Abstract
Jun dimerization protein-2 (JDP-2) is a progesterone receptor (PR) coregulatory protein that acts by inducing structure and transcriptional activity in the disordered amino-terminal domain (NTD) of PR. JDP-2 can also potentiate the partial agonist activity of the PR antagonist RU486 by mechanisms that have not been defined. Functional mutagenesis experiments revealed that a subregion of the NTD (amino acids 323-427) was required for the partial agonist activity of RU486 induced by PR interaction with JDP-2. However, this subregion was not required for JDP-2 enhancement of the activity of progestin agonists. Mutation of phosphorylation sites within this region of the NTD showed that phosphorylation of serine 400 was required for the partial agonist activity of RU486 stimulated by JDP-2, but was not required for activity of hormone agonist, either in the presence or absence of JDP-2. Cyclin-dependent kinase 2 (Cdk2)/cyclin A is a novel PR coregulator that binds the NTD and acts by phosphorylating steroid receptor coactivator-1 and modulating steroid receptor coactivator-1 interaction with PR. Cdk2/cyclin A also potentiated the partial agonist activity of RU486; however, phosphorylation of serine 400 was not required, indicating that JDP-2 and Cdk2/cyclin A act by distinct mechanisms. We conclude that PR bound to RU486 and associated with JDP-2 adopts an active conformation in a subregion of the NTD requiring phosphorylation of serine 400 that is distinct from that promoted by progestin agonists. These data underscore the structural flexibility of the NTD of PR, and the ability of steroid ligands together with interacting proteins to affect the conformation and activity of the NTD.
This study demonstrates that conformational flexibility and site-specific phosphorylation of the amino-terminal domain of progesterone receptor are important determinants for cellular responses to different ligands.
Progesterone receptor (PR) is expressed from a single gene as two protein isoforms, full-length PR-B and N-terminally truncated PR-A. Both isoforms have identical DNA-binding domains (DBDs) and ligand-binding domains (LBDs) and differ only by the extended amino-terminal domain (NTD) of PR-B (1,2). As with other steroid/nuclear receptors, PR contains two transcriptional activation domains or functions, activation function (AF)1 located in the NTD and AF2 in the carboxy-terminal LBD (3,4). The unique amino-terminal sequences of PR-B [amino acids (aa) 1–165] contains a third transcription activation domain, AF3 (5). AF2 mediates transcriptional activation through interaction in a hormone agonist-dependent manner with different classes of well-characterized coactivators. The amino-terminals AF1 and AF3 are less well characterized. Despite similar DNA- and ligand-binding activities, PR-A and PR-B have distinct transcriptional activities and functions in vivo (6,7,8). PR-B is generally a strong activator of gene transcription, whereas PR-A can also act as a repressor of other steroid receptors including estrogen receptor (ER). A transferable inhibitory domain (ID) located in the first approximately 150 amino acids of the NTD of PR-A is responsible for repression activity, suggesting that ID within the context of PR-B is inactivated by an intramolecular interaction with the unique NTD extension of PR-B (9,10).
PR is phosphorylated by multiple kinases on as many as 10 sites that are mostly contained within Ser-Pro motifs located throughout the NTD (see review in Ref. 11). Hormone binding in cells induces phosphorylation of selected sites and increases others that are basally phosphorylated in the absence of hormone (11,12,13). The functional role of all phosphorylation sites in PR has yet to be defined. However, selected phosphorylation sites have been characterized and shown to have direct modulatory effects on PR including acceleration of receptor protein turnover in the cell, nuclear-cytoplasmic shuttling of PR, complex formation with coregulatory proteins, transcriptional activity of either unliganded or progestin-bound PR, and directing receptors to selected target gene promoters (14,15,16,17,18,19,20,21). Phosphorylation of PR serine 294 is strongly induced by progestins or by treatment of breast cancer cells with growth factors such as epidermal growth factor and heregulin (11,12,13,15,16,18,19,22). Phosphorylation of serine 294 by cross talk with growth factor-signaling pathways is mediated by p42/p44 MAPKs, and this promotes nuclear accumulation of PR-B in the absence of ligand and potentiates transcriptional activation of PR induced by progestin, resulting in hyperactive PR that responds to low levels of hormone. Progestin-dependent phosphorylation of serine 294 is not mediated by p42/p44 MAPK but by unknown kinases, is required for maximal ligand-dependent transcriptional activity of PR, and is linked to down-regulation of PR in response to progestins by targeting receptors for ubiquitination and proteosome-mediated degradation (15,16,18). The effect of serine 294 phosphorylation on transcriptional activity of PR appears to be indirect through a regulatory interplay between phosphorylation and sumoylation of K388 in the NTD of receptor. Phosphorylation of serine 294 blocks sumoylation of PR resulting in a reversal of a repressive effect of sumoylation on transcriptional activity of PR (19). The serine 294 site along with another hormone-dependent site at serine 162, present in the unique amino-terminal segment of PR-B, are highly regulated during progression through the cell cycle. These sites are phosphorylated predominantly during S phase in a manner that correlates with maximal transcriptional activity of PR and with recruitment of coactivators to PR target genes (21). Another hormone-dependent phosphorylation site at serine 345 in the NTD was shown to be required for PR interaction with, and transactivation of, selected endogenous target genes such as epidermal growth factor receptor and p21 through PR tethering to Sp1 sites in the promoter/enhancer regions (23). Selected sets of endogenous PR target genes have also shown to be sensitive to the serine 294 phosphorylation/K388 sumolyation state of PR (19,24). These data with serine 294 and 345 sites taken together suggest a role for phosphorylation in directing receptors to selected gene targets, a concept further supported by studies demonstrating that glucocorticoid receptor phosphorylation affects selective subsets of endogenous target genes (25). Serine 400 of PR is basally phosphorylated and is increased upon hormone treatment and cyclin-dependent kinase 2 (CDK2)/cyclin E was shown to phosphorylate this site in vitro and within intact cells by overexpression of CDK2 (17,26). Although phosphorylation of serine 400 was not required for progestin-dependent transcriptional activity of PR, it was found to be necessary for ligand-independent activity of PR mediated by overexpression of an activated CDK2 (17).
