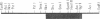Abstract
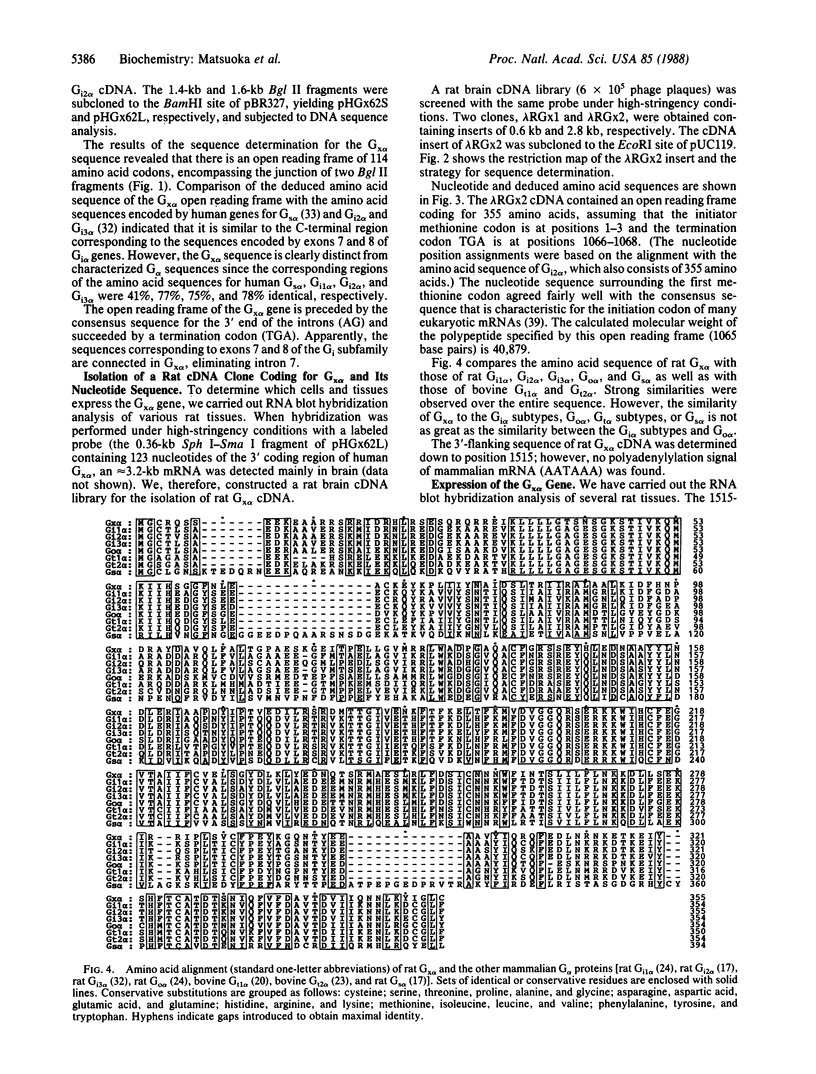
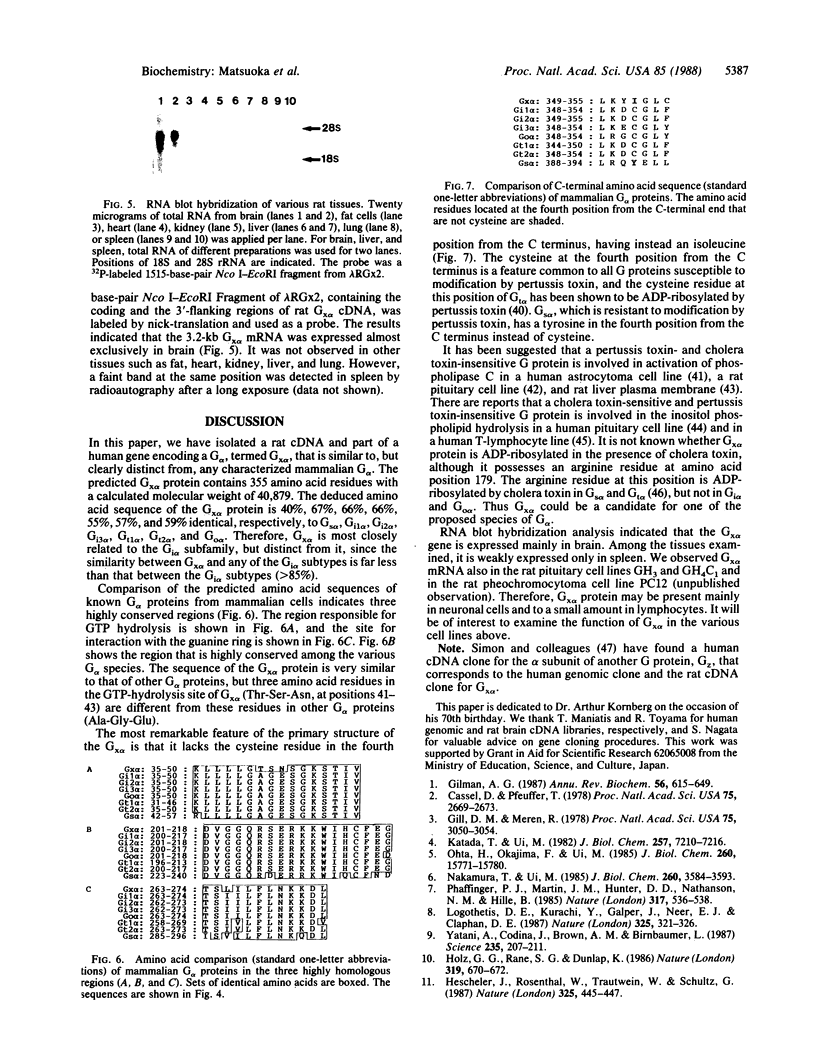
We have isolated cDNA clones from rat C6 glioma cells coding for several guanine nucleotide-binding regulatory protein (G protein) alpha subunits (G alpha). The cDNA clones were then used to isolate human chromosomal genes. Among human genomic clones isolated by cross-hybridization with the rat cDNA for the alpha subunit of the inhibitory G protein Gi2, termed Gi2 alpha, a clone designated lambda HGi62 was found to contain a sequence that is highly homologous but distinct from any of the known G alpha sequences, and we have tentatively designated this sequence Gx alpha. We have searched a rat brain cDNA library with the Gx alpha sequence and isolated a cDNA clone containing a rat sequence similar to human Gx alpha. The cDNA contained a single open reading frame of 1065 nucleotides coding for a protein of 355 amino acids with a calculated molecular weight of 40,879. The amino acid sequence of rat Gx alpha shows 66% and 40% similarity with rat Gi2 alpha and rat Gs alpha (the alpha subunit of the stimulatory G protein), respectively. By RNA blot hybridization analysis, mRNA of approximately 3.2 kilobases was detected mainly in brain. Interestingly, the deduced amino acid sequence of Gx alpha predicts that the Gx alpha protein may be refractory to modification by pertussis toxin since the cysteine residue in the fourth position from the C terminus of pertussis toxin-sensitive G alpha is replaced by isoleucine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beals C. R., Wilson C. B., Perlmutter R. M. A small multigene family encodes Gi signal-transduction proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7886–7890. doi: 10.1073/pnas.84.22.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bray P., Carter A., Guo V., Puckett C., Kamholz J., Spiegel A., Nirenberg M. Human cDNA clones for an alpha subunit of Gi signal-transduction protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5115–5119. doi: 10.1073/pnas.84.15.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Didsbury J. R., Ho Y. S., Snyderman R. Human Gi protein alpha-subunit: deduction of amino acid structure from a cloned cDNA. FEBS Lett. 1987 Jan 26;211(2):160–164. doi: 10.1016/0014-5793(87)81428-x. [DOI] [PubMed] [Google Scholar]
