Abstract
The current statistics associated with breast cancer continue to show a relatively high recurrence rate together with a poor survival for aggressive metastatic disease. These findings reflect, in part, the pharmaceutical intractability of processes involved in the metastatic process and highlight the need to identify additional drug targets for the treatment of late-stage disease. In the current study, we report that ligand activation of the aryl-hydrocarbon receptor (AhR) inhibits multiple aspects of the metastatic process in a panel of breast cancer cell lines that represent the major breast cancer subtypes. Specifically, it was observed that treatment with exogenous AhR agonists significantly inhibited cell invasiveness and motility in the Boyden chamber assay and inhibited colony formation in soft agar regardless of estrogen receptor (ER), progesterone receptor, or human epidermal growth factor receptor 2 status. Knockdown of the AhR using small interfering RNA duplexes demonstrated that the inhibition of invasiveness was receptor dependent and that endogenous receptor activity was protective in each cell type examined. The inhibition of invasiveness and anchorage-independent growth correlated with the ability of exogenous AhR agonists to promote differentiation. Finally, exogenous AhR agonists were able to promote differentiation in a putative mammary cancer stem cell line. Cumulatively, these results suggest that the AhR plays an important role in mammary epithelial differentiation and, as such, represent a promising therapeutic target for a range of phenotypically distinct human breast cancers.
The AhR inhibits invasive properties and promotes differentiation of breast cancer cells; thus, the receptor represents a promising therapeutic target for human breast cancers.
Breast cancer is currently the most prevalent malignancy among women in industrialized countries and comprises 30% of all cancers found in women. The statistics indicate that one in eight women will develop breast cancer at some point in her lifetime, and nearly 200,000 women will be diagnosed with breast cancer in the United States each year (1,2). For metastatic breast cancer specifically, it is estimated that nearly 155,000 women in the United States are currently living with the disease, and this number is expected to increase by 5% over the next several years (3). Despite the availability of adjuvant therapies targeting estrogen and growth factor-signaling pathways, the incidence and mortality of breast cancer have not declined at the same rate as other major causes of death, which highlights the need for new therapeutic targets and treatment modalities.
The aryl-hydrocarbon receptor (AhR) was initially identified in early toxicology studies that observed an increase in mono-oxygenase activity after exposure to polyaromatic hydrocarbons. Genetic and biochemical studies mapped the response to a single autosomal locus and led the eventual cloning of the AhR (4,5,6,7). Molecular characterization of the AhR across vertebrate and invertebrate species demonstrated that the receptor is highly conserved (8) and plays a significant role in tissue development (9,10,11,12,13). In the mouse, for instance, targeted disruption of the AhR results in altered immune function (14), ovarian follicle development (15), seminal vesicle maintenance (16), vascular remodeling (17), and mammary development (18).
The cancer-related effects of AhR activation have been primarily characterized using xenobiotic ligands. Similar to the nuclear hormone receptors, these studies have identified context- and tissue-specific effects that include tumor promotion in certain tissues and a decreased tumor incidence in others. In occupational exposures to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), workers had an increased risk for all combined cancers and for lung cancer (19). A separate epidemiological study of a population exposed to TCDD after an industrial accident found a significant decrease in breast and endometrial cancers (20). More recently, a report following a population exposed to dioxin emissions from a municipal waste incinerator noted a significant decrease in incidence of invasive breast cancer in women living in the highest exposed zone (21). The tissue-related differences in the tumor response in humans were also observed in rodent studies. In a recent comprehensive rodent cancer bioassay study, an increased tumor incidence was observed in the liver, lung, and oral mucosa, whereas a significant decrease in tumors was observed in the mammary gland, pituitary, and thyroid (22). A reduction in 7,12-dimethylbenz[a]anthracene-induced mammary tumors has also been observed after treatment with TCDD (23).
Mechanistic studies on the protective effects of AhR agonists in breast cancer have indicated that this receptor is engaged in the regulation of several distinct processes. It has been demonstrated that the protective effects of AhR ligands on tumor growth are related to the ability of the receptor to antagonize estrogen receptor α (ERα) signaling (24,25,26,27,28). The functional consequences of the antagonistic AhR-ER cross talk are apparent in both in vitro and in vivo models in which TCDD was shown to completely reverse the proliferative effects of estrogens (29,30). In addition to the antagonistic effects on ERα signaling, the AhR also regulates key processes required for breast cancer cell growth, cell cycle control, chemokine signaling, and cell migration (31). Previous studies have demonstrated that TCDD can down-regulate both CXCR4 and CXCL12 in a breast cancer cell line and decrease cell migration toward a CXCL12 gradient (32).
The initial identification of the AhR as the receptor for polyaromatic hydrocarbons has contributed to its perceived role as a xenobiotic sensor. However, a growing body of evidence indicates that the AhR modulates critical aspects of cellular function that may support pursuit of the receptor as a pharmaceutical target. The objectives of this study were to 1) characterize AhR activity in a panel of human breast cancer cell lines that represent the major breast cancer subtypes; 2) define the relationship between AhR activation and processes involved in tumor progression and metastasis; and 3) investigate the mechanisms that contribute to the protective effects of AhR agonists in breast cancer cells.
Results
AhR is expressed in normal breast and in a range of human breast tumor types
Previous studies have examined whether the AhR is expressed and transcriptionally active in a variety of different breast cancer cell lines (reviewed in Ref. 33). However, few studies have characterized the expression of the AhR in human breast tumor samples. This knowledge is important for determining whether the receptor may be a relevant target in human breast cancer. To address this question, quantitative RT-PCR (qPCR) was used to compare expression of AhR mRNA in normal breast tissue with that in human breast tumors of various stages. Expression was measured in tissues derived from 48 patients with various stages of breast cancer ranging from normal (disease-free) to stage III (advanced; metastatic). Although marginal increases were observed in stage 0 and I, expression of AhR mRNA did not drop significantly below that in normal breast tissue (Fig. 1). In addition to the expression of the AhR mRNA, the presence of AhR protein has been demonstrated in a limited number of human breast tumors samples (http://www.proteinatlas.org).
Figure 1.