PR is an important therapeutic target for contraception and various female endocrine disorders and tumors; thus, PR antagonists have been developed and used clinically in these capacities (8,27). The most widely used PR antagonist is the steroid analog RU486 (Mifapristone). RU486 has a high affinity for PR, competes with progesterone, and promotes many of the same receptor activation steps as progesterone including dissociation from protein chaperones, dimerization, site-specific phosphorylation, and binding to progesterone response element (PRE) DNA. PR bound to DNA in the presence of RU486 has an impaired ability to transmit a transcriptional signal, due in part to an altered conformation in AF2 that interferes with coactivator interaction and promotes recruitment of corepressors such as silencing mediator of retinoid and thyroid hormone receptor (SMRT) or nuclear receptor corepressor (NCoR) (8,28,29,30,31,32,33,34). Steroid antagonists such as RU486 exhibit partial agonist/antagonists activities in a cell and promoter-dependent manner that has led to their definition as selective receptor modulators. Partial agonist activity of RU486 has been detected with PR-B, not with PR-A, has been shown to require the NTD, and can be modulated by the relative level of cellular expression of corepressors NCoR/SMRT and certain coactivators (35). We and others have shown that cross talk with cAMP-dependent protein phosphorylation signal transduction cascades can also potentiate the partial agonist activity of RU486. This cross talk does not appear to alter phosphorylation of PR itself, but rather promotes dissociation of the corepressors NCoR and SMRT from PR, suggesting that interacting coregulatory proteins are the targets of protein phosphorylation mediated by cAMP (33,36,37).
The coactivators/corepressors described to be involved in modulating RU486 activity have predominantly been those that interact with the carboxyl-terminal hinge or LBD (35). However, the partial agonist activity of RU486 is dependent on the NTD, suggesting that carboxyl-terminal-interacting cofactors cannot entirely explain the activity of selective PR modulators. We previously identified a small basic region leucine zipper protein, termed “jun dimerization protein-2” (JDP-2), that functions as a coactivator of the PR NTD independent of AF2 (38,39). The NTD of steroid receptors is an intrinsically disordered region consisting largely of random coil in aqueous solution with minimal evidence of stable secondary or tertiary structure. However, the NTD has the propensity to fold in the presence of natural osmolytes or in response to complexing with other proteins (40,41,42,43,44). The ability of JDP-2 to stimulate transcriptional activity of the NTD of PR correlated with an increase in α-helical content as detected by circular dichroism spectroscopy, suggesting that JDP-2 acts by inducing or stabilizing a more ordered conformation of the NTD. Furthermore JDP-2 induced folding of the NTD is mediated by an interdomain communication, because the direct interaction site for JDP-2 is with residues in the core DBD and the carboxyl-terminal extension (CTE) (39,45). The CTE is a nonconserved short flexible region on the immediate C-terminal side of the conserved second zinc finger of the core DBD. The CTE was originally termed the hinge but has more recently been demonstrated to be a short flexible region that binds other proteins or the minor groove of DNA flanking hormone-response elements in the major groove (45).
In addition to enhancing the transcriptional activity of PR in the presence of hormone agonist, we previously reported that JDP-2 can potentiate the partial agonist activity of RU486 (38). In this study we analyzed the mechanism by which JDP-2 influences the activity of RU486. We show that a distinct subregion of the NTD and phosphorylation of PR serine 400 are required. In contrast, hormone agonist utilizes different segments of the NTD independent of serine 400 phosphorylation. These results support the concept that the NTD of PR exists in multiple conformations, which can be stabilized by the combined effects of steroidal ligands and interacting proteins to generate distinct transcriptional activation surfaces in the presence of hormone agonists and antagonists.
Results
JDP-2 potentiation of the partial agonist activity of RU486 is mediated by the NTD of PR
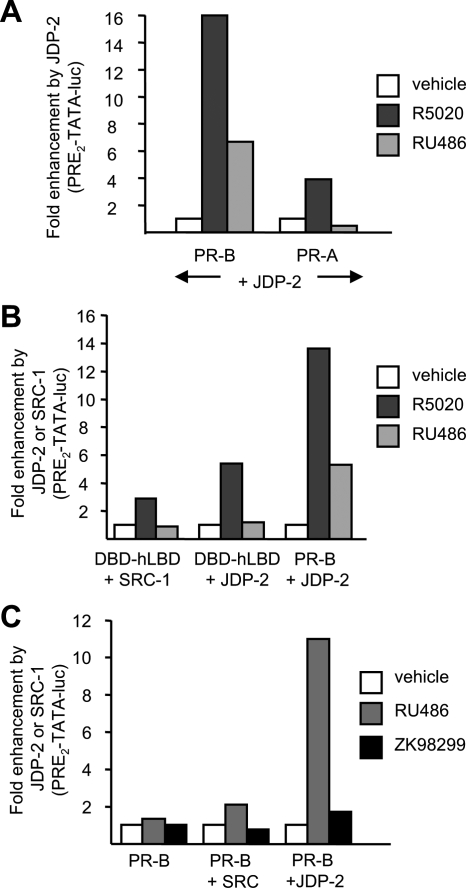
In cell-based transcription assays, we previously showed that ectopic expression of JDP-2 resulted in RU486 induction of PR-B-mediated transactivation of PRE-luciferase (Luc) reporter genes, whereas little or no induction by RU486 was observed in the absence of transfected JDP-2 (38). However, our previous studies examined the influence of JDP-2 on RU486 activity mediated by PR-B only. To determine effects on PR-A, COS-1 cells were cotransfected with PR-A or PR-B plus a PRE2-TATA-Luc reporter gene in the absence (empty vector control) or presence of a JDP-2 expression plasmid. JDP-2 was compared with the AF2 steroid receptor coactivator-1 (SRC-1). Cells were treated for 24 h with the progestin agonist R5020 (10 nm), or RU486 (10 nm), and relative luciferase activity was determined after normalization for transfection efficiency with a constitutive β-galactosidase reporter. Data were presented as fold enhancement of luciferase activity by ectopic expression of JDP-2 or SRC-1. JDP-2 did not promote activation of either PR-A or PR-B in the absence of ligand. However, it strongly enhanced hormone agonist (R5020)-dependent transcriptional activity of PR-B and PR-A, albeit the effect was greater on PR-B (16-fold) than PR-A (4-fold). JDP-2 also promoted a 7-fold RU486-dependent induction of PRE2-TATA-Luc mediated by PR-B, whereas no RU486 induction was mediated by PR-A either in the absence or presence of JDP-2, consistent with the previously defined functional activity of RU486 (Fig. 1A).