- Didsbury J. R., Snyderman R. Molecular cloning of a new human G protein. Evidence for two Gi alpha-like protein families. FEBS Lett. 1987 Jul 13;219(1):259–263. doi: 10.1016/0014-5793(87)81228-0. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Yoshimoto K. K., Eversole-Cire P., Simon M. I. Identification of a GTP-binding protein alpha subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc Natl Acad Sci U S A. 1988 May;85(9):3066–3070. doi: 10.1073/pnas.85.9.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Toyama R., Kozasa T., Tsukamoto T., Matsuoka M., Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988 May 15;263(14):6656–6664. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Katada T., Oinuma M., Kusakabe K., Ui M. A new GTP-binding protein in brain tissues serving as the specific substrate of islet-activating protein, pertussis toxin. FEBS Lett. 1987 Mar 23;213(2):353–358. doi: 10.1016/0014-5793(87)81521-1. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T., Itoh H., Tsukamoto T., Kaziro Y. Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2081–2085. doi: 10.1073/pnas.85.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lo W. W., Hughes J. A novel cholera toxin-sensitive G-protein (Gc) regulating receptor-mediated phosphoinositide signalling in human pituitary clonal cells. FEBS Lett. 1987 Aug 17;220(2):327–331. doi: 10.1016/0014-5793(87)80840-2. [DOI] [PubMed] [Google Scholar]
- Lo W. W., Hughes J. Receptor-phosphoinositidase C coupling. Multiple G-proteins? FEBS Lett. 1987 Nov 16;224(1):1–3. doi: 10.1016/0014-5793(87)80410-6. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Lucas D. O., Bajjalieh S. M., Kowalchyk J. A., Martin T. F. Direct stimulation by thyrotropin-releasing hormone (TRH) of polyphosphoinositide hydrolysis in GH3 cell membranes by a guanine nucleotide-modulated mechanism. Biochem Biophys Res Commun. 1985 Oct 30;132(2):721–728. doi: 10.1016/0006-291x(85)91192-1. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Martin M. W., Harden T. K., Brown J. H. Pertussis toxin does not inhibit muscarinic-receptor-mediated phosphoinositide hydrolysis or calcium mobilization. Biochem J. 1985 May 1;227(3):933–937. doi: 10.1042/bj2270933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R., Codina J., Crozat A., Kidd V., Woo S. L., Birnbaumer L. Identification by molecular cloning of two forms of the alpha-subunit of the human liver stimulatory (GS) regulatory component of adenylyl cyclase. FEBS Lett. 1986 Sep 29;206(1):36–42. doi: 10.1016/0014-5793(86)81336-9. [DOI] [PubMed] [Google Scholar]