AhR is expressed in normal breast and in a range of human breast tumors. Tissues were derived from 48 patients, and RNA was isolated and provided as first-strand cDNAs on the TissueScan Breast Tissue pPCR Array Panel I (Origene Technologies; BCRT-01). qPCR was used to analyze hAhR expression in the 48 independent patient-derived tissue samples. Data were normalized to β-actin, and samples in each tissue type (Normal or stage 0–III) were averaged. Graphic data are depicted as relative fold expression over the average of values obtained using normal breast samples (set at 1). Error bars represent sd among independent samples of each tissue type. The distribution of the 48 patient samples represented as a function of breast tissue tumor grade was: normal breast: two samples; stage 0: five samples; stage I: 10 samples; stage II, 20 samples; stage III, 11 samples.
AhR responsiveness is a characteristic of both ER-negative and ER-positive breast cancer cells
The majority of human breast tumors can be categorized based upon their expression of ERα, the progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). To reflect these categories in the current studies, four human breast cancer cell lines were selected: MDA MB-231 (ER−, PR−, HER2−), SKBR3 (ER−, PR−, HER2+), MCF-7 (ER+, PR+, HER2−), and ZR-75-1 (ER+, PR+, HER+). The presence of AhR protein in each of these cell lines was verified by Western blotting (data not shown). The biological activity of the AhR was assessed based on the induction of cytochrome P450 (CYP)1A1 mRNA using qPCR. This analysis demonstrated that CYP1A1 was highly up-regulated by TCDD in all cell lines (Fig. 2, A–C). In addition to CYP1A1, the expression of two genes associated with breast cancer metastasis, CXCR4 and matrix metalloproteinase-9 (MMP-9), was significantly repressed by TCDD in both ER− and ER+ breast cancer cells. Exposure to TCDD repressed both the basal expression and 17β- estradiol (E2)-dependent induction of both genes. Together, these results demonstrate that the AhR is biologically active in all four cell lines examined and that ligand activation of the receptor by TCDD suppresses the expression of sentinel genes associated with breast cancer cell metastasis. Finally, we observed that the AhR displays a mutually antagonistic relationship with ER, a result that is consistent with that reported previously in different model systems (24,25,26,27,28).
Figure 2.
Dioxin responsiveness is a characteristic of both ER-negative and ER-positive breast cancer cells. A, CYP1A1, CXCR4, and MMP-9 in SKBR3 or MDA MB-231 ER-negative breast cancer cells after 24-h exposure to 10 nm TCDD. Cells were harvested for total RNA, which was used for qPCR. Tabular data are represented as fold induction over vehicle (Veh) (set at 1). B and C, Fold induction of CYP1A1, CXCR4, and MMP-9 in MCF-7 or ZR-75-1 ER-positive breast cancer cells. For panels A–C, data represent the average of triplicate amplification reactions for each condition in a representative experiment; n = 3 independent assays. *, P < 0.05 for comparison between Veh and TCDD. +, P < 0.05 for comparison between Veh and E2. a, P < 0.05 for comparison between E2 and E2 + TCDD.
AhR agonists inhibit invasiveness of human breast cancer cells
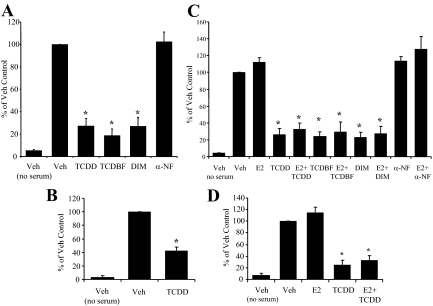
Metastasis requires the acquisition of cellular characteristics that enable motility and invasiveness (35,36). The ability of AhR agonists to inhibit these activities in vitro was assessed using a modified Boyden chamber system. In both ER− and ER+ breast cell lines, TCDD treatment resulted in a consistent 55% (MDAMB-231) to 70% (SKBR3, MCF-7, ZR-75-1) reduction in the number of invaded cells (Fig. 3, A–D). Similar results were achieved when cells were exposed to the AhR agonists, tetrachlorodibenzofuran (TCDBF) and diindolylmethane (DIM). In contrast, the AhR antagonist α-naphthoflavone (α-NF) had no effect (Fig. 3, A and C). In addition, no significant changes were observed with E2 treatment (Fig. 3, C and D), which is consistent with previous reports that ER activation does not protect against cancer cell spread (37). Cell viability assays confirmed that the inhibitory effects seen with the AhR ligands were not due to cell toxicity or cell death (data not shown). Taken together, these studies indicate that AhR agonists (representing several different chemotypes) are capable of inhibiting breast cancer cell invasion in a manner that is not significantly impacted by ER, PR, or HER2 status.
Figure 3.
AhR agonists inhibit invasiveness of human breast cancer cells. A, Invasiveness of SKBR3 cells after 24-h exposure to vehicle (Veh) or multiple AhR ligands (TCDD, 10 nm; TCBDF, 10 nm; and DIM, 20 μm) and the AhR antagonist α-NF. Graphic data are represented as percent of Veh control (set at 100%). B, Invasiveness of MDA MB-231 cells after 24-h exposure to Veh or TCDD (10 nm). C, Invasiveness of MCF-7 cells after 24-h exposure to Veh or multiple AhR ligands (TCDD, 10 nm; TCBDF, 10 nm; and DIM, 20 μm) and the AhR antagonist α-NF in the presence and absence of 10 nm E2. D, Invasiveness of ZR-75-1 cells after 24-h exposure to Veh or TCDD (10 nm) in the presence and absence of 10 nm E2. For panels A–D, n = 3–4 independent assays, and bars are mean ± sd. *, P < 0.05 compared to vehicle.