Figure 1.
JDP-2 potentiation of the partial agonist activity of RU486 is dependent on the NTD of PR. A, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) and phPR-B (1.5 ng) or phPR-A (15 ng) together with pCR3.1-JDP-2 (100 ng) or an empty vector. B, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) and DBD-hLBD (10 ng) or phPR-B (1.5 ng) together with pCR3.1-SRC-1 (200 ng) or pCR3.1-JDP-2 (137 ng). C, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) and phPR-B (1.5 ng) together with pCR3.1-SRC-1 (150 ng) or pCR3.1-JDP-2 (100 ng). Cells were treated for 24 h with vehicle, 10 nm R5020, 10 nm RU486, or 100 nm ZK98299 as indicated in panels A–C. Fold luciferase induction was calculated as a ratio of relative luciferase activation of ligand-treated cells divided by the corresponding vehicle-treated samples. Fold enhancement was calculated by setting luciferase induction of each receptor construct in each treatment group to 1.0, and corresponding values in the presence of JDP-2 or SRC-1 were calculated as folds relative to 1.0. This is a single representation of triplicate experiments.
To determine whether the effect of JDP-2 on RU486 activity required the NTD of PR, full-length PR-B was compared with a truncated PR construct lacking the NTD and retaining the DBD-hinge, and LBD (DBD-hLBD). Cotransfection with JDP-2 enhanced hormone agonist-dependent transactivation mediated by the DBD-hLBD construct; however, the response was lower (5-fold) than that observed with full-length PR (14-fold) (Fig. 1B). In contrast, JDP-2 did not enable the amino-terminal truncated PR to activate transcription in the presence of RU486 (Fig. 1B). It should be noted that although JDP-2 can enhance the transcriptional activity of AF2 in the LBD in response to hormone agonist, we previously showed that it synergizes with SRC-1 at AF2, whereas the effect of JDP-2 on activity of the NTD is independent of SRC-1 (38,39). As further evidence of the importance of the NTD for the partial agonist activity of RU486, cotransfection of cells with SRC-1 failed to potentiate RU486-dependent activity of the two-domain DBD-hLBD PR and had only a minimal effect on full-length PR-B bound to RU486 (Fig. 1, B and C). JDP-2 did not alter the activity of another PR antagonist ZK98299 (Onapristone). ZK98299 exhibited no agonist activity in the presence of coexpressed JDP-2 or SRC-1, whereas RU486 under the same conditions gave a 10-fold induction in the presence of cotransfected JDP-2 (Fig. 1C). This differential response to JDP-2 is consistent with ZK98299 behaving as a more pure antagonist than RU486 (29,30,33,46).
Partial agonist activity of RU486 requires a region of the NTD of PR between AF1 and ID
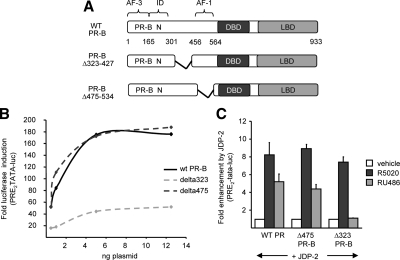
Although the NTD of PR is required for partial agonist activity of RU486, the regions and mechanisms involved have not been defined. We previously showed, by use of internal deletion and truncation mutations in the context of PR-B, that enhancement of NTD activity by JDP-2 in the presence of hormone agonist was not mediated by a single element including the previously defined minimal AF1 (aa 456-564 adjacent to the DBD; see Fig. 2A). Multiple regions were required, suggesting that JDP-2 acts by facilitating the folding of different elements of the NTD (39). Activation curves analyzing hormone agonist induction of PRE2-TATA-Luc at varying concentrations of transfected wild-type PR-B and PR-B with internal deletions in the NTD confirmed our previous results (39). Deletion of aa 475-534 (Δ475), which removes the majority of the previously defined AF1, had no influence on receptor activity as compared with wild-type PR-B, whereas deletion of aa 323-427 (Δ323) between AF1 and ID substantially reduced agonist-dependent receptor activity. Despite the fact that the region between aa 323-427 was required for full hormone-agonist dependent activity of PR-B in the absence of JDP-2, neither this region nor aa 475-534 were required for enhancement by JDP-2. Ectopically expressed JDP-2 gave a similar fold enhancement of hormone agonist (R5020) induction of reporter gene activity mediated by PR-B, PR-BΔ475-534, or PR-BΔ323-427 (Fig. 2C).
Figure 2.
NTD (N) sequence between AF1 and ID is required for JDP-2 potentiation of the partial agonist activity of RU486. A, Schematic illustration of PR-B internal deletion constructs. B, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) together with varying doses of wild-type or deletion PR-B constructs expressed from pCDNA1 (1–12.5 ng). Cells were treated with vehicle or R5020 (10 nm) for the final 24 h of transfection. C, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) together with a single dose of phPR-B expressing wild-type PR-B, Δ323 PR-B, or Δ475 PR-B (1.5 ng each) in the presence or absence of pCR3.1-JDP-2 (100 ng). Cells were treated for the final 24 h of transfection with vehicle, or 10 nm R5020 or RU486, as indicated. Fold luciferase induction by ligands and fold enhancement by JDP-2 were calculated as in Fig. 1. Values are averages ± sem of at least three independent experiments. WT, Wild type.
These internal deletion mutants were used to further define the regions of the NTD required for JDP-2 potentiation of partial agonist activity of RU486. Similar to results with hormone agonist, deletion of aa 475-543 (AF1) had no effect on JDP-2 potentiation of the partial agonist activity of RU486. Ectopic expression of JDP-2 resulted in a 5-fold RU486 induction mediated by either PR-B or PR-BΔ475-534 (Fig. 2C). However, deletion of the region between AF1 and ID (Δ323-427) completely eliminated JDP-2 potentiation of RU486 activity, whereas this same region had no effect on JDP-2 enhancement of hormone agonist-dependent transcription activity of PR (Fig. 2C). To determine whether this region is sufficient to mediate the partial agonist activity of RU486 induced by JDP-2, receptor constructs were generated that contain most of this region, with or without the adjacent AF1, linked directly to a DBD-LBD two-domain PR fragment (Fig. 3A). These PR polypeptides were unable to mediate RU486-induced transactivation in the presence of coexpressed JDP-2 (Fig. 3B), indicating that the subregion of the NTD between aa 323-427 is not sufficient for RU486 agonist activity in the absence of other sequences in the NTD.
