose gel. These experiments confirmed the enhanced association of the proximal PR region with PR48, PR168, PR221, and PR311. In addition, we were able to detect amplified products with PR205, PR306, and PR318 but not PR89 or PR220 (Fig. 7D). A set of control primers, which amplified DNA sequence 6 kb upstream of the PR-B transcription start site, demonstrated that each of the DNA preparations was similarly amplified (control).
Role of ERα in interaction of proximal and distal PR sites
The capacity of E2 to selectively enhance the interaction of proximal and distal PR regions suggested that ERα may serve as a nucleating factor and foster chromatin reorganization. To determine whether this was the case, 3C assays were performed with MCF-7 cells, which endogenously express ERα, and C4-12 cells (51), an MCF-7-derived cell line, which does not express ERα (Fig. 8A). E2 induced an increase in PR levels in MCF-7 but not in C4-12 cells. Furthermore, although an amplified product was produced in 3C assays when MCF-7 cells had been treated with E2 and the digested chromatin had been ligated (Fig. 8B, lane 8), no amplicons were produced when C4-12 cells were used (lanes 1–4). In addition, few or no amplicons were produced when MCF-7 cells had been treated with ethanol (lane 6), and no amplicons were produced when the ligase was omitted from the reactions (lanes 5 and 7). However, when control primers were used, an appropriately sized product was observed in each lane demonstrating that each DNA preparation was similarly amplified (control). These findings demonstrate that the proximal PR region interacts with PR168, PR221, and PR311 when MCF-7 cells have been treated with E2 but that no interaction occurred when the cells failed to express ERα, thereby demonstrating the essential role of ERα in organizing chromatin structure and regulating PR gene expression.
Figure 8.
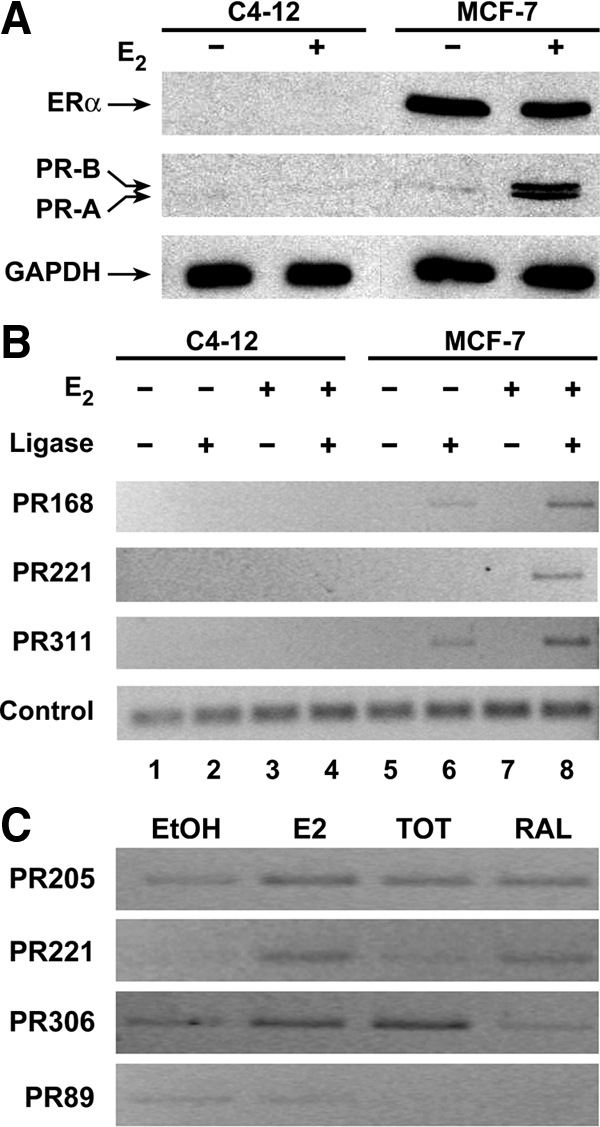
ERα- and ligand-dependent association of proximal and distal PR regions. A and B, MCF-7 cells, which endogenously express ERα, and a daughter MCF-7 cell line, C4-12, which do not express ERα, were treated with ethanol or 10 nm E2 and used in Western blot analysis (A) to examine ERα, PR, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and 3C assays (B) to examine the affiliation of proximal and distal PR regions. Control primers were used to demonstrate that the DNA preparations were similarly amplified. C, MCF-7 cells were treated with ethanol, 10 nm E2, or 100 nm TOT or RAL for 24 h and then subjected to 3C analysis. The data shown are representative of three independent experiments, which produced similar results.
Ligand-specific regulation of chromatin structure
The selective ER modulators tamoxifen and raloxifene (RAL) have been extraordinarily useful clinically in decreasing the incidence and recurrence of breast cancer (52,53) and maintaining bone mineral density (54,55), and yet little is known about their effects at the level of the gene. We previously showed that 4-hydroxytamoxifen (TOT) and RAL differentially influence PR gene expression using transient transfection assays (26) and reasoned that these two selective ER modulators might also differentially alter PR chromatin structure. To delineate whether TOT and RAL differentially affected PR chromatin structure, MCF-7 cells were treated with 10 nm E2 or 100 nm TOT or RAL, and 3C assays were performed. Again, E2 enhanced the association of PR205, PR221, and PR306 with the proximal PR gene (Fig. 8C). Although both TOT and RAL enhanced the association of PR205 with the proximal PR gene region, only RAL enhanced the association of PR221 and only TOT enhanced the association of PR306 with the proximal PR gene region. In contrast, none of the hormone treatments affected the association of PR89 with the proximal PR gene. Thus, the effects of E2, TOT, and RAL on PR chromatin structure are ligand and site specific.