Knockdown of AhR reverses inhibition of motility and invasiveness and increases endogenous levels of invasiveness
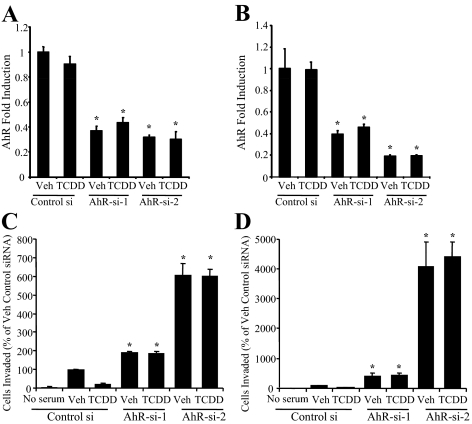
The inhibition of motility and invasiveness by different classes of agonists suggests that the effects noted are receptor dependent. To confirm the requirement for AhR in these processes and to probe the specific role(s) for ligand in this process, we evaluated invasiveness in SKBR3 and MDA MB-231 breast cancer cells after treatment with either of two small interfering RNA (siRNA) duplexes directed against the receptor siRNA 1 targeting AhR (AhR-si-1) and AhR-si-2. A reduction in AhR mRNA was confirmed using qPCR analysis and showed approximately 60% (AhR-si-1) and 60–75% (AhR-si-2) reduction in receptor expression (Fig. 4, A and B). In cells transfected with a control, nontargeting siRNA duplex, TCDD treatment significantly inhibited invasiveness (Fig. 4, C and D). The effects of TCDD were completely reversed in cells treated with either of the two siRNA duplexes targeting the AhR. In cells treated with the dimethylsulfoxide vehicle, invasiveness was significantly increased in both cell lines transfected with AhR-si-1, and even further with AhR-si-2. The increase in invasiveness observed after the addition of the two siRNA duplexes correlated with the degree of knockdown as assessed by qPCR (Fig. 4, A–D). Together these studies suggest that the inhibition of motility and invasiveness by exogenous AhR agonists are receptor mediated, and furthermore raise the possibility that endogenous receptor activity may confer some level of protection against metastasis.
Figure 4.
AhR expression and activation is associated with protection against breast cancer cell invasiveness. qPCR analysis of endogenous AhR expression. SKBR3 cells (panel A) and MDA MB-231 cells (panel B) were transfected either a nontargeting siRNA duplex (Control si) or one of two independent AhR siRNAs (AhR-si-1, AhR-si-2). After 24 h, cells were treated with vehicle (Veh) or TCDD (10 nm) for 24 h. Cells were harvested 48 h after transfection for total RNA, which was used for qPCR. Graphic data are represented as fold induction over vehicle (set at 1). Data points represent the average of triplicate amplification reactions for each condition in a representative experiment (n = 3 independent assays). Invasiveness of SKBR3 cells (panel C) and MDA MB-231 cells (panel D) in the modified Boyden chamber invasion assay. Before assay, cells were transfected with siRNAs for 48 h and administered Veh or TCDD (10 nm) for 24 h. Graphic data are represented as percent of Veh control (set at 100%). For panels C and D, a representative experiment (n = 3 independent assays) is shown. In panels A–D, *, P < 0.05 for comparison between Control si and AhR-si-1 and Control si and AhR-si-2 for each treatment (Veh or TCDD).
AhR agonists inhibit colony formation of breast cancer cells
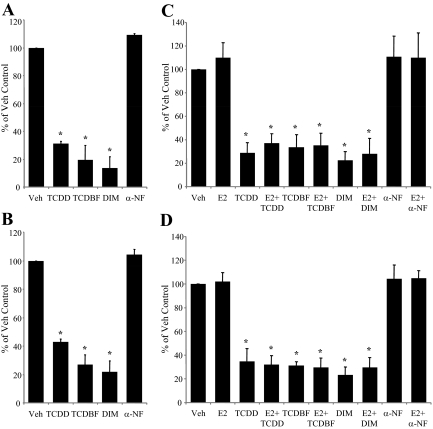
Another step in cancer progression is the ability of a cancer cell to grow in the absence of adhesion to a surface (i.e. anchorage-independent growth). The molecular mechanisms involved in anchorage-independent growth mirror those involved in aggressive tumor growth in vivo, and this step is considered to be the best in vitro correlate of tumorigenicity (38). To evaluate the effects of AhR agonists on anchorage-independent growth, each of the four breast cancer cell lines under study were plated in soft agarose, and the number of colonies was measured after 21 d. In ER− cells, all AhR agonists tested resulted in a significant reduction in colonies, whereas treatment with the AhR antagonist α-NF had no effect (Fig. 5, A and B). Likewise, in the ER+ cells, the AhR agonists exhibited a similar ability to suppress colony formation, and cotreatment with E2 did not appear to have an effect in the system (Fig. 5, C and B). As an additional control, colony formation in HeLa cells, which lack AhR expression and activity, was not affected by any of the AhR ligands (supplemental Fig. 1 published as supplemental data on The Endocrine Society's Journals Online web site at http://endojournals.mend.org). Thus, treatment with exogenous AhR agonists, acting through their cognate receptor, clearly inhibits anchorage-independent growth of breast cancer cells regardless of ER, PR, or HER2 status.
Figure 5.
AhR agonists inhibit colonization of breast cancer cells. Results from the soft-agar colony formation assay. Anchorage-independent growth of SKBR3 cells (A) and MDA MB-231 cells (B) after continuous exposure to multiple AhR ligands for 21 d (TCDD, 10 nm; TCBDF, 10 nm; and DIM, 20 μm) and the AhR antagonist α-NF (10 μm). Cells were plated in six-well plates in soft agar containing AhR ligand and overlaid with media containing ligand. Graphic data are represented as percent of Vehicle (Veh) control (set at 100%). For A and B, n = 5 independent assays for TCDD and TCDF; n = 3 independent assays for DIM and α-NF. Anchorage-independent growth of MCF-7 cells (C) or ZR-75-1 cells (D) after continuous exposure to multiple AhR ligands as described above, in the presence or absence of E2 (10 nm) (for C and D, n = 3 independent assays). For A–D, bars are mean ± sd. *, P < 0.01. All independent assays contained triplicate wells for each treatment condition.
AhR agonists induce markers of breast epithelial cell differentiation
Among the hallmarks of aggressive cancer cells are a persistent replicative potential, insensitivity to antigrowth signals, and a poorly differentiated phenotype (39). Certain cancer therapies function by inducing cell cycle arrest, blocking replication, and promoting differentiation of cancer cells into cells resembling their tissue of origin (40,41). Typical endpoints associated with epithelial cell differentiation include alterations in cell morphology, cytoskeleton rearrangement, changes in cell-cell junctions, and the expression of lineage-specific features (42,43).
