Abstract
The expression of the TRH gene in the paraventricular nucleus (PVH) of the hypothalamus is required for the normal production of thyroid hormone (TH) in rodents and humans. In addition, the regulation of TRH mRNA expression by TH, specifically in the PVH, ensures tight control of the set point of the hypothalamic-pituitary-thyroid axis. Although many studies have assumed that the regulation of TRH expression by TH is at the level of transcription, there is little data available to demonstrate this. We used two in vivo model systems to show this. In the first model system, we developed an in situ hybridization (ISH) assay directed against TRH heteronuclear RNA to measure TRH transcription directly in vivo. We show that in the euthyroid state, TRH transcription is present both in the PVH and anterior/lateral hypothalamus. In the hypothyroid state, transcription is activated in the PVH only and can be shut off within 5 h by TH. In the second model system, we employed transgenic mice that express the Cre recombinase under the control of the genomic region containing the TRH gene. Remarkably, TH regulates Cre expression in these mice in the PVH only. Taken together, these data affirm that TH regulates TRH at the level of transcription in the PVH only and that genomic elements surrounding the TRH gene mediate its regulation by T3. Thus, it should be possible to identify the elements within the TRH locus that mediate its regulation by T3 using in vivo approaches.
The TRH gene is directly regulated by T3 at the level of transcription only in the paraventricular hypothalamus using two different in vivo models.
Regulation of the hypothalamic-pituitary-thyroid (HPT) axis is essential to maintain thyroid hormone (TH) levels within a fine range, a requirement for processes such as cellular development, metabolism, energy expenditure, and temperature regulation (1). Normal function of the HPT axis in both rodents and man requires the synthesis of TRH in the paraventricular nucleus (PVH) of the hypothalamus and its secretion into the portal capillary system (2,3). Indeed, TRH knockout mice and humans with mutations in the TRH receptor have central hypothyroidism (4,5). To tightly regulate systemic TH levels, TRH mRNA expression and peptide production are negatively regulated by the active form of TH, 3,5,3′-triiodothyronine, T3 (6). Thus, the HPT axis is self-regulating through this feedback mechanism. Although many studies from a number of laboratories, including our own, have used the TRH promoter as a useful reagent to examine negative regulation by T3, the molecular mechanism by which T3 mediates regulation of TRH gene expression is unknown, and it remains unclear whether T3 regulates the TRH gene at the level of transcription in vivo.
T3 regulates gene transcription and expression via its cognate nuclear receptors, the TH receptor (TR) isoforms, which bind to TH response elements (TREs) in the regulatory regions of target genes (7). Although there are three TR isoforms, mouse knockout studies have established that the TRβ2 isoform is required for regulation of TRH gene expression by T3 (8). On positively regulated T3 targets, the TR interacts with the nuclear corepressors: nuclear receptor corepressor and silencing mediator of retinoic acid and TRs to mediate ligand-independent repression (9,10,11,12,13,14). In the presence of T3, the corepressors are released and the TR recruits a coactivator complex that leads to transcriptional activation (15,16). In contrast, on negative T3 targets, such as TRH, the role of corepressors and coactivators is undefined. Cell culture models studying a variety of negatively regulated T3 targets have produced conflicting results (17,18,19). However, in these model systems, negative targets are transcriptionally activated in the absence of ligand and the addition of T3 leads to their repression. Moreover, cell culture models have identified a cis acting site in the TRH promoter termed Site 4, which binds the TR and appears to mediate negative regulation by T3 in cell culture reporter systems (20,21,22). Its role in vivo has never been substantiated.
Mouse models have provided some insight into the mechanisms by which T3 negatively regulates TRH, such as establishing that TRβ2 must bind to DNA to regulate TRH gene expression (8,23). Furthermore, members of the steroid receptor coactivator family appear to play a paradoxical role in ligand-dependent repression by T3 because steroid receptor coactivator 1 knockout mice have inappropriately high circulating TSH levels in the face of elevated TH levels, which suggests that defects in TRH regulation are present (24,25). Taken together, these studies suggest that TRH is a transcriptional target of T3, but further in vivo studies are required to better understand the mechanism. The importance of understanding negative regulation is underscored by the fact that the most common test used to interpret thyroid function in humans is the TSH assay, whose value is the result of negative regulation of the TRH and TSH subunit genes by T3.
To further delineate how TRH is regulated by T3, we have developed a methodology to examine TRH transcription in vivo. Also, we have developed a novel transgenic model using a TRH bacterial artificial chromosome (BAC) to explore the role of TRH genomic elements in mediating negative regulation by T3. Our results demonstrate that TRH is regulated at the level of transcription by T3 only in the PVH, but not in other regions of the hypothalamus. In addition, we show that genomic regions surrounding the TRH gene mediate its negative regulation by the TR and T3. These data reveal the TRH gene as an ideal in vivo target to use in understanding the mechanisms of negative regulation by T3 through the TR.