Identification of cis elements in the distal PR gene regions
It is clear that estrogen-responsive genes are regulated not simply by EREs but by the combined input from an array of regulatory cis elements and their associated trans-acting factors. To identify other potential transcription factor binding sites, computational analysis was used to scan each of the eight distal PR gene regions. The most abundant cis elements identified were sites for the forkhead proteins FoxD1, FoxF2, FoxI1, and FoxL1. Although the association of Fox F2 with PR205 and PR211 was increased, the association of FoxF2 with PR48 was decreased when MCF-7 cells had been treated with E2 (Fig. 9). In contrast, the association of Fox F2 with PR221 was unaltered by exposure of MCF-7 cells to E2, thereby demonstrating that FoxF2 is recruited to specific distal PR gene regions. We also found that more FoxF2 was associated with the ERE-containing region of the pS2 gene when MCF-7 cells had been treated with E2 than when cells had been treated with ethanol. Thus, as previously reported for FoxA1 (2,56), FoxF2 association with the pS2 ERE is enhanced by exposure of MCF-7 cells to E2.
Figure 9.
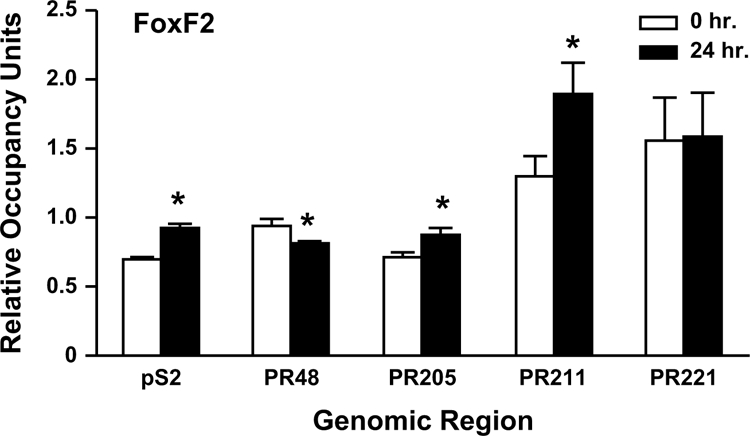
Binding of FOX F2 to the distal PR gene regions. ChIP assays were performed in MCF-7 cells that had been treated with ethanol or 10 nm E2 using a FOX F2-specific antibody. qPCR was used to detect the number of genomic copies for the indicated gene region and corrected for the number of 36B4 genomic copies to obtain the relative occupancy units. Data were from three independent experiments, which had been done in triplicate, were combined and are presented as the mean ± sem. Statistical differences in the absence and in the presence of E2 were calculated using a Student's t test: *, P < 0.05.
Although we detected numerous potential FoxL1 binding sites and a limited number of Sp1 sites in the distal PR regions, we were unable to detect any difference in the association of FoxL1 or Sp1 with these regions (data not shown). These findings emphasize the importance of combining experimental approaches with bioinformatic methods so that the association of a transcription factor with putative binding sites can be verified.
Discussion
We previously reported that AP-1 and Sp1 sites within 1 kb of the PR-B transcription start site are involved in conferring estrogen responsiveness to the PR gene (25,26,27,28,29,30). We now show that regions from 48–311 kb upstream of the PR-B transcription start site also play a role in regulating PR gene expression. PR48, PR168, PR205, PR221, and PR311 associate with ERα, AIB1, p300, and AcH4, confer estrogen responsiveness to a heterologus promoter, and interact with the proximal PR gene, suggesting that these regions are directly involved in the E2-mediated expression of the endogenous PR gene.
Role of EREs in regulating PR gene expression
Years of investigation have emphasized the importance of EREs in regulating transcription of estrogen-responsive genes (reviewed in Ref. 57). We and others have demonstrated that transcription factor binding sites can be far removed from the proximal promoter and still play a role in regulating gene expression (1,2,3,4,5,6,7,8,9,10). Brown and co-workers recently reported that the majority of ERα binding sites identified in a genome-wide screen were more than 50 kb from any transcription start site (3). Thus, although the overwhelming majority of studies completed to date have investigated 1–2 kb of 5′-flanking sequence to identify cis elements involved in regulating transcription, our studies and others (1,2,3,4,5,56) suggest that it is no longer sufficient to screen proximal gene regions to identify DNA sequences involved in regulating transcription. Long-range transcriptional control is not unique to ERα and has been observed with other transcription factors as well (6,7,8,9,10).