Figure 3.
The sequence region of the PR NTD (N) between aa 323 and aa 475 is necessary, but not sufficient, for the partial agonist activity of RU486. A, Schematic illustration of PR-B constructs including WT-PR-B, truncation 350-DhLBD, and 323-427-DhLBD constructs. B, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) together with single concentrations of WT-PR-B and PR constructs in the presence or absence of pCR3.1-JDP-2 (100 ng). Cells were treated for the final 24 h with vehicle or 10 nm RU486, as indicated. Fold enhancement by JDP-2 was calculated as in Fig. 1. Values are averages ± sem of at least three independent experiments. WT, Wild type; DhLBD, DBD-hLBD.
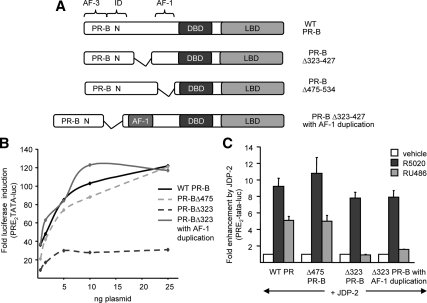
We considered the possibility that the region between aa 323 and 427 simply acts as a spacer between ID and AF1, such that its removal inhibits PR activity by inactivating AF1, or other NTD sequences, by placing the repression activity of ID too close. To test this idea, an additional AF1 sequence (aa 455-554) was inserted into PR-BΔ323-427, effectively replacing the deleted 323-427 sequence with a duplicated AF1 (aa 455-554) (Fig. 4A). As shown in Fig. 4B, replacement of 323-427 with a second AF1 restored the impaired hormone agonist (R5020)-induced transactivation mediated by PR-BΔ323-427 to levels comparable to that mediated by wild-type PR-B. Transactivation did not appreciably exceed that of wild-type PR-B, suggesting that the duplicated AF1 does not synergize with other receptor activation regions. Rather, these data suggest that the PR-BΔ323-427 internal deletion reduced the agonist-dependent activity of the NTD by changing proximity of the ID to AF1. However, insertion of an additional AF1 as a spacer did not restore the partial agonist activity of RU486 stimulated by JDP-2. PR-BΔ323-427 with the AF1 duplication was not activated by RU486 in cells with ectopically expressed JDP-2, as compared with RU486 induction of target gene expression mediated by wild-type PR-B or PR-BΔ475 (9.2- and 11-fold coactivation, respectively) (Fig. 4C). These results, taken together, show that a specific subregion of the NTD of PR between aa 323 and aa 427 is required, but not sufficient, for the partial agonist activity of RU486 induced by JDP-2 and that other regions of the NTD are required for JDP-2 coactivation of PR in the presence of hormone agonist.
Figure 4.
NTD (N) between AF1 and ID acts as a spacer for hormone agonist activity. A, Schematic illustration of PR-B constructs including WT-PR-B, internal deletions (Δ323-475) (Δ475-534), and AF1 duplication. B, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) together with phPR-B expressing wild-type or PR-B constructs depicted in panel A (0.5–25 ng). Cells were treated with vehicle or 10 nm R5020 for the final 24 h of transfection. C, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) together with single concentrations (1.5) of WT-PR-B and PR constructs in the presence or absence of pCR3.1-JDP-2 (100 ng). Cells were treated for the final 24 h with vehicle, 10 nm R5020, or RU486, as indicated. Fold luciferase induction by ligand and fold enhancement by JDP-2 was calculated as in Fig. 1. Values are averages ± sem of at least three independent experiments. WT, Wild type.
Phosphorylation of serine 400 is important for the partial agonist activity of RU486
The region of the NTD between aa 323 and aa 427 required for potentiation of the agonist activity of RU486 contains two phosphorylation sites; ligand-dependent serine 345 (induced by hormone agonist or RU486) and constitutive serine 400, the phosphorylation of which is increased in response to binding ligands in cells (Fig. 5A). The kinase that phosphorylates serine 345 is unknown, but serine 400 is a substrate for cyclinA/Cdk2 in vitro (26) and cyclinE/Cdk2 in vivo (17). To determine whether these phosphorylation sites are required for JDP-2 enhancement of hormone agonist or RU486 activity, serine 345 or serine 400 was replaced with an alanine or glutamic acid in the context of full-length PR-B to mimic unphosphorylated and phosphorylated receptor, respectively. The mutants were compared with wild-type PR-B for transcriptional activity in cell-based cotransient transfection assays. Functional analysis with varying doses of transfected wild-type or serine mutant PR-B expression plasmids, together with a PRE2-TATA-Luc reporter gene, showed that progestin-agonist (R5020)-dependent activation curves of PR-B345ala and PR-B400glu are comparable to that of wild-type PR-B (Fig. 5B). The activation curve of PR-B400ala is slightly right shifted compared with other PRs, suggesting a decreased transcription sensitivity; however, the maximal activity at the highest concentration of transfected receptor was not decreased. Thus, phosphorylation of serine 345 or 400 had no major impact on hormone agonist-dependent PR-mediated transactivation under these conditions.
Figure 5.
Phosphorylation of serine 400 in NTD (N) of PR influences JDP-2 potentiation of partial agonist activity of RU486. A, Schematic illustration of characterized serine phosphorylation sites in PR. Hormone-induced sites include serines 102, 294, and 345; other sites are basally phosphorylated and increased by ligand treatment of cells. B, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) together with phPR-B expressing wild-type or serine 345 or serine 400 phosphorylation mutants (1.5–25 ng). Cells were treated with vehicle or 10 nm R5020 for the final 24 h of transfection. C, COS-1 cells were transfected with PRE2-TATA-Luc (200 ng) together with 1.5 ng of phPR-B expressing wild-type PR-B or phosphorylation mutants, in the presence or absence of pCR3.1-JDP-2 (100 ng). Cells were treated for the final 24 h of transfection with vehicle or 10 nm R5020 or RU486, as indicated. Fold luciferase induction by ligand and fold enhancement by JDP-2 were calculated as in Fig. 1. Values are averages ± sem of at least three independent experiments. h, Hinge; WT, wild type.