Materials and Methods
Animals
Animal care
The Institutional Animal Care and Use Committee at the Beth Israel Deaconess Medical Center (BIDMC) approved all animal experiments. All mice described below were housed at the BIDMC animal facility, maintained on a 14-h light, 10-h dark cycle, and fed a rodent chow diet (Harlan Teklad F6 Rodent Diet, Harlan Teklad Laboratories, Indianapolis, IN) unless otherwise noted.
TRH transcription experiments
Twelve C57BL/6 mice at 7 wk of age were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were acclimated to BIDMC for 1 wk. For the next 3 wk, four mice remained on chow diet and eight mice were placed on a low iodine (LI) diet supplemented with 0.15% propylthiouracil (PTU) (PTU/LI, TD.95125, LoI/PTU; Harlan Teklad Laboratories). At the end of this period, mice were killed as described below except for four mice on the PTU/LI diet that received one dose of T3 (Sigma, St. Louis, MO) by ip injection (1 μg/1 g of body weight in PBS) and then were killed 5 h after the injection.
Generation of TRH-Cre Mice
The TRH-Cre transgene was made from a BAC (Genome Systems in the vector pBeloBAC 11) containing the TRH gene with at least 30 KB of 5′ and 40 KB of 3′ flanking sequence. The cDNA encoding the Cre recombinase was inserted downstream of the endogenous ATG used in exon 2 of the TRH gene using bacterial recombineering techniques (26,27). The insertion of the Cre recombinase disrupted the TRH gene in the transgene only. The resulting recombinant BAC DNA was purified by cesium chloride centrifugation and used to make TRH-Cre transgenic mice. A number of founders were isolated, and two subsequent lines (F5: TRH-Cre and F40: TRH-Cre) were used in the following studies. Genotyping of all mice was performed on genomic DNA isolated from tail snips. Primers were designed such that both the recombinant and endogenous allele could be detected in the same reaction.
To test the functionality of the Cre recombinase, the Founder 5 and Founder 40 lines of TRH-Cre mice (F5: TRH-Cre and F40: TRH-Cre) were crossed with Rosa-26-GFP reporter mice (The Jackson Laboratory). Male mice were euthanized by transcardiac perfusion as described below. To test the regulation of the transgene by TH, F40: TRH-Cre mice (n = 3) remained on chow diet, whereas control littermate wild-type (WT) (n = 3) and F40: TRH-Cre mice (n = 6) were placed on PTU/LI diet for 3 wk. Mice were killed as described below except for half of the F40: TRH-Cre mice (n = 3) on PTU/LI diet. These mice were given a dose of T3 (Sigma) for 3 d by ip injection (1 μg/100 g of body weight in PBS) and then killed 14 h after the last injection. To ascertain whether the TRH-Cre transgene disrupted endogenous TRH mRNA expression, ISH for TRH mRNA was performed on the F40: TRH-Cre mice from above and control littermate WT mice placed on chow diet, PTU/LI diet for 3 wk, or PTU/LI diet for 3 wk plus 3 d of ip injection of T3 (as described above).
Histology
All mice were euthanized via transcardiac perfusion. Mice were given an ip injection of ketamine and xylazine (80 mg ketamine/1 kg body weight, 12 mg xylazine/1 kg body weight in saline). Upon complete anesthetization, blood samples were taken by cardiac puncture; serum was separated via centrifugation and frozen at −80 C. Mice were then perfused through the heart with saline followed by 10% neutral buffered formalin. Brains were dissected, postfixed in 10% neutral buffered formalin for 4–6 h, dehydrated in 20% sucrose in diethylpyrocarbonate (DEPC)-treated PBS, and stored at 4 C overnight. Brains were cut in coronal 25-μm sections into four equal series on a freezing microtome, and then the tissue was stored in antifreeze solution (50% formalin, 30% glycerol, 20% ethylene glycol) at −20 C until processed.
ISH
Riboprobe preparation
To determine the expression of TRH mRNA and TRH heteronuclear RNA (TRH hnRNA) expression, S35 radiolabeled and digoxigenin riboprobes were prepared using linearized plasmid according to the manufacturer directions using the Promega T3/T7/SP6 Riboprobe kit (Promega Corp., Madison, WI). The murine cDNA for TRH mRNA, a gift from Masanobu Yamada (Gunma University School of Medicine, Maebashi, Japan), has been previously described, and the antisense riboprobe was generated with SP6 (28). The template DNA for TRH hnRNA was created by genomic PCR and confirmed by sequencing and the antisense riboprobe was generated with SP6.