It is important to note that each of the distal PR regions we characterized had one or more EREs and that each of these EREs was comprised of one consensus ERE half-site and one half-site that deviated from the consensus sequence by 1–4 bp (Fig. 1B). Our transient transfection assays demonstrated that some of these EREs were capable of conferring estrogen responsiveness to a heterologous promoter and, in agreement with previous studies (57,58,59,60), that the capacity of individual imperfect EREs to increase transcription varied. Two EREs, PR168/ERE1 (GGTCAtcaTGACA) and PR311/ERE1 (TGTCAgtgTGACC), differed from the consensus, palindromic ERE sequence (GGTCAnnnTGACC) (61) by a single base pair (underlined). The PR regions containing these EREs were the most potent enhancers in our transient transfection experiments. However, closer examination reveals that four of the five nucleotides in PR311/ERE2 (GGTCAtttTTTTG) differ from a consensus ERE half-site, and yet this ERE appears to be as important in regulating transcription as the near-consensus PR311/ERE1. Thus, although one consensus ERE half-site is required to confer estrogen responsiveness, the sequence of the other ERE half-site can vary by 1–4 bp and still be transcriptionally active, demonstrating that the sequence of an ERE cannot necessarily be used to predict its effectiveness in influencing ERα-mediated gene expression. Furthermore, the mere presence of a palindromic ERE does not ensure it is functional.
Recruitment of ERα and coregulatory proteins to distal PR regions
ERα, AIB1, p300, and AcH4 were associated with PR48, PR168, PR205, PR221, and PR311 (Table 1). However, the association of coregulatory proteins with the three other distal regions was more selective. PR+4 was associated with ERα but not AIB1, p300, or AcH4. PR306 was associated with ERα and AIB1 but not p300 or AcH4. ERα and AIB1, but not p300, were recruited to PR211 after cells had been treated with hormone, suggesting that AIB1, but not p300, may be involved in enhancing histone acetylation in this region. These findings illustrate the individual properties of the distal PR regions and suggest that each PR region makes distinct contributions to provide the flexibility needed for the fine-tuned regulation of gene expression. Overall, our studies show that the ability of a distal PR region to 1) associate with ERα, AIB1, p300, and AcH4, 2) confer estrogen responsiveness to a heterologous promoter, and 3) affiliate with the proximal PR gene are remarkably interrelated.
Table 1.
Summary of results
| PR+4 | PR48 | PR168 | PR205 | PR211 | PR221 | PR306 | PR311 | |
|---|---|---|---|---|---|---|---|---|
| ERα | + | +++ | +++ | +++ | + | +++ | +++ | ++ |
| AIB1 | − | ++ | + | ++ | + | ++ | + | + |
| p300 | − | + | ++ | ++ | − | ++ | − | + |
| AcH4 | − | + | ++ | ++ | ++ | ++ | − | ++ |
| Tfx | − | ++ | +++ | ++ | − | ++ | + | +++ |
| 3C | N/A | ++ | +++ | + | − | +++ | + | ++ |
The relative changes in association of ERα, AIB1, p300, or AcH4 with the PR gene regions in the absence and in the presence of E2, E2-induced transcription of the distal PR gene regions in transient transfection assays (Tfx), and affiliation of proximal and distal PR gene regions in 3C assays are shown. N/A, Comparison is not applicable.
Effects of ligand on PR gene structure
Although E2 enhanced the association of PR48, PR168, PR205, PR221, PR306, and PR311 with the proximal gene, four of the distal gene regions (PR168, PR220, PR221, and PR311) were associated with the proximal PR region in the absence of E2, albeit to a lower extent than in the presence of E2. Our studies suggest that specific regions of the PR gene are poised for transcription even in the absence of hormone and may be involved in maintaining the low level of PR expression that is observed in MCF-7 cells that have not been exposed to E2. Our findings are similar to a previous study in MCF-7 cells that showed that the basal promoter region of the endogenous pS2 gene is poised for transcription in the absence and in the presence of hormone but that E2 and TOT induce dramatically different patterns of protection in the ERE-containing region of this gene (62). Thus, one mechanism by which these ligands differentially influence gene expression is through inducing ligand- and site-specific alterations in chromatin structure.
Role of chromatin structure in gene expression
Genomic organization requires chromosome compaction but must also allow for dynamic reorganization at the level of the local response element. Little is known about the large-scale organization of chromatin or how this organization facilitates genome management. However, results from our BAC clone controls may provide some insight in this regard. We were interested to find that some of the purified BAC clone DNA fragments had dramatically different ligation efficiencies. Such data make it tempting to posit that the native PR DNA sequence helps to drive chromatin structure. In fact, it would make sense for a cell to use these intramolecular forces to help organize genes and control transcriptional processes.
In addition to the intrinsic structure imposed by DNA sequence, histones and nuclear proteins provide a second layer of control over DNA structure and help to ensure that genes such as the PR gene are accessible. The association of multiple distal PR regions with AIB1 and p300, which possess histone acetyltransferase activity, may help to ensure that histone acetylation is enhanced and that an extended region of this gene is accessible to transcription factors involved in regulating gene expression.
Addition of hormone imposes a third layer of control on PR DNA structure, resulting in discrete, localized changes in chromatin organization. This limited, localized reorganization would allow a cell to maximize speed and minimize error and energy expenditure when responding to hormone. Furthermore, the degree of chromatin compaction required to accommodate 3 billion base pairs of DNA and a plethora of nuclear proteins make it hard to envision massive rearrangements of long stretches of DNA in response to an external signal. Given that six of the seven distal PR regions interact with the proximal PR gene region in the presence of E2 and that four of the distal PR regions interact with the proximal PR gene region in the absence of E2, one would anticipate that the proximal and distal gene regions would be in relatively close proximity where they would be able to interact.