To determine whether phosphorylation of serines 345 and 400 are required for JDP-2 potentiation of either R5020 or RU486 activity, wild-type PR-B, PR-B345ala, PR-B400ala, or PR-B400glu plasmids at amounts that generate similar levels of submaximal R5020-dependent transactivation by receptor alone were transfected together with a PRE2-TATA-Luc reporter gene in the presence or absence of ectopically expressed JDP-2. Mutation of serine 345 or serine 400 had no appreciable effect on JDP-2 enhancement of hormone agonist (R5020)-dependent transactivation mediated by PR (Fig. 5C). Substitution of alanine for serine 345 also had no effect on JDP-2 potentiation of RU486-dependent transactivation; however, an alanine substitution for serine 400 substantially reduced JDP-2 potentiation of RU486 activity (Fig. 5C). Glutamic acid substitution for serine 400 had no effect on JDP-2 potentiation of the partial agonist activity of RU486 (Fig. 5C), suggesting that phosphorylation of serine 400 affects PR activity primarily through negative charge. These data, taken together with functional mutagenesis of the NTD, suggests the region between ID and AF1 in combination with phosphorylation of serine 400 constitutes a specific activation surface in the presence of RU486 and the coregulatory protein JDP-2.
We previously reported that the cyclin-dependent kinase Cdk2/cyclin A interacts with the NTD of PR and functions as a coactivator (47). Cyclin A/Cdk2 is recruited to target gene promoters in a ligand-dependent manner, and the ability of cyclin A to serve as a coactivator is dependent upon its capacity to associate with Cdk2, although independent of its phosphorylation of PR. To test whether cyclin A/Cdk2 could also potentiate the partial agonist activity of RU486, and, if so, whether phosphorylation of serine 400 was required, we performed cotransfection experiments with cyclin A/Cdk2. Transfection of Hela cells with either cyclin A or Cdk2 alone resulted in a weak RU486 induction of a reporter gene activity (Fig. 6A). However, coexpression of cyclin A and Ckd2 together resulted in a greater induction (∼5-fold) of reporter gene activation by RU486, whereas minimal induction was obtained with cells cotransfected with a vector control (Fig. 6A). In contrast to results with JDP-2, alanine substitution for serine 400 in PR-B did not abrogate the partial agonist activity of RU486 potentiated by cyclin A/Cdk2; instead it was enhanced (Fig. 6B). Thus, the partial agonist activity of RU486 under these conditions is not dependent on phosphorylation of serine 400, indicating the coactivation function of cyclin A/Cdk2 is mediated by a distinct mechanism from that of JDP-2.
Figure 6.
Cyclin A/Cdk2 potentiation of partial agonist activity of RU486. HeLa cells were transfected with PR-B (panels A and B) or PR-B/S400A (panel B) along with 1 μg empty vector or 0.5 μg cyclin A, 0.5 μg Cdk2 or cyclin A + Cdk2 together, and 0.25 μg of E1B-TATA-luciferase reporter. The cells were treated 24 h after transfection with 10 nm RU486 for an additional 24 h. The cells were harvested, and luciferase activity was measured and normalized to β-Gal activity. The results are expressed as fold change from vector-transfected vehicle-treated samples. Values are averages ± sem of three independent experiments.
Discussion
RU486 exhibits partial agonist activity that is dependent on the NTD of PR and varies with cell type and target gene promoters. In keeping with its role as a coactivator of the NTD of PR, we previously reported that JDP-2 potentiates the transcriptional agonist activity of RU486 (38). In this paper we sought to define the sequence region of PR involved and the mechanism by which JDP-2 induces the partial agonist activity of RU486. This effect of JDP-2 is substantial, because it resulted in RU486-induced transactivation equal to that observed with hormone agonist-occupied PR in the absence of expressed JDP-2. Potentiation of the partial agonist activity of RU486 by JDP-2 also conforms to the known functional properties of PR antagonists, because the NTD of PR-B was required and ZK98299 maintained its antagonist activity in the presence of JDP-2 (Fig. 1). These results suggest that the cellular level and availability of JDP-2 may be an important determinant of the pharmacology of RU486.
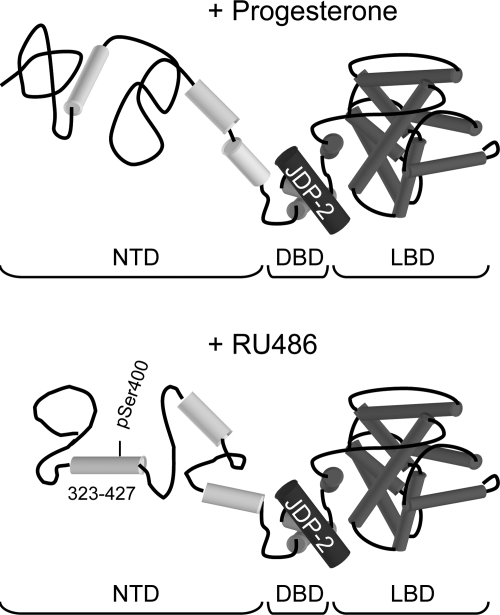
We previously showed, by limited proteolytic digestion assay and circular dichroism spectroscopy, that the NTD of PR is disordered in aqueous solution, consisting largely of random coil, and that JDP-2 interaction with the DBD, through an interdomain communication, induced stable secondary structure in the NTD. We further showed that increased α-helical content of the NTD induced by JDP-2 correlated with greater transcriptional activity of the PR (39). By functional analysis of deletion and truncation mutations, we were unable to define a minimal sequence region of the NTD responsible for mediating JDP-2 enhancement of hormone agonist-dependent transcriptional activity of PR, leading us to conclude that JDP-2 stabilizes folding of multiple elements required for the transcriptional activity of the NTD (39). Consistent with this concept we found that AF1, originally defined as a minimal region of the NTD with transcriptional activity when linked to a heterologous DBD (4), is not required for hormone agonist-dependent transcriptional activity within the context of full-length PR (39). Thus AF1, as originally defined, does not appear to be sufficient for mediating transcriptional activity of the NTD in the context of full-length PR and its own DBD. We show here, based on internal deletion mutations in the context of full-length PR-B, that a previously uncharacterized region of the NTD (aa 323-427) between ID and AF1 was required for JDP-2 potentiation of the partial agonist activity of RU486, but was not required for JDP-2 enhancement of the transcriptional activity of PR occupied by hormone agonist. Although deletion of aa 323-427 between ID and AF1 reduced overall hormone agonist activity in the absence of ectopically expressed JDP-2, the fold enhancement by JDP-2 was the same for wild-type PR-B and PR-BΔ323-427. Replacement of deleted aa 323-427 with duplicated AF1 (aa 475-534) was able to rescue receptor-dependent hormonal agonist activity but not JDP-2 enhancement of the partial agonist activity of RU486. These data imply that the region between ID and AF1 has a specific role in mediating the partial agonist activity of antagonists, but functions as a spacer with respect to activity of hormone agonists. We conclude that the partial agonist activity of RU486 in the presence of JDP-2 involves induction of a conformation in the NTD that is distinct from that induced in the agonist-occupied receptor (Fig. 7).