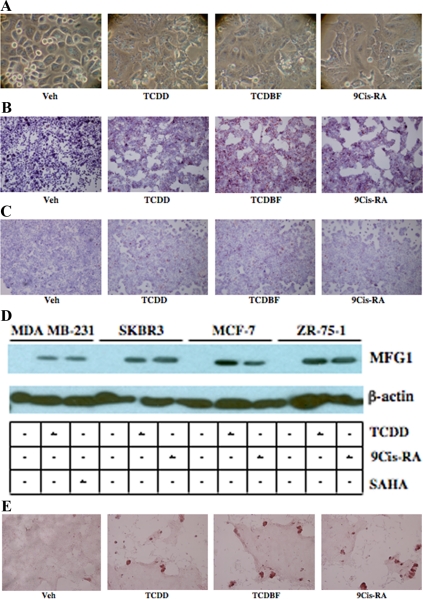
Although a number of studies have shown that exogenous AhR agonists can inhibit proliferation, little is known about the effect of AhR agonists on breast cancer differentiation. Thus, the ability of exogenous AhR agonists to induce morphological changes consistent with mammary epithelial differentiation was examined in SKBR3 cells by light microscopy. Cells administered TCDD or TCDBF for 48 h displayed morphological changes including a flat appearance, loss of rounded shape, increased cytoplasmic-nuclear ratio, and high degree of adherence compared with vehicle-treated cells (Fig. 6A). These changes were comparable to those observed in cells administered 9-cis-retinoic acid (9-cisRA), an agent known to induce differentiation of breast cancer and other cancer cell types (44,45,46).
Figure 6.
Activation of AhR induces markers of breast epithelial cell differentiation. A, SKBR3 cells were seeded in six-well plates and treated with vehicle (Veh), TCDD (10 nm), TCDBF (10 nm), or 9-cisRA (1 μm) for 48 h. Cells were imaged by light microscopy. SKBR3 cells (B) and MCF-7 cells (C) were seeded in six-well plates and treated for 48 h as described above. Cells were fixed, stained with Oil Red O, counterstained with hematoxylin, and imaged by light microscopy. Red granules represent cellular lipid deposits. For A–C, a representative experiment (n =3 independent experiments each) is shown in each case. D, SKBR3, MDA MB-231, MCF-7, and ZR-75-1 cells were treated for 48 h with Veh (lanes 1, 4, 7, and 10), 10 nm TCDD (lanes 2, 5, 8, and 11), 500 nm SAHA (lane 3), or 1 μm 9-cisRA (lanes 6, 9, and 12). Cells were lysed and whole-cell extracts were prepared and used for SDS-PAGE and Western blotting using a rabbit antihuman antibody to MFG1. A mouse antihuman β-actin antibody was used as a control for total protein between different samples. Shown is a representative experiments (n = 4 independent experiments). E, LA7 cells were seeded in four-well chamber slides and treated with TCDD, TCDBF, or 9-cisRA as described above for 48 h. Cells were fixed, stained with Oil Red O, counterstained with hematoxylin, and imaged by light microscopy.
Apart from morphological changes, differentiation of breast cancer cells results in the expression of lineage-specific features including the intracellular accumulation of lipids and milk proteins (40,43,47). To examine the effect of exogenous AhR agonists on cellular lipid content, SKBR3 and MCF-7 cells were treated with TCDD and TCDBF for 48 h. After treatment, the cells were fixed, stained with Oil Red O, and examined by light microscopy. In both the ER+ and ER− cell lines, treatment with exogenous AhR agonists caused an increase in cell lipid content that was comparable to 9-cisRA (Fig. 6B). To assess changes in milk protein production, the expression of κ-casein mRNA and milk fat globulin 1 (MFG1) protein (a component of the milk fat globule membrane) were measured using qPCR and Western blotting, respectively. As shown in Table 1, TCDD treatment induced large increases in κ-casein mRNA in SKBR3, MCF-7, and ZR-75-1 cells. The changes were comparable to that seen with 9-cisRA. In MDA MB-231 cells, a relatively modest increase in κ-casein mRNA was observed compared with the other cell lines. The less dramatic effect of TCDD on MDA MB-231 cells is likely cell specific, because these cells displayed no change in κ-casein expression after treatment with 9-cisRA and a minimal response to the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) (Table 1 and data not shown). Similar to the effects on κ-casein expression, administration of TCDD resulted in a marked increase in MFG1 protein. The TCDD-related effects on MFG1 were also comparable to that seen with either SAHA or 9-cisRA (Fig. 6D). Taken together, these observations indicate that treatment with exogenous AhR agonists induce phenotypic and biochemical changes in breast cancer cells that are consistent with breast epithelial cell differentiation. Furthermore, the AhR agonists appear to be as effective as agents such as 9-cisRA and SAHA that are currently in clinical trials for treatment of breast cancer.
Table 1.
AhR agonist TCDD markedly enhances expression of κ-casein in breast cancer cells
| Fold induction of κ-casein in four breast cancer cell lines
|
|
|
|
|---|---|---|---|
| Veh | TCDDa | 9-CisRA | |
| MDA MB-231 | 1 | 137 | 346b |
| SKBR3 | 1 | 5.64 × 108 | 1.76 × 109 |
| MCF-7 | 1b | 3.43 × 107 | 1.01 × 108 |
| ZR-75-1 | 1 | 1.75 × 107 | 4.57 × 107 |
qPCR analysis of endogenous κ-casein expression. MDA MB-231 cells were treated for 48 h with Veh, TCDD (10 nm) or SAHA (500 nm). SKBR3, MCF-7, and ZR-75-1 cells were treated for 48 h with Veh, TCDD (10 nm), or 1 mm 9-cisRA. Cells were harvested for total RNA, which was used for qPCR. Tabular data are represented as fold induction over vehicle for each cell line (set at 1). Data represent the average of triplicate amplification reactions for each condition in a representative experiment (n = 3 independent assays).
P < 0.01 for comparison between Veh and TCDD.
For MDA MB-231 cells 500 nm SAHA was substituted for 9-CisRA.
AhR agonists induce differentiation of putative breast cancer stem cells
According to the cancer stem cell hypothesis, tumors arise from a subpopulation of cancer cells that retain stem cell-like characteristics (48). Although the hypothesis is still controversial, there is considerable interest in developing therapeutic agents capable of targeting cancer stem cells (49). Given the results in the preceding experiments showing differentiation across multiple human breast cancer cell lines, the effects of exogenous AhR agonists on a putative rat mammary cancer stem cell line (LA7) were examined. The LA7 line has been previously characterized and possesses the hallmark features of cancer stem cells (50). Treatment with TCDD or TCDBF for 48 h caused significant morphological changes in LA7 cells (Fig. 6E) including the dome formation that is characteristic of early-stage lactogenic differentiation in the mammary gland (51). Staining with Oil Red O further revealed the accumulation of lipid droplets in a manner comparable to 9-cisRA (Fig. 6E). The results demonstrate that exogenous AhR agonists can differentiate putative mammary cancer stem cells and suggest that the AhR regulates key genes involved in mammary development and differentiation.