The PR gene has tremendous biological significance, not only for its role in mammary gland development and maintenance of reproductive competence but also for its use as a breast cancer marker. Our analysis of this gene is the most complete of any estrogen-responsive gene examined to date and reveals that multiple cis elements spread over more than 300 kb help to provide the input needed to maintain basal PR expression and respond to hormone with exquisite precision.
Materials and Methods
Cell culture
MCF-7 cells were maintained in phenol red-containing MEM supplemented with 5% calf serum in a humidified incubator with 5% CO2. Before hormone treatment, cells were transferred to phenol red-free MEM containing 5% charcoal dextran-treated calf serum for a minimum of 72 h. C4-12 cells (51), kindly provided by Geoffrey Greene (University of Chicago, Chicago, IL) were maintained on MEM with 10% fetal bovine serum and then transferred to phenol red-free MEM containing 10% charcoal dextran-treated fetal bovine serum for 3C assays. MCF-7 cells were also cultured in these media when they were directly compared with C4-12 cells in 3C assays.
RNA isolation
MCF-7 cells were seeded into six-well plates for 48 h and then treated with ethanol or 10 nm E2 for 45 min, 2 h, or 24 h. RNA was isolated and cDNA was synthesized as described (63). Quantitative real-time PCR (qPCR) was performed using iQSybr Green Supermix and the iCycler PCR thermocycler (Bio-Rad, Hercules, CA) with gene-specific primers for the PR (5′-GTG CCT ATC CTG CCT CTC AAT C-3′and 5′-CCC GCC GTC GTA ACT TTC G-3′), TRPC6 (5′-TGA GGA TGA CGC TGA TGT G-3′ and 5′-ACT CGG CAC CAG ATT GAA G-3′), and 36B4 (5′-GTG TTC GAC AAT GGC AGC AT-3′ and 5′-GAC ACC CTC CAG GAA GCG A-3′) genes. Data from three independent experiments were normalized and combined to determine gene expression changes using the ΔΔCt method as described (64).
Western blots
MCF-7 cells were seeded into six-well plates for 48 h and then treated with ethanol or 10 nm E2 for 45 min, 2 h, or 24 h. Cells were harvested, resuspended in lysis buffer [20 mm Tris (pH 8), 200 mm NaCl, 1 mm EDTA, and 0.2% Nonidet P-40 (NP-40)], and passed through a syringe with a 25-gauge needle 10 times. Cellular debris was pelleted, and the supernatant was fractionated on a 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane as described (28). Proteins were detected with a TRPC6, ERα (sc-19196 and sc-543, respectively; Santa Cruz Biotechnology, Santa Cruz, CA), PR (RM-9102; Lab Vision Corp., Fremont, CA), or GAPDH (TAB1001; Open Biosystems, Huntsville, AL) antibody. Horseradish peroxidase-conjugated secondary antibodies and a chemiluminescent detection system were used as described (28).
ChIP assays
MCF-7 cells were seeded into 150-mm plates and incubated for 96 h. The cells were treated with ethanol or 10 nm E2 for the times indicated. ChIP assays were carried out essentially as recommended by Millipore (Billerica, MA) except that the pelleted cells were washed three times in lysis buffer [10 mm Tris (pH 7.5), 10 mm NaCl, 3 mm MgCl2, and 0.5% NP-40], resuspended in micrococcal nuclease buffer [10 mm Tris (pH 7.5), 10 mm NaCl, 3 mm MgCl2, 10 mm CaCl2, and 4.5% NP-40], and treated with 50 U micrococcal nuclease (USB, Cleveland, OH) for 10 min before sonication. The samples were incubated overnight with a nonspecific antibody directed against fluorescein (Immunological Resource Center, University of Illinois, Urbana, IL) and then incubated with protein A beads (GE Healthcare, Piscataway, NJ). After removal of the beads, the precleared samples were incubated with ERα, AIB1, p300 (sc-8002, sc-5765, and sc-585, respectively; Santa Cruz), AcH4 (04-557; Millipore), or FoxF2 (Ab 23306; Abcam, Cambridge, MA) antibody for 2 h at 4 C. Protein A beads were added and incubated overnight at 4 C. The beads were washed using previously described buffer conditions (41), DNA was purified, and qPCR was used to detect the relative change in the number of genomic copies using iQSybr Green Supermix and the iCycler PCR thermocycler according to the manufacturer's directions (Bio-Rad, Hercules, CA). Standard curves using 1,000, 5,000, 10,000, 50,000, and 100,000 genomic copies were run with each primer set during each experiment. The primers used for amplification were pS2 (5′-CCC GTG AGC CAC TGT TGT C-3′ and 5′-CCT CCC GCC AGG GTA AAT AC-3′), PR+4 (5′-TTG GTT CTG CTT CGG AAT CTG-3′ and 5′-CCT CCT CTC CTC ACT CTT GG-3′), PR48 (5′-AAA TAG GGC AAA GGG AAC AG-3′ and 5′-CCC ACA CTT AAC CCA ATC C-3′), PR168 (5′-GAT GAC AGA AGG AGA AGT TAG AAG-3′and 5′-ATA TGG CAT TGA AGC AAC AGG-3′), PR205 (5′-AAA GAG AGT GAG TCA TTT GTG-3′ and 5′-CAG GAG ATC CGT GAG TTC-3′), PR211 (5′-GGG AAT TAG GGA AGG ATA AAG AGG-3′ and 5′-CCA ACC ACT ACA TTG TCA TCT ATC-3′), PR221 (5′-GGG AAA TTG CCT CTC CTC ACT TTG-3′ and 5′-CCA AGG ATT AGG GCA GTT CAG AAG-3′), PR306 (5′-GAG CAT TAT GAC CTG AGA AG-3′ and 5′-CAA TTT GCC AAT GAA GGA TAG-3′), PR311 (5′-ATG ACA TCA GCA GCA GTG-3′ and 5′-GAA AGA ACA CAC CAA CCT G-3′), PR95 (5′-CAG GCT ATT TCT CAG GTC AG-3′ and 5′-GAC AAA CAC ATT CCC AAA CC-3′), PR110 (5′-AAG AGT GAA CAG CAG CAA G-3′ and 5′-CAG CAG GAT GTG GGT AGG-3′), PR176 (5′-GAAGAACTGCTTGAGACC-3′ and 5′-GTA TAT TTC CCA TCC CAT TAT C-3′), PR309 (5′-GGT GGG AGG ATG AAG GAG TG-3′ and 5′-ACC TAT TAG AAG CCA GTG TTG AC-3′), and 36B4 (5′-GTG TTC GAC AAT GGC AGC AT-3′ and 5′-GAC ACC CTC CAG GAA GCG A-3′).