Figure 7.
Model of JDP-2 potentiation of RU486 activity. JDP-2 interaction with the DBD, through interdomain allosteric coupling, induces distinct structures in the NTD of PR bound to progesterone or RU486.
Two phosphorylation sites of PR (serine 345 and serine 400) are located within the region of the NTD required for JDP-2 potentiation of the partial agonist activity of RU486. Mutation of serine 400 to a nonphosphorylatable residue (alanine) substantially reduced JDP-2 potentiation of RU486 agonist activity, whereas mutation of serine 400 had no affect on hormone agonist-dependent PR-mediated transcription. These data suggest that phosphorylation of serine 400 contributes to an active conformation of the NTD induced by RU486 in the presence of JDP-2, perhaps through a charge effect because substitution of a glutamic acid for serine 400 did not effect the partial agonist activity of RU486. Alternatively, phosphorylation could modulate interaction of this surface with another protein. It was reported earlier that serine 400 of PR is phosphorylated in vitro (26) and in vivo (17) by Cdk2/cyclin E. Furthermore, phosphorylation of serine 400 was shown to be unnecessary for transcriptional activity of PR in the presence of progestin agonist, but was required for transcriptional activity of unliganded PR stimulated by overexpression of a constitutively active mutant of Cdk2 (17). The present results are consistent with the report of Lange et al. (17), in that PR serine 400 was not required for the activity of R5020, either in the absence or presence of JDP-2. The influence of phosphorylation of PR serine 400 on the activity of RU486 was not examined in these previous studies (17).
Other proteins that interact with PR have been described to influence the agonist/antagonist properties of RU486, but they appear to work by a different mechanism than JDP-2. L7/SPA, another small basic region leucine zipper protein, was reported to enhance RU486 agonist activity (34). However, L7/SPA had no effect on R5020-dependent PR transactivation, and it interacts with the carboxyl-terminal hinge and LBD of PR rather than the DBD, suggesting that it alters coacativator/corepressor interactions with the AF2 LBD in the presence of RU486, rather than modulating the NTD. We show here that ectopic expression of cyclinA/Cdk2, another NTD-dependent PR coactivator (47), can also potentiate the agonist activity of RU486. However, phosphorylation of serine 400 was not important, suggesting that cyclin A/Cdk2 does not have the same requirements as JDP-2 (Fig. 6). Thus, it appears that multiple PR-interacting proteins are capable of modulating the activity of RU486, each by different mechanisms.
Functionally distinct subregions within the ERα NTD have also been described to mediate the activity of estradiol (E2) and the ER antagonist tamoxifen (48). The NTD of ER is much shorter than PR, consisting of only 184 amino acid residues vs. 534 amino acids, yet exhibits similar properties. Deletion of the amino-terminal most 40 residues was reported to have no effect on ER activity, whereas deletion of aa 41-64, either by truncation or internal deletion, abrogated tamoxifen-dependent transactivation but left E2 activation intact. Receptor E2-transactivation proved to be more complex, because aa 41-109 were sufficient to mediate E2 activity, but combinations of other regions, such as aa 1-87 and 109-180, mediated comparable agonist activity (48). Therefore, partial agonist activity of tamoxifen mapped to a single minimal element, whereas activity of agonist appears to utilize multiple segments of the NTD. Thus it is tempting to speculate that the hormone agonist activity mediated by the NTD of ER and PR represents a sum total of, or synergy between, multiple elements and their mediating factors, where as partial agonist activity of antagonists is mediated by a single minimal element while simultaneously silencing other elements. This mechanism could explain, in part, why the partial agonist activities of tamoxifen and RU486 are generally weaker than those of hormone agonists.
Previous studies have shown that RU486 and other antagonists induce a unique conformation in the C-terminal helix 12 of the receptor LBD (31,49). Helix 12 in the antagonist conformation blocks p160 coactivator interaction with AF2 surfaces. The partial agonist activity of RU486 has previously been thought to be mediated simply by the constitutive activity of the NTD that fails to be effectively silenced by RU486 (28). The present results suggest a different mechanism whereby RU486, in conjunction with JDP-2, induces an active conformation of the NTD that exposes protein interaction surfaces that are distinct from those presented by hormone agonist (Fig. 7). These results also highlight the importance of advancing our understanding of the structure, mechanism, and interacting proteins that regulate the activity of the NTD because this will ultimately be essential for the development of selective receptor modulators with more highly refined tissue and target gene specificities.
Materials and Methods
R5020 (Promegestone) was obtained from New England Nuclear Life Sciences Products (Boston, MA). ZK98299 (Onapristone), RU486 (Mifepristone), and other steroid hormones were purchased from Sigma (St. Louis, MO).
Plasmid construction
Multiple plasmid constructs were used to analyze effects of JDP-2 and cyclin A/Cdk2 on transcriptional activity of PR. pCR3.1-rat JDP-2, under the control of the cytomegalovirus promoter, were previously described (38). Cyclin A, Cdk2, and a matching empty vector, pCDLSRα296, were described previously (47). PR expression vectors and reporter plasmids have been described previously including phPR-B and phPR-A expressing full-length human PR isoforms under the control of the Simian virus 40 promoter, pCDNA1-PR-B, plasmid human (ph)PR-B containing point mutations of serine 345 and serine 400, and PR DBD-hLBD (aa 546-933) domains under the control of the Rous sarcoma virus promoter in pABΔGal (38). Reporter gene constructs PRE2-TATA-LUC and E1b-Luc were previously described (39).