Discussion
Approximately 20–30% of all women first diagnosed with localized breast cancer eventually develop regional or distant metastases. The 5-yr survival rate for breast cancer with local lymph node involvement is 84%, whereas the survival rate for cases with distant metastases is only 27% (3). The relatively high recurrence rate together with the poor survival for aggressive metastatic breast cancer suggests that additional therapeutic approaches are necessary for the treatment of this disease.
Metastasis is a complex process that involves cellular dissociation, invasion, motility, colonization, and proliferation (35,36). In the present study, the AhR was found to inhibit multiple aspects of this process in human breast cancer cells regardless of ER, PR, or HER2 status. Treatment with exogenous AhR agonists significantly inhibited cell invasiveness and motility in the Boyden chamber assay and inhibited colony formation in soft agar. Knockdown of the AhR using siRNA duplexes demonstrated that although exogenous agonists afforded a heightened level of protection against invasion, there was an increase in invasiveness that occurred upon knockdown of the receptor alone. These data suggest the possibility that an endogenous AhR ligand exists within the cells (or in the media) and/or that the activity of the receptor is regulated by signaling pathways that are operative in the cellular models of breast cancer under study. Given these findings, it might be expected that AhR expression and activity might be selected against during breast cancer progression. However, measurement of AhR mRNA did not show a significant decrease in more aggressive stage II and III tumors, suggesting that if dysregulation of the AhR does confer a selective advantage, then this might occur as a consequence of posttranscriptional modifications that alter receptor activity. Alternatively, previous research has demonstrated a role for endogenous AhR activity in facilitating cell cycle progression (52). As a result, the selection process may be balancing the apparent advantage of inhibiting AhR activity for invasion, motility, and colonization with the need for growth and proliferation. Importantly, the role of the AhR in facilitating cell cycle progression is only manifest in the absence of an exogenous ligand because treatment with AhR agonists inhibits cell cycle progression (31,53,54,55). As a result, it is anticipated that AhR agonists could be useful for the treatment of breast cancer and would allow inhibition of both the metastatic processes and cell proliferation.
The ability of exogenous AhR agonists to inhibit processes related to breast cancer metastasis, in both ER− and ER+ cells, indicates that the mechanism is not limited to antagonistic action on ERα signaling. Rather, the present studies suggest that the underlying mechanism for the protective effects is transcriptional regulation of genes involved in mammary epithelial differentiation. This observation is consistent with previous reports demonstrating a role for AhR in mammary gland development. In rodent models, targeted disruption of AhR results in a significant reduction in estrus-induced terminal end bud formation whereas treatment with AhR agonists have been shown to inhibit mammary gland development by suppressing epithelial cell growth and inducing permanent differentiation (18,56,57).
The popular view of the role of AhR in physiology suffers from the toxicological origins of the receptor. In reality, the AhR may be viewed in the same light as other cellular receptors (e.g. ER, androgen receptor, and peroxisome proliferator-activated receptor) with a normal physiological role that can be disrupted by xenobiotic chemicals rather than a receptor that evolved primarily as a xenobiotic sensor. As a pharmacological treatment for breast cancer, the therapeutic benefit of any AhR agonist must be balanced against its adverse effects (i.e. reasonable therapeutic index). For the tumor-promoting effects of AhR agonists, a rodent cancer bioassay using a stop-exposure design found no significant increase in any tumors after exposure to TCDD for 30 wk followed by vehicle exposure for the remainder of the 2-yr study (22). Notably, a marginal reduction in the baseline incidence of mammary tumors was observed in the same animals (P = 0.064). For non-cancer-related endpoints, it has been proposed that selective AhR modulators exist that are capable of activating the specific signaling pathways involving the protective effects of the AhR in breast cancer while limiting activation of those pathways leading to the adverse effects (58). Evidence for the feasibility of this concept is mounting. First, nontraditional AhR ligands have been identified that are capable of activating the receptor without association with the ligand-binding pocket (59). Second, similar to steroid hormone receptors (60), different ligands induce unique conformational changes in the AhR that facilitate interaction with different transcriptional cofactors (61). On a functional level, the AhR agonists I3C, DIM, and a series of DIM analogs were shown to effectively inhibit the growth of estrogen-dependent and -independent breast cancer cells and tumors in a manner comparable to TCDD (62,63,64,65,66). Structure-activity studies using the DIM analogs showed that specific methyl and dihalo substitutions could further enhance the anticancer effects above those substantial effects seen for DIM alone (62,63,65). AhR agonists can also be optimized for tissue-selective responses. The AhR agonist, 6-methyl-1,3,8-trichlorodibenzofuran, was found to synergize with tamoxifen to inhibit growth of mammary tumors in rodents while inhibiting the undesirable estrogenic effects of tamoxifen in the uterus and did not interfere with the bone-protective actions of tamoxifen in ovariectomized animals (65). Importantly, effective doses of DIM and 6-methyl-1,3,8-trichlorodibenzofuran, did not produce the adverse side effects observed with TCDD exposure (34,62,65,67).
In summary, the present studies demonstrate that the AhR and its agonists may confer protective effects in multiple breast cancer subtypes by inhibiting invasive and metastatic features and inducing differentiation. These results support previous epidemiological and rodent studies showing a decrease in breast cancer incidence after exposure to AhR agonists. Given the current challenges in treating aggressive metastatic breast cancer, the clinical development of selective AhR modulators may provide an effective, broad-based alternative to current adjuvant therapies.
Materials and Methods
Biochemicals
TCDD and TCDBF were purchased from AccuStandard (New Haven, CT). α-NF, E2, dimethylsulfoxide, 9-cisRA, and SAHA were purchased from Sigma Chemical Co. (St. Louis, MO). DIM was a gift from Dr. Jim Joseph (Duke University Medical Center, Durham, NC). Oligonucleotides were obtained from Invitrogen (Carlsbad, CA). siRNA duplexes were purchased from Dharmacon (Lafayette, CO). qPCR reagents were obtained from Applied Biosystems, Inc. (Foster City, CA). Oil Red O was purchased from Poly Scientific (Bay Shore, NY).