Plasmids
PR+4 and PR48 were amplified from RPCI11-762MI BAC clone DNA (Invitrogen, Palo Alto, CA) and PR168, PR205, PR211, PR221, PR306, and PR311 were amplified from CTD2148-J14 BAC clone DNA (Invitrogen) with Pfu DNA polymerase (Stratagene, La Jolla, CA) according to the manufacturer's specifications. The site-specific primer sets incorporated a 5′ BglII and a 3′ MluI restriction enzyme sites. Amplified DNA was fractionated on a 1.25% agarose gel. The bands were excised, and DNA was isolated using the DNA Extraction Montage kit (Millipore), digested with BglII and MluI (Invitrogen), and ligated to a BglII/MluI-cut pGL3-Promoter vector (Promega, Madison, WI). Consensus ERE half-site W, 5′-GGTCA-3′, were converted to 5′-AATCA-3′ using the QuikChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The luciferase coding region of the pGL3 control vector was removed and replaced with a renilla coding sequence to produce the control reporter plasmid, pRenilla, which contains the same backbone as the reporter plasmid. All plasmids were sequenced to verify that the DNA sequence was correct, and large-scale plasmid DNA was prepared using the QiaMaxiPrep Kit (QIAGEN Inc., Valencia, CA).
Transient transfections
MCF-7 cells that had been seeded into 24-well plates for 24 h were transfected with 1 μg of an SV40 luciferase reporter plasmid with or without a distal PR gene region or a 2EREpS2 luciferase reporter plasmid (65) and 1 ng pRenilla (Invitrogen) or 0.5 g β-galactosidase reporter plasmid using Lipofectin and incubated for 8 h according to the manufacturer's specifications. The cells were treated with ethanol or 10 nm E2 for 24 h. Luciferase activity was quantified using the dual luciferase assay kit (Promega) and normalized for the amount of renilla activity.
3C assays
3C assays were carried out essentially as described (66). Briefly, MCF-7 cells were seeded into150-mm plates for 96 h and then treated with ethanol or 10 nm E2 for 24 h. The cells were exposed to formaldehyde to form protein-protein and protein-DNA cross-links and then lysed. The cross-linked chromatin was isolated, digested with EcoR1, diluted, and incubated without or with 100 Weiss units of T4 ligase (Invitrogen, Carlsbad, CA) for 4 h at 16 C and 0.5 h at room temperature. The DNA was purified and subjected to qPCR to detect proximal-distal PR ligation products. iQSybr Green Supermix and the iCycler PCR thermocycler were used as described by the manufacturer (Bio-Rad). Melt curves were examined in each experiment for each primer set to ensure that a single product was present. Negative controls included a region that contained four EREs but was not associated with ERα (PR95) and 16 intervening DNA sequences that contain no ERα binding sites. Processing each sample in the absence and in the presence of ligase helped to ensure that the amplification products were ligase dependent. The primers used for qPCR analysis to amplify the ligated proximal and distal PR regions were PR48 (5′-TGG AAG TTA GGC AGA TAG-3′ and 5′-TAA TAA TGG TTA GTG GAC AG-3′), PR95 (5′-TGG AAG TTA GGC AGA TAG-3′ and 5′-GGA AGT ATA GTG GCT ACC-3′), PR168 (5′-GTG GAA GTT AGG CAG ATA GAG-3′ and 5′-CTT GTG GAA TAG TGT CAA TAC G-3′), PR205 (5′-GGA AGT TAG GCA GAT AGA G-3′ and 5′-AAG TGA CTG GTA ACA AGA G-3′), PR211 (5′-ATT TGG AGT TGG TTG TTC-3′ and 5′-GTG TGA TGT TAG ATT CCG-3′), PR221 (5′-GAA GTT AGG CAG ATA GAG-3′ and 5′-TTG CTT AAT ACT TAT TGA GG-3′), PR306 (5′-GGA AGT TAG GCA GAT AGA GG-3′ and 5′-AGT GCT TAT GCT CCA