PR internal-deletion constructs were all cloned in several steps with descriptions available upon request. For pCDNA1- and phPR-B-Δ475-534, an in-frame deletion between aa 474 and 535 was constructed. pCDNA- and phPR-B-Δ323-427 were constructed through sequential digestion to creating the following juncture: PR aa R322DHMR (428). pABΔgal-350-DBD-hLBD and pABΔgal-350-428-DBD-hLBD were constructed through insertion of PR aa 350-545 or 350-428, respectively, upstream of PR DBD-hLBD (aa 546-933) in pABΔgal. phPR-BΔ323/AF1-duplication was constructed through insertion of the AF1 fragment (PR aa 455-554) into phPR-B-Δ323-427 at aa 441 to result in an additional AF1 region within PRΔ323 at aa 441, just after the Δ323 internal deletion and upstream of the native AF1 region. All cloning junctures and deletion mutations were verified by DNA sequencing, and equal PR protein expression was confirmed by Western blot analysis.
Cell culture and transfection
COS-1 cells maintained in DMEM supplemented with 5% fetal bovine serum (FBS) (Hyclone-Millipore, Billerica, MA) and Hela cells in DMEM plus 10% FBS as previously described (50) were plated in six-well dishes (Falcon, Franklin Lakes, NJ) at a density of 1.6 × 105. At 24 h after plating, cells were transfected with constitutively active pCH110 β-galactosidase expression vector as an internal control for transfection efficiency, PRE2-TATA-Luc progesterone-responsive luciferase reporter plasmids, and varying amounts of PR or PR domain expression vectors together with pCR3.1-SRC-1 or pCR3.1-JDP-2, using Lipofectamine and Plus reagents (Life Technologies, Inc., Gaithersburg, MD) according to manufacturer's instructions. pCR3.1 empty vector was added to maintain a constant number of moles of cytomegalovirus promoter. After transfection, cells were grown in DMEM or MEM supplemented with 5% charcoal-stripped FBS for 24 h and then treated in the same medium for 16–20 h with 10 nm R5020 or RU486 or 100 nm ZK98299. At 48 h after transfection, cells were washed [40 mm Tris (pH 7.4), 150 mm NaCl, 1 mm EDTA] and lysed directly in the well using 0.3 ml lysis buffer [20 mm K2HPO4 (pH 7.8), 5 mm MgCl2, 0.5% Triton X-100]. Lysates were centrifuged at 13,000 × g for 5 min at 4 C to pellet cell particulates, and luciferase and β-galactosidase assays were performed as previously described, except that 10 μl or 5 μl of cell lysates were used for luciferase or β-galactosidase assays, respectively (30,50). Luciferase was normalized to β-galactosidase activity, and relative luciferase activity was calculated by setting the normalized values obtained with vehicle-treated reporter alone (no hormone, no PR) to 1.0, and all other values were calculated as a fold increase relative to 1.0. Fold luciferase induction was calculated as a ratio of relative luciferase activity of hormone-treated samples divided by relative luciferase activity of corresponding vehicle-treated samples. Fold coactivation was calculated by setting relative luciferase activity or fold luciferase induction of each receptor or receptor domain construct to 1.0, and corresponding values in the presence of JDP-2 were calculated as folds relative to 1.0.
Footnotes
This work was supported, in part, by National Institutes of Health Grants DK49030 and CA46938 (to D.P.E.), and CA05739 (to N.L.W.).
Current address for S.E.W.: Duke University Medical Center, Department of Pharmacology and Cancer Biology, Durham, North Carolina 27710.
Current address for R.N.: GTX, Inc., Drug Discovery, Memphis, Tennessee 38163.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 11, 2009
Abbreviations: aa, Amino acids; AF, activation function; CDK2, cyclin-dependent kinase 2; CTE, carboxyl-terminal extension; DBD, DNA-binding domain; DBD-hLBD, DBD-hinge, and LBD; E2, estradiol; ER, estrogen receptor; FBS, fetal bovine serum; ID, inhibitory domain; JDP-2, jun dimerization protein-2; LBD, ligand-binding domain; NCoR, nuclear receptor corepressor; NTD, amino-terminal domain; ph, plasmid human; PR, progesterone receptor; PRE, progesterone response element; SMRT, silencing mediator of retinoid and thyroid hormone receptor; SRC-1, steroid receptor coactivator-1.