Mammalian cell culture
Cell lines were obtained from American Type Culture Collection (Manassas, VA). MDA MB-231, SKBR3, MCF-7, and ZR-75 (human breast adenocarcinoma) cells were maintained in DMEM supplemented with 8% fetal bovine serum (FBS), 0.1 mm nonessential amino acids, and 1 mm sodium pyruvate (Invitrogen). LA7 (rat mammary carcinoma) cells were maintained in DMEM supplemented with 5% FBS (Invitrogen), and 4.5 g/liter glucose, 0.005 mg/ml insulin, and 20 mm HEPES (Sigma). All cell lines were grown in a 37 C incubator with 5% CO2.
RNA isolation and qPCR
For gene expression analysis in the cell culture studies, SKBR3, MDA MB-231, MCF-7, and ZR-75-1 cells were seeded in six-well plates. After 24 h or 48 h, cells were administered ligands for 24 h in phenol-red free DMEM supplemented with 8% charcoal-stripped FBS [charcoal-dextran (C/D) FBS] (Hyclone Laboratories, Inc., Logan, UT). Cells were harvested and total RNA was isolated using the RNeasy kit (QIAGEN, Valencia, CA). RNA (1 μg) was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit synthesis kit (Applied Biosystems). The ABI PRISM 7900HT Real Time PCR System was used to amplify and quantitate levels of target gene cDNA. qPCRs were performed using 0.1 μl of cDNA, 10 mm gene-specific primers, and 2× SYBR Green Mastermix (Applied Biosystems). Data are the mean ± sem of three biological replicates performed in triplicate. The following primers were used: CYP1A1, 5′-CGGCCACGGAGTTTCTTCT-3′ (forward) and 5′-GGGTCAGCATGTGCCCAAT-3′ (reverse); CXCR4, 5′-TACACCGAGGAAATGGGCTCA-3′ (forward) and 5′-AGATGATGGAGTAGATGGTGGG-3′(reverse); MMP-9, 5′-GGGACGCAGACATCGTCATC-3′(forward) and 5′-TCGTCATCGTCGAAATGGGC-3′(reverse); and κ-casein, 5′-TTTGGCTGTGGAGGTTCAAAA-3′ (forward) and 5′-TTGGCACATAATACATTGGGACA-3′ (reverse).
AhR expression in human breast tumors was analyzed using the TissueScan Breast Tissue pPCR Array Panel I according to the manufacturer's protocols (Origene Technologies, Rockville, MD).Reactions were amplified as above using β-actin primers provided by the manufacturer and the following primers for human AhR:
5′-ACATCACCTACGCCAGTCG-3′ (forward) and
5′-CTCTATGCCGCTTGGAAGGAT-3′ (reverse).
Mammalian cell siRNA transfection
Validated siRNAs directed against a control nontargeting sequence or two different regions of AhR were obtained from Dharmacon. For transfections, SKBR3 and MDA MB-231 cells were plated in six-well plates for 24 h. Before transfection, media was replaced with phenol red-free MEM containing 8% charcoal/dextran (C/D) filtered serum (Hyclone). Cells were transfected 48 h with 50 nmol/liter siRNA final concentration using DharmaFECT-2 transfection reagent (Dharmacon) according to the manufacturer's protocol.
AhR expression in mammalian cells transfected with siRNAs was analyzed 48 h after transfection using the reagents and methods described above for qPCR.
Breast cancer cell invasion assays
Cell invasion was assessed using a modified Boyden chamber invasion assay. Briefly, cells were plated in six-well dishes in phenol red-free DMEM + 8% C/D FBS. On d 3, cells were treated with chemicals in DMEM containing 0.225% fatty acid-free BSA (Sigma) and 10 mm HEPES. After 24 h, cells were dissociated, counted, and plated in BSA-containing media supplemented with chemical treatments in the top chambers of Matrigel invasion chambers in a 24-well plate format (BD Biosciences, Bedford, MA). Lower chambers contained media or media with chemical treatment in the presence of 5% serum. After 16 h, top chambers were removed, noninvaded cells were swabbed from the top surface of the membrane, and membranes were fixed for 1 h in 2% formaldehyde/0.2% glutaraldehyde and stained with 5% crystal violet in 20% methanol. Remaining cells (those that had invaded through the membrane) were counted using a light microscope. A minimum of three fields was counted for each condition, and values were averaged. Duplicate chambers were used for every treatment condition, and each experiment was repeated at least three times (n = 3–4 independent assays). For invasion assays in siRNA-transfected cells, cells were transfected with siRNAs on d 1 of assay and retransfected on d 3 according to the methods described above.
Colony-forming assays
A 1:1 mixture of 1% agarose and 2× DMEM (Sigma) containing 16% FBS, 2 mm sodium pyruvate, 9 g/liter glucose and 2× penicillin/streptomycin (Invitrogen) was used to form a bottom layer in six-well plates. This was overlaid with cells suspended in a 1:1 mixture of 0.7% agarose and 2× DMEM containing supplements as above together with 2× concentration of appropriate ligand treatment. Cell dilutions were as follows: 4000 cells/ml for SKBR3 cells and 8000–16,000 cells/ml for MDA MB-231, MCF-7, ZR-75-1, and HeLa cells. After the top layer had dried, agarose was overlaid with 1 ml DMEM + 8% FBS containing 1× ligand treatment. Cells were grown in the agarose suspension for 3 wk. The overlaying media were changed twice per week. Colonies were visualized on phase-contrast and dissecting microscopes after staining with 0.005% crystal violet. A minimum of three fields was counted for each well, and values were averaged. Triplicate wells were used for every treatment condition, and each experiment was repeated at least three times (n = 3–5 independent assays).
Western immunoblotting
MDA MB-231, SKBR3, MCF-7, and ZR-75-1 cells were seeded in six-well plates and treated with ligands for 48 h. Whole-cell extracts were harvested using M-PER Mammalian Protein Extraction Reagent according to the manufacturer's protocol (Thermo Scientific, Waltham, MA). Protein concentration of whole-cell extracts was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Proteins (20 μg/sample) were resolved on 12% Tris-glycine gels (Invitrogen) and transferred to polyvinylidene difluoride membrane (Bio-Rad). Blots were probed with an antibody to human milk fat globulin 1 [MFG-06] (Abcam, Cambridge, MA). A β-actin antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used as a loading control.
Evaluation of differentiation phenotype and markers
SKBR3 cells, MCF-7, or LA7 cells were seeded in four-well chamber slides and treated for 48 h with ligands. Unstained SKBR3 cells were imaged by light microscopy on an Olympus BX51TF inverted microscope and photographed with a Nikon digital camera. In other experiments, the Oil Red O in Propylene Glycol Method for Fats (Poly Scientific) was used to fix and stain cells. Cells were counterstained with hematoxylin. Cells were imaged and photographed as above.