AGG-3′), and PR311 (5′-AGT TGG TTG TTC TAG TAA ATA G-3′ and 5′-GCA TTT CCT CTA CAG ACT C-3′). Sixteen additional primers sets were used to examine intervening and flanking DNA regions, including PR3 (5′-TTA GAT ATG TGG AAG TTA GGC-3′ and 5′-GGG AGT AGG GAG TTA TGC-3′), PR4 (5′-TGG AAG TTA GGC AGA TAG AGG-3′ and 5′-GTG TCT GTG TCT GTT CAA ATC-3′), PR7 (5′-TGT GGA AGT TAG GCA GAT AG-3′ and 5′-TCT ACT CAT TAG GAA CAC TGG-3′), PR9 (5′-AGG ATT TGG AGT TGG TTG TTC-3′ and 5′-AGA AGT AAG AGT AGG GAG ATG G-3′), PR35 (5′-AAG TTA GGC AGA TAG AGG-3′ and 5′-CTC ACA TCA TTT CCC TTC-3′), PR64 (5′-GGA AGT TAG GCA GAT AGA GG-3′ and 5′-CAG TCT TGG GAG ATT CAC G-3′), PR86 (5′-TAT GTG GAA GTT AGG CAG ATA G-3′ and 5′-GCT GTA AAG GCT GAG ACG-3′), PR89 (5′-GGA AGT TAG GCA GAT AGA G-3′ and 5′-TGA GAT GGA GTT AGA ATA GC-3′), PR122 (5′-GGA TTT GGA GTT GGT TGT TC-3′ and 5′-GCT GAG TAA GAC GAG GAG-3′), PR184 (5′-GTG GAA GTT AGG CAG ATA G-3′ and 5′-GGA ATA GTT TGA GTA GGA TTA G-3′), PR220 (5′-GGT TGT TCT AGT AAA TAG-3′ and 5′-AAA GTA ATG AGG TAA ATC-3′), PR230 (5′-GAA GTT AGG CAG ATA GAG G-3′ and 5′-CAT TTC ATT TGT TTA CTA CTC C-3′), PR242 (5′-TGG AAG TTA GGC AGA TAG AG-3′ and 5′-GAG TAT GTG AAA TGT AAA GAG G-3′), PR263 (5′-TTA GAT ATG TGG AAG TTA GGC-3′ and 5′-GTT CTC ATT CTG CTG GAT G-3′), PR300 (5′-AGG CAG ATA GAG GAA AGG-3′ and 5′-GCA TAA CTG ATT GGT AGC-3′), and PR318 (5′-TAG GCA GAT AGA GGA AAG-3′ and 5′-AAC CTT GGA TTA ACT TGG-3′).
To ensure that appropriately sized amplicons were produced at the PR regions of interest, additional primer sets were designed, DNA was amplified, and the resulting amplicons were run on agarose gels. Primer sets used to amplify the ligated products included forward primer 1 (5′-ATC TGG GGA GGG GCT ATA TG-3′) and reverse primers PR48 (5′-GGA CAG TTT TGC CTT CAA GC-3′), PR89 (5′-CAA AGG GCC CCA ATC TAA AG-3′), PR168 (5′-CCA CTT GGT TGC TAT TGG TG-3′), PR205 (5′-GGC AAG AAA ATT CAT CAC TGG-3′), PR221 (5′-GGA CTG TCC ATC TGC AAT TT-3′), PR306 (5′-TGC TCC AAG GTT TTC TTG CT-3′), PR311 (5′-AAA TTT GCC ACC AAA CAA CG-3′), and PR318 (5′-TGC GCT GTC TAC ATC AAA GG-3′). Forward primer 2 (5′-TGT GGA AGT TAG GCA GAT AGA GG-3′) was used with PR220 (5′-CAC CCA TAT GGT ACA GCT TCA A-3′).
To test the efficiency of each primer set, a library of all possible ligation products was created as described (66) using the BAC clones RP11-762MI, CTD2148-J14, and CTD2023-D14 (Invitrogen, Carlsbad, CA). BAC clone libraries were used in both real-time PCR and agarose gel analyses.
Computational motif analysis
Each distal PR gene region was searched for transcription factor binding sites using position weight matrices from the JASPAR CORE database (67) at a relative profile score threshold of 70%. The predicted transcription factor binding site output was analyzed to obtain the frequency of occurrence using an in-house tool command language (TCL) script executed in Visual Molecular Dynamics (VMD) (68) within the Multiseq bioinformatics software package (69). Twenty random 850-bp DNA sequences were generated using an in-house TCL script with an equal probability for each base and searched to obtain the average background frequency of each predicted transcription factor binding site. Predicted transcription factor binding sites present in each of the PR gene regions were determined by subtracting the average number of transcription factor binding sites present in the 20 random DNA sequences from the number of transcription factor binding sites in each of the distal PR regions.