- Medynski D. C., Sullivan K., Smith D., Van Dop C., Chang F. H., Fung B. K., Seeburg P. H., Bourne H. R. Amino acid sequence of the alpha subunit of transducin deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Nukada T., Tanabe T., Takahashi H., Noda M., Haga K., Haga T., Ichiyama A., Kanagawa K., Hiranaga M., Matsuo Primary structure of the alpha-subunit of bovine adenylate cyclase-inhibiting G-protein deduced from the cDNA sequence. FEBS Lett. 1986 Mar 3;197(1-2):305–310. doi: 10.1016/0014-5793(86)80347-7. [DOI] [PubMed] [Google Scholar]
- Nukada T., Tanabe T., Takahashi H., Noda M., Hirose T., Inayama S., Numa S. Primary structure of the alpha-subunit of bovine adenylate cyclase-stimulating G-protein deduced from the cDNA sequence. FEBS Lett. 1986 Jan 20;195(1-2):220–224. doi: 10.1016/0014-5793(86)80164-8. [DOI] [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Russell D. W., Harris B. A., Smigel M. D., Gilman A. G. Deduced primary structure of the alpha subunit of the GTP-binding stimulatory protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1251–1255. doi: 10.1073/pnas.83.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suki W. N., Abramowitz J., Mattera R., Codina J., Birnbaumer L. The human genome encodes at least three non-allellic G proteins with alpha i-type subunits. FEBS Lett. 1987 Aug 10;220(1):187–192. doi: 10.1016/0014-5793(87)80900-6. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Liao Y. C., Alborzi A., Beiderman B., Chang F. H., Masters S. B., Levinson A. D., Bourne H. R. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Nukada T., Nishikawa Y., Sugimoto K., Suzuki H., Takahashi H., Noda M., Haga T., Ichiyama A., Kangawa K. Primary structure of the alpha-subunit of transducin and its relationship to ras proteins. Nature. 1985 May 16;315(6016):242–245. doi: 10.1038/315242a0. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Van Dop C., Tsubokawa M., Bourne H. R., Ramachandran J. Amino acid sequence of retinal transducin at the site ADP-ribosylated by cholera toxin. J Biol Chem. 1984 Jan 25;259(2):696–698. [PubMed] [Google Scholar]
- Van Meurs K. P., Angus C. W., Lavu S., Kung H. F., Czarnecki S. K., Moss J., Vaughan M. Deduced amino acid sequence of bovine retinal Go alpha: similarities to other guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3107–3111. doi: 10.1073/pnas.84.10.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Yatsunami K., Khorana H. G. GTPase of bovine rod outer segments: the amino acid sequence of the alpha subunit as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4316–4320. doi: 10.1073/pnas.82.13.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]