Radioactive ISH
ISH has been adapted from previously described techniques (29,30,31). Brain sections were mounted on to Superfrost Plus slides (Thermo Fisher Scientific, Pittsburgh, PA), air-dried, and stored at −20 C until further use. Prehybridization and hybridization steps used DEPC-treated solutions. Prehybridization washes of the tissue included fixation in 4% formaldehyde in PBS for 30 min at 4 C, a 10-min incubation in 0.25% acetic anhydride in 0.1 m tetraethylammonium chloride (TEA), dehydration in a series of increasing concentrations of ethanol, clearance in xylenes for 15 min, rehydration in decreasing concentrations of ethanol, exposure of hybridization substrates by microwaving in prewarmed sodium citrate buffer (95–100 C, pH 6.0) for 10 min at 20% power in a Panasonic (Secaucus, NJ) NN-969 Genius Premier microwave (1100 W), and finished with dehydration in a series of increasing concentrations of ethanol. Following air-drying, slides were applied with 120 μl of S35-labeled riboprobes diluted to 106 cpm/ml in hybridization solution [50% formamide (Thermo Fisher Scientific, Fairlawn, NJ), 2× standard saline citrate (SSC), 1× Denhardt’s (Sigma), 10% (wt/vol) dextran sulfate, 400 μg/ml salmon sperm DNA, and 500 mm dithiothreitol (Promega Corp.)]. Coverslips were added to each slide, and the tissue was incubated at 57 C for 12–16 h. After hybridization, coverslips were removed in 1× SSC and washed for 15 min four times in 1× SSC. Slides were incubated at 37 C for 30 min in RNase wash buffer (0.5 m NaCl, 0.01 m Tris, and 0.01 mm EDTA) containing 100 μg/ml RNase A (Sigma). Sections were rinsed in decreasing concentrations of SSC containing 0.25% dithiothreitol (two washes of 2× SSC for 30 min at 50 C, two washes of 0.2× SSC for 30 min at 55 C, and two washes of 0.2× SSC for 30 min at 60 C). Sections were next dehydrated in increasing concentrations of ethanol (50, 70, 80, and 90%) containing 0.3 m NH4OAc followed by a 100% ethanol wash and air-drying. Slides were then placed in x-ray film cassettes with BioMax film (Eastman Kodak, Rochester, NY) for 16–18 h (TRH mRNA) or 2 d (TRH hnRNA). Slides were dipped in K5 Ilford photographic emulsion (Polysciences, Inc., Warrington, PA), dried, and stored at 4 C with desiccant in light-blocking boxes for 7 (TRH mRNA) or 21 d (TRH hnRNA). Slides were developed with Dekol developer and Fixer (Eastman Kodak), counterstained with thionin, dehydrated in increasing concentrations of ethanol, cleared with xylenes, and coverslipped with Cytoseal 280 (Richard-Allan Scientific, Kalamazoo, MI). Sense riboprobe controls for both TRH mRNA and TRH hnRNA were run to confirm specificity of the procedures.
Analysis of digital images was adapted from previously described techniques (32). Images were acquired on Zeiss Axioimager.Z1 with Axiovision 4.5 software (Oberkochen, Germany). Dark-field digital images of each hypothalamic region [PVH, anterior and lateral hypothalamus, dorsomedial hypothalamus (DMH)] of the brain were taken with the same exposure time, brightness, and contrast. The ×10 magnification images were quantified using ImageJ (Public Domain, Developed at the National Institute of Mental Health, Bethesda, MD), where the same threshold was used for comparison sets. The total number of positive pixels per unit area (pixel density) was calculated using at least 50 single-cell measurements and subtracting background from areas not expressing TRH mRNA or hnRNA. For both the mRNA and hnRNA probes, pixel density per mouse in each region of the hypothalamus was averaged from a minimum of three matching sections. Each experimental condition (euthyroid, hypothyroid, and hyperthyroid) in each region of the hypothalamus was tested in four animals. Data are presented as sample means relative to control euthyroid mice ± sem. The significance was tested using one-way ANOVA with Tukey’s post hoc analysis.
Digoxigenin-labeled ISH
For ISH on brain sections from WT and transgenic TRH-Cre animals, free-floating tissue sections were fixed with 4% formaldehyde, incubated in 0.25% acetic anhydride in 0.1 m TEA, then hybridized for 16–18 h at 59 C in buffer [50% formamide, 5× SSC, 5× Denhardt’s (Sigma), 0.5 mg/ml salmon sperm DNA, 0.25 mg/ml and Escherichia coli tRNA] containing digoxigenin-labeled riboprobe to TRH mRNA. Prehybridization and hybridization steps used DEPC-treated solutions. After hybridization washes included decreasing concentrations of SSC at 59 C. Sections were incubated overnight in sheep antidigoxigenin conjugated to alkaline phosphatase (1:7500), followed by incubation in nitroblue tetrazolium choloride. Tissue was mounted on Superfrost Plus slides (Thermo Fisher Scientific), and slides were coverslipped with Cytoseal 280 (Richard-Allan Scientific). Light-field images were captured using the Zeiss Axioimager.Z1 with Axiovision 4.5