Acknowledgments
We thank Abhi Rao and Dan Thorngren for assistance in the preparation of this manuscript; Chin Yo Lin for providing information about the locations of PR48, PR205, and PR221 before publication; and Karen Kieser for the pS2 vector.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants DK 0601463 and DK53884 (to A.M.N.) and predoctoral support to J.B.M. from the NIH Cell and Molecular Biology Training Grant 5T32GM007283.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 1, 2009
Abbreviations: AcH4, Acetylated histone H4; AIB1, amplified in breast cancer 1; BAC, bacterial artificial clone; 3C, chromosome conformation capture; ChIP, chromatin immunoprecipitation; ERα, estrogen receptor α; ERE, estrogen response element; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NP-40, Nonidet P-40; PR, progesterone receptor; qPCR, quantitative real-time PCR; RAL, raloxifene; SV40, simian virus 40; TOT, 4-hydroxytamoxifen.
References
- Creekmore AL, Ziegler YS, Bonéy JL, Nardulli AM 2007 Estrogen receptor α regulates expression of the breast cancer 1 associated ring domain 1 (BARD1) gene through intronic DNA sequence. Mol Cell Endocrinol 267:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Vega VB, Lin CY, Lai KS, Kong SL, Xie M, Su X, Teh HF, Thomsen JS, Yeo AL, Sung WK, Bourque G, Liu ET 2006 Multiplatform genome-wide identification and modeling of functional human estrogen receptor binding sites. Genome Biol 7:R82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET 2007 Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet 3:e87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR 2007 Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet 3:e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, Liu J, Zhao XD, Chew JL, Lee YL, Kuznetsov VA, Sung WK, Miller LD, Lim B, Liu ET, Yu Q, Ng HH, Ruan Y 2006 A global map of p53 transcription-factor binding sites in the human genome. Cell 124:207–219 [DOI] [PubMed] [Google Scholar]
- Zeller KI, Zhao X, Lee CW, Chiu KP, Yao F, Yustein JT, Ooi HS, Orlov YL, Shahab A, Yong HC, Fu Y, Weng Z, Kuznetsov VA, Sung WK, Ruan Y, Dang CV, Wei CL 2006 Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci USA 103:17834–17839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR 2007 Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 21:2005–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G, Hirst M, Bainbridge M, Bilenky M, Zhao Y, Zeng T, Euskirchen G, Bernier B, Varhol R, Delaney A, Thiessen N, Griffith OL, He A, Marra M, Snyder M, Jones S 2007 Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods 4:651–657 [DOI] [PubMed] [Google Scholar]
- Kumar V, Chambon P 1988 The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell 55:145–156 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Korach KS 1994 Insights from the study of animals lacking functional estrogen receptor. Science 266:1524–1527 [DOI] [PubMed] [Google Scholar]
- Subbiah MTR 1998 Mechanisms of cardioprotection by estrogens. Proc Soc Exp Biol Med 217:23–29 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD 1996 Mechanisms of estrogen action during neural development: mediation by interactions with the neurotophins and their receptors? J Steroid Biochem Mol Biol 56:169–178 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Alexander PS 1983 In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology 113:2195–2201 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP 1993 Human progesterone receptor A form Is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ 2001 Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol 179:97–103 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW 2002 Reproductive functions of progesterone receptors. Recent Prog Horm Res 57:339–355 [DOI] [PubMed] [Google Scholar]
- Cui X, Schiff R, Arpino G, Osborne CK, Lee AV 2005 Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23:7721–7735 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClellend RA, Manning DL, Nicholson RI 1995 Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst 87:746–750 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG 2005 Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast 14:458–465 [DOI] [PubMed] [Google Scholar]
- Graham JD, Yeates C, Balleine RL, Harvey SS, Milliken JS, Bilous AM, Clarke CL 1996 Progesterone receptor A and B protein expression in human breast cancer. J Steroid Biochem Mol Biol 56:93–98 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM 2003 Estrogen receptor α and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol 201:165–175 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM 2005 Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors α and β. J Biol Chem 280:347–354 [DOI] [PubMed] [Google Scholar]
- Petz LN, Ziegler YS, Schultz JR, Nardulli AM 2004 Fos and Jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site. Mol Endocrinol 18:521–532 [DOI] [PubMed] [Google Scholar]
- Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM 2004 Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Biochem Mol Biol 88:113–122 [DOI] [PubMed] [Google Scholar]
- Petz LN, Ziegler YS, Loven MA, Nardulli AM 2002 Estrogen receptor α and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology 143:4583–4591 [DOI] [PubMed] [Google Scholar]
- Petz LN, Nardulli AM 2000 Sp1 binding sites and an estrogen resonse element half-site are involved in regulation of the human progesterone receptor A promoter. Mol Endocrinol 14:972–985 [DOI] [PubMed] [Google Scholar]
- Cheng AS, Jin VX, Fan M, Smith LT, Liyanarachchi S, Yan PS, Leu YW, Chan MW, Plass C, Nephew KP, Davuluri RV, Huang TH 2006 Combinatorial analysis of transcription factor partners reveals recruitment of c-MYC to estrogen receptor-α responsive promoters. Mol Cell 21:393–404 [DOI] [PubMed] [Google Scholar]
- Wang Q, Carroll JS, Brown M 2005 Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell 19:631–642 [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE 2006 An introduction to TRP channels. Annu Rev Physiol 68:619–647 [DOI] [PubMed] [Google Scholar]