Supplementary Material
Acknowledgments
We thank Dr. Jim Joseph (Duke University Medical Center) for the gift of AhR agonist DIM. We thank members of the Thomas laboratory and the Hamner Institutes for their valuable scientific input.
Footnotes
This work was supported by the Hamner Institutes for Health Sciences Pilot Projects Initiative and by National Institutes of Health Grant R37DK48807 (to D.P.M.).
Present address for J.M.H.: Campbell University, School of Pharmacy and Health Sciences, PO Box 1090, Buies Creek, North Carolina 27516.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2009
Abbreviations: AhR, Aryl-hydrocarbon receptor; AhR-si-1, siRNA 1 targeting AhR; C/D, charcoal-dextran; 9-cisRA, 9-cis-retinoic acid; CYP, cytochrome P450; DIM, diindolylmethane; E2, 17β-estradiol; ER, estrogen receptor; FBS, fetal bovine serum; HER2, human epidermal growth factor receptor 2; MFG1, milk fat globulin 1; MMP, matrix metalloproteinase; α-NF, α-naphthoflavone; PR, progesterone receptor; qPCR, quantitative RT-PCR; SAHA, suberoylanilide hydroxamic acid; siRNA, small interfering RNA; TCDBF, tetrachlorodibenzofuran; TCDD, tetrachlorodibenzo-p-dioxin.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ 2008 Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group 2009 United States cancer statistics: 1999–2005 Incidence and mortality web-based report. Atlanta, GA. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. (http://apps.nccd.cdc.gov/uscs) [Google Scholar]
- Bernard-Marty C, Cardoso F, Piccart MJ 2004 Facts and controversies in systemic treatment of metastic breast cancer. Oncologist 9:617–632 [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson JC 1982 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol 22:517–554 [DOI] [PubMed] [Google Scholar]
- Burbach KM, Poland A, Bradfield CA 1992 Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA 89:8185–8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Sogawa K, Watanabe N, Chujoh Y, Matsushita N, Gotoh O, Funae Y, Fujii-Kuriyama Y 1992 cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun 184:246–253 [DOI] [PubMed] [Google Scholar]
- Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA 1993 Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol 44:911–917 [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA 2002 Rules for making human tumor cells. N Engl J Med 347:1593–1603 [DOI] [PubMed] [Google Scholar]
- Duncan DM, Burgess EA, Duncan I 1998 Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev 12:1290–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME 2002 Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact 141:131–160 [DOI] [PubMed] [Google Scholar]
- Kim MD, Jan LY, Jan YN 2006 The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev 20:2806–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Powell-Coffman JA 2004 The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol 270:64–75 [DOI] [PubMed] [Google Scholar]
- Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C 2006 Stochastic spineless expression creates the retinal mosaic for colour vision. Nature 440:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ 1995 Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726 [DOI] [PubMed] [Google Scholar]
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y 2005 Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol 25:10040–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Shima Y, Owaki A, Mimura J, Oshima M, Fujii-Kuriyama Y, Morohashi KI 2008 Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice. Sex Dev 2:1–11 [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA 2000 Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA 97:10442–10447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hushka LJ, Williams JS, Greenlee WF 1998 Characterization of 2,3,7,8-tetrachlorodibenzofuran-dependent suppression and AH receptor pathway gene expression in the developing mouse mammary gland. Toxicol Appl Pharmacol 152:200–210 [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer 1997 IARC monographs on the evaluation of carcinogen risks to humans. Cheney J, ed. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. Vol 69. Lyon, France: World Health Organization; 1–15 [PMC free article] [PubMed] [Google Scholar]
- Bertazzi PA, Zocchetti C, Guercilena S, Consonni D, Tironi A, Landi MT, Pesatori AC 1997 Dioxin exposure and cancer risk: a 15-year mortality study after the “Seveso accident.” Epidemiology 8:646–652 [PubMed] [Google Scholar]
- Viel JF, Clément MC, Hägi M, Grandjean S, Challier B, Danzon A 2008 A Dioxin emissions from a municipal solid waste incinerator and risk of invasive breast cancer: a population-based case-control study with GIS-derived exposure. Int J Health Geogr 7:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP 2006 Toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Washington, DC: US Department of Health and Human Services National Toxicology Program [Google Scholar]
- Holcomb M, Safe S 1994 Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett 82:43–47 [DOI] [PubMed] [Google Scholar]
- Wang F, Hoivik D, Pollenz R, Safe S 1998 Functional and physical interactions between the estrogen receptor Sp1 and nuclear aryl hydrocarbon receptor complexes. Nucleic Acids Res 26:3044–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Porter W, Samudio I, Vyhlidal C, Kladde M, Safe S 1999 Transcriptional activation of c-fos protooncogene by 17β-estradiol: mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol Endocrinol 13:1511–1521 [DOI] [PubMed] [Google Scholar]
- Klinge CM, Bowers JL, Kulakosky PC, Kamboj KK, Swanson HI 1999 The aryl hydrocarbon receptor (AHR)/AHR nuclear translocator (ARNT) heterodimer interacts with naturally occurring estrogen response elements. Mol Cell Endocrinol 157:105–119 [DOI] [PubMed] [Google Scholar]
- Ricci MS, Toscano DG, Mattingly CJ, Toscano Jr WA 1999 Estrogen receptor reduces CYP1A1 induction in cultured human endometrial cells. J Biol Chem 274:3430–3438 [DOI] [PubMed] [Google Scholar]
- Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S 2007 Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature 446:562–566 [DOI] [PubMed] [Google Scholar]
- Gierthy JF, Bennett JA, Bradley LM, Cutler DS 1993 Correlation of in vitro and in vivo growth suppression of MCF-7 human breast cancer by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Res 53:3149–3153 [PubMed] [Google Scholar]
- Oenga GN, Spink DC, Carpenter DO 2004 TCDD and PCBs inhibit breast cancer cell proliferation in vitro. Toxicol In Vitro 18:811–819 [DOI] [PubMed] [Google Scholar]