References
- Li X, O'Malley BW 2003 Unfolding the action of progesterone receptors. J Biol Chem 278:39261–39264 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P 1989 The human estrogen receptor has two independent non-acidic transcriptional activation functions. Cell 59:477–487 [DOI] [PubMed] [Google Scholar]
- Meyer ME, Quirin-Stricker C, Lerouge T, Bocquel MT, Gronemeyer H 1992 A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem 267:10882–10887 [PubMed] [Google Scholar]
- Giangrande PH, McDonnel DP 1999 The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 45:291–313 [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM 2000 Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell SE, Edwards DP 2005 Mechanisms controlling agonist and antagonist potential of selective progesterone receptor modulators (SPRMs). Semin Reprod Med 23:9–21 [DOI] [PubMed] [Google Scholar]
- Hovland AR, Powell RL, Takimoto GS, Tung L, Horwitz KB 1998 An N-terminal inhibitory function, IF, suppresses transcription by the A-isoform but not the B-isoform of human progesterone receptors. J Biol Chem 273:5455–5460 [DOI] [PubMed] [Google Scholar]
- Giangrande PH, Pollio G, McDonnell DP 1997 Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J Biol Chem 272:32889–32900 [DOI] [PubMed] [Google Scholar]
- Weigel NL, Moore NL 2007 Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol 21:2311–2319 [DOI] [PubMed] [Google Scholar]
- Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP 2000 Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol 14:52–65 [DOI] [PubMed] [Google Scholar]
- Knotts TA, Orkiszewski RS, Cook RG, Edwards DP, Weigel NL 2001 Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem 276:8475–8383 [DOI] [PubMed] [Google Scholar]
- Takimoto GS, Hovland AR, Tasset DM, Melville MY, Tung L, Horwitz KB 1996 Role of phosphorylation on DNA binding and transcriptional functions of human progesterone receptors. J Biol Chem 271:13308–13316 [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB 2000 Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degration by the 26S proteasome. Proc Natl Acad Sci USA 97:1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA 2003 Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol 17:628–642 [DOI] [PubMed] [Google Scholar]
- Pierson-Mullany LK, Lange CA 2004 Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol 24:10542–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Horwitz KB, Lange CA 2001 Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol 21:6122–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Faivre EJ, Lange CA 2007 Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol 21:2890–2906 [DOI] [PubMed] [Google Scholar]
- Daniel AR, Knutson TP, Lange CA 2009 Review: signaling inputs to progesterone receptor gene regulation promoter selectivity. Mol Cell Endocrinol 308:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Edwards DP, Weigel NL 2005 Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol 25:2885–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labriola L, Salatino M, Proietti CJ, Pecci A, Coso OA, Kornblihtt AR, Charreau EH, Elizalde PV 2003 Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol Cell Biol 23:1095–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre EJ, Daniel AR, Hillard CJ, Lange CA 2008 Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol 22:823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Lange CA 2009 Protein kinases mediate ligand-independent derepression of sumoylated progesterone receptors in breast cancer cells. Proc Natl Acad Sci USA 106:14287–14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, Rogatsky I, Logan SK, Garabedian MJ 2008 Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol 22:1754–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Beck CA, Poletti A, Clement 4th JP, Prendergast P, Yip TT, Hutchens TW, Edwards DP, Weigel NL 1997 Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol 11:823–832 [DOI] [PubMed] [Google Scholar]
- Spitz IM, Croxatto HB, Robbins A 1996 Antiprogestins: mechanism of action and contraceptive potential. Annu Rev Pharmacol Toxicol 36:47–81 [DOI] [PubMed] [Google Scholar]
- Meyer ME, Pronon A, Ji JW, Bocquel MT, Chambon P, Gronemeyer H 1990 Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J 9:3923–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L, Cato AC, Henderson D, Ryffel GU 1991 Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res 19:1227–12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass EK, Leonhardt SA, Nordeen SK, Edwards DP 1998 The antagonists RU486 and ZK98299 stimulate progesterone receptor binding to deoxyribonucleic acid in vitro and in vivo, but have distinct effects on receptor conformation. Endocrinology 139:1905–1919 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Allan GF, Schrader WT, Tsai MJ, McDonnell DP, O'Malley BW 1992 The mechanism of RU486 antagonism in dependent on the conformation of the carboxyl-terminal tail of the human progesterone receptor. Cell 69:703–713 [DOI] [PubMed] [Google Scholar]
- Leonhardt S, Edwards D 2002 Mechanism of action of progesterone antagonists. Exp Biol Med 227:969–980 [DOI] [PubMed] [Google Scholar]
- Wagner BL, Norris JD, Knotts TA, Weigel NL, McDonnell DP 1998 The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol 18:1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung L, Horwitz KB 1997 The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR and SMRT. Mol Endocrinol 11:693–705 [DOI] [PubMed] [Google Scholar]
- Liu Z, Auboeuf D, Wong J, Chen JD, Tsai SY, Tsai MJ, O'Malley BW 2002 Coactivator/corepressor ratios modulate PR-mediated transcription by the selective receptor modulator RU486. Proc Natl Acad Sci USA 99:7940–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CA, Weigel NL, Moyer ML, Nordeen SK, Edwards DP 1993 The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci USA 90:4441–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius CA, Tung L, Takimoto GS, Horwitz KB 1993 Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem 268:9262–9266 [PubMed] [Google Scholar]
- Wardell SE, Boonyaratanakornkit V, Adelman JS, Aronheim A, Edwards DP 2002 Jun dimerization protein 2 functions as a progesterone receptor N-terminal domain coactivator. Mol Cell Biol 22:5451–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell SE, Kwok SC, Sherman L, Hodges RS, Edwards DP 2005 Regulation of the amino-terminal transcription activation domain of progesterone receptor by a cofactor-induced protein folding mechanism. Mol Cell Biol 25:8792–8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Thompson EB 2003 Transactivation functions of the N-terminal domains of nuclear hormone receptors: protein folding and coactivator interactions. Mol Endocrinol 17:1–10 [DOI] [PubMed] [Google Scholar]
- Bain DL, Franden MA, McManaman JL, Takimoto GS, Horwitz KB 2001 The N-terminal region of human progesterone B-receptors. J Biol Chem 276:23825–23831 [DOI] [PubMed] [Google Scholar]
- Kumar R, Volk DE, Li J, Lee JC, Gorenstein DG, Thompson EB 2004 TATA box binding protein induces structure in the recombinant glucocorticoid receptor AF1 domain. Proc Natl Acad Sci USA 101:16425–16430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wärnmark A, Wikström A, Wright AP, Gustafsson JA, Härd T 2001 The N-terminal regions of estrogen receptor α and β are unstructured and show different TBP binding properties. J Biol Chem 276:45939–45944 [DOI] [PubMed] [Google Scholar]
- McEwan IJ, Lavery D, Fischer K, Watt K 2007 Natural disordered sequences in the amino terminal domain of nuclear receptors: lessons from the androgen and glucocorticoid receptors. Nucl Recept Signal 5:e001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KK, Roemer SC, Jones DN, Churchill ME, Edwards DP 2009 A progesterone receptor co-activator (JDP2) mediates activity through interaction with residues in the carboxyl-terminal extension of the DNA binding domain. J Biol Chem 284:24415–24424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto GS, Tasset DM, Eppert AC, Horwitz KB 1992 Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc Natl Acad Sci USA 89:3050–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Adigun AA, Edwards DP, Weigel NL 2005 Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol 25:264–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney EM, Katzenellenbogen BS 1996 Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcriptional activation. J Biol Chem 271:24172–24178 [DOI] [PubMed] [Google Scholar]
- Xu J, Nawaz Z, Tsai SY, Tsai MJ, O'Malley BW 1996 The extreme C terminus of progesterone receptor contains a transcriptional repressor domain that functions through a putative corepressor. Proc Natl Acad Sci USA 93:12195–12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi M, Taraseviciene L, Nordeen SK, Allegretto EA, Edwards DP 1998 High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol 18:4471–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]