- Nardulli AM, Greene GL, O'Malley BW, Katzenellenbogen BS 1988 Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen's effect on progesterone receptor synthesis and degradation. Endocrinology 122:935–944 [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, McDonald WH, Yates JR, Nardulli AM 2006 Protein disulfide isomerase serves as a molecular chaperone to maintain estrogen receptor α structure and function. Mol Endocrinol 20:1982–1995 [DOI] [PubMed] [Google Scholar]
- El Marzouk S, Schultz-Norton JR, Likhite VS, McLeod IX, Yates JR, Nardulli AM 2007 Rho GDP dissociation inhibitor α interacts with estrogen receptor α and influences estrogen responsiveness. J Mol Endocrinol 39:249–259 [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Gabisi VA, Ziegler YS, McLeod IX, Yates JR, Nardulli AM 2007 Interaction of estrogen receptor α with proliferating cell nuclear antigen. Nucleic Acids Res 35:5028–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Norton JR, Walt KA, Ziegler YS, McLeod IX, Yates JR, Raetzman LT, Nardulli AM 2007 The deoxyribonucleic acid repair protein flap endonuclease-1 modulates estrogen-responsive gene expression. Mol Endocrinol 21:1569–1580 [DOI] [PubMed] [Google Scholar]
- Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM 2008 Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol Endocrinol 22:1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM 1997 Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569–580 [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L 1996 Nuclear receptor coactivators and corepressors. Mol Endocrinol 10:1167–1177 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2002 Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA 2004 Histones and histone modifications. Curr Biol 14:R546–R551 [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y 1996 The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953–959 [DOI] [PubMed] [Google Scholar]
- Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL 2006 Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311:844–847 [DOI] [PubMed] [Google Scholar]
- Oduro AK, Fritsch MK, Murdoch FE 2008 Chromatin context dominates estrogen regulation of pS2 gene expression. Exp Cell Res 314:2796–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N 2002 Capturing chromosome conformation. Science 295:1306–1311 [DOI] [PubMed] [Google Scholar]
- Oesterreich S, Zhang P, Guler RL, Sun X, Curran EM, Welshons WV, Osborne CK, Lee AV 2001 Re-expression of estrogen receptor α in estrogen receptor α-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res 61:5771–5777 [PubMed] [Google Scholar]
- Jordan VC, Dix CJ 1979 Effect of oestradiol benzoate, tamoxifen and monohydroxytamoxifen on immature rat uterine progesterone receptor synthesis and endometrial cell division. J Steroid Biochem 11:285–291 [DOI] [PubMed] [Google Scholar]
- Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC 1988 Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res 48:812–815 [PubMed] [Google Scholar]
- Jordan VC, Phelps E, Lindgren JU 1987 Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat 10:31–35 [DOI] [PubMed] [Google Scholar]
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL 1992 Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 326:852–856 [DOI] [PubMed] [Google Scholar]
- Laganière J, Deblois G, Giguère V 2005 Functional genomics identifies a mechanism for estrogen activation of the retinoic acid receptor alpha1 gene in breast cancer cells. Mol Endocrinol 19:1584–1592 [DOI] [PubMed] [Google Scholar]
- Klinge CM 2001 Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JR, Greene GL, Nardulli AM 1998 Estrogen response elements function as allosteric modulators of estrogen receptor conformation. Mol Cell Biol 18:1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven MA, Wood JR, Nardulli AM 2001 Interaction of estrogen receptors α and β with estrogen response elements. Mol Cell Endocrinol 181:151–163 [DOI] [PubMed] [Google Scholar]
- Wood JR, Likhite VS, Loven MA, Nardulli AM 2001 Allosteric modulation of estrogen receptor conformation by different estrogen response elements. Mol Endocrinol 15:1114–1126 [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L, Ryffel GU, Heitlinger E, Cato ACB 1988 A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res 16:647–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Petz LN, Ziegler YS, Wood JR, Potthoff SJ, Nardulli AM 2000 Regulation of the estrogen-responsive pS2 gene in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol 74:157–168 [DOI] [PubMed] [Google Scholar]
- Curtis CD, Likhite VS, McLeod IX, Yates JR, Nardulli AM 2007 Interaction of the tumor metastasis suppressor nonmetastatic protein 23 homologue H1 and estrogen receptor α alters estrogen-responsive gene expression. Cancer Res 67:10600–10607 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS 2002 Antagonists selective for estrogen receptor α. Endocrinology 143:941–947 [DOI] [PubMed] [Google Scholar]
- Miele A, Gheldof N, Tabuchi TM, Dostie J, Dekker J 2006 Mapping chromatin interactions by chromosome conformation capture. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, eds. Current protocols in molecular biology. New York: Greene Publishing Associates and Wiley Interscience; 21.11.1–21.11.20 [DOI] [PubMed] [Google Scholar]
- Bryne JC, Valen E, Tang MH, Marstrand T, Winther O, da Piedade I, Krogh A, Lenhard B, Sandelin A 2008 JASPAR, the open access database of transcription factor-binding profiles: new content and tools in the 2008 update. Nucleic Acids Res 36:D102–D106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K 1996 VMD: visual molecular dynamics. J Mol Graph 14:33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- Roberts E, Eargle J, Wright D, Luthey-Schulten Z 2006 MultiSeq: unifying sequence and structure data for evolutionary analysis. BMC Bioinformatics 7:382 [DOI] [PMC free article] [PubMed] [Google Scholar]