- Gomez-Duran A, Carvajal-Gonzalez JM, Mulero-Navarro S, Santiago-Josefat B, Puga A, Fernandez-Salguero PM 2009 Fitting a xenobiotic receptor into cell homeostasis: how the dioxin receptor interacts with TGFβ signaling. Biochem Pharmacol 77:700–712 [DOI] [PubMed] [Google Scholar]
- Hsu EL, Yoon D, Choi HH, Wang F, Taylor RT, Chen N, Zhang R, Hankinson O 2007 A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol Sci 98:436–444 [DOI] [PubMed] [Google Scholar]
- Safe S, Wormke M 2003 Inhibitory aryl hydrocarbon receptor-estrogen receptor α cross-talk and mechanisms of action. Chem Res Toxicol 16:807–816 [DOI] [PubMed] [Google Scholar]
- Bradlow HL 2008 Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In vivo (Athens, Greece) 22: 441–445 [PubMed] [Google Scholar]
- Chiang AC, Massagué J 2008 Molecular basis of metastasis. N Engl J Med 359:2814–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA 2008 Mechanisms of malignant progression. Carcinogenesis 29:1092–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer BL, Best KL, Goode RL, Heiman ML, Hoover DM, Robertson DW, Sarosdy MF, Shaar CJ, Tanzer LR, Merriman RL 1992 Comparative antitumor effects of hormonal ablation, estrogen agonist, estrogen cytotoxic derivative, and antiestrogen in the PAIII rat prostatic adenocarcinoma. Cancer Res 52:4663–4671 [PubMed] [Google Scholar]
- Yang JJ, Kang JS, Krauss RS 1998 Ras signals to the cell cycle machinery via multiple pathways to induce anchorage-independent growth. Mol Cell Biol 18:2586–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA 2000 The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- Münster PN, Srethapakdi M, Moasser MM, Rosen N 2001 Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res 61:2945–2952 [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW 2006 Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784 [DOI] [PubMed] [Google Scholar]
- Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M 1993 Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci USA 90:1746–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chang CJ, Bacus SS, Hung MC 1995 Suppressed transformation and induced differentiation of HER-2/neu-overexpressing breast cancer cells by emodin. Cancer Res 55:3890–3896 [PubMed] [Google Scholar]
- Hansen LA, Sigman CC, Andreola F, Ross SA, Kelloff GJ, De Luca LM 2000 Retinoids in chemoprevention and differentiation therapy. Carcinogenesis 21:1271–1279 [PubMed] [Google Scholar]
- Schneider SM, Offterdinger M, Huber H, Grunt TW 2000 Activation of retinoic acid receptor alpha is sufficient for full induction of retinoid responses in SK-BR-3 and T47D human breast cancer cells. Cancer Res 60:5479–5487 [PubMed] [Google Scholar]
- Okuno M, Kojima S, Matsushima-Nishiwaki R, Tsurumi H, Muto Y, Friedman SL, Moriwaki H 2004 Retinoids in cancer chemoprevention. Curr Cancer Drug Targets 4:285–298 [DOI] [PubMed] [Google Scholar]
- Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM 2001 The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res 61:8492–8497 [PubMed] [Google Scholar]
- Polyak K, Hahn WC 2006 Roots and stems: stem cells in cancer. Nat Med 12:296–300 [DOI] [PubMed] [Google Scholar]
- Schmidt C 2008 Drug makers chase cancer stem cells. Nat Biotechnol 26:366–367 [DOI] [PubMed] [Google Scholar]
- Zucchi I, Sanzone S, Astigiano S, Pelucchi P, Scotti M, Valsecchi V, Barbieri O, Bertoli G, Albertini A, Reinbold RA, Dulbecco R 2007 The properties of a mammary gland cancer stem cell. Proc Natl Acad Sci USA 104:10476–10481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi I, Bini L, Albani D, Valaperta R, Liberatori S, Raggiaschi R, Montagna C, Susani L, Barbieri O, Pallini V, Vezzoni P, Dulbecco R 2002 Dome formation in cell cultures as expression of an early stage of lactogenic differentiation of the mammary gland. Proc Natl Acad Sci USA 99:8660–8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink CJ 2003 Aryl hydrocarbon receptor-mediated cell cycle control. Prog Cell Cycle Res 5:261–267 [PubMed] [Google Scholar]
- Elferink CJ, Ge NL, Levine A 2001 Maximal aryl hydrocarbon receptor activity depends on an interaction with the retinoblastoma protein. Mol Pharmacol 59:664–673 [DOI] [PubMed] [Google Scholar]
- Greenlee WE, Hushka LJ, Hushka DR 2001 Molecular basis of dioxin actions: evidence supporting chemoprotection. Toxicol Pathol 29:6–7 [DOI] [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL 2009 The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol 77:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL 2002 Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci 67:63–74 [DOI] [PubMed] [Google Scholar]
- Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP 2004 A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci 78:248–257 [DOI] [PubMed] [Google Scholar]
- Safe S, Qin C, McDougal A 1999 Development of selective aryl hydrocarbon receptor modulators for treatment of breast cancer. Expert Opin Investig Drugs 8:1385–1396 [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA 2008 The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 2005 Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interventions 5:343–357 [DOI] [PubMed] [Google Scholar]
- Hestermann EV, Brown M 2003 Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol Cell Biol 23:7920–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, McDougal A, Wang F, Safe S 1998 Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 19:1631–1639 [DOI] [PubMed] [Google Scholar]
- McDougal A, Sethi Gupta M, Ramamoorthy K, Sun G, Safe SH 2000 Inhibition of carcinogen-induced rat mammary tumor growth and other estrogen-dependent responses by symmetrical dihalo-substituted analogs of diindolylmethane. Cancer Lett 151:169–179 [DOI] [PubMed] [Google Scholar]
- Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S 2000 Indole-3-carbinol is a negative regulator of estrogen receptor-α signaling in human tumor cells. J Nutr 130:2927–2931 [DOI] [PubMed] [Google Scholar]
- McDougal A, Gupta MS, Morrow D, Ramamoorthy K, Lee JE, Safe SH 2001 Methyl-substituted diindolylmethanes as inhibitors of estrogen-induced growth of T47D cells and mammary tumors in rats. Breast Cancer Res Treat 66:147–157 [DOI] [PubMed] [Google Scholar]
- Hong C, Kim HA, Firestone GL, Bjeldanes LF 2002 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis 23:1297–1305 [DOI] [PubMed] [Google Scholar]
- Leibelt DA, Hedstrom OR, Fischer KA, Pereira CB, Williams DE 2003 Evaluation of chronic dietary exposure to indole-3-carbinol and absorption-enhanced 3,3′-diindolylmethane in Sprague-Dawley rats. Toxicol Sci 74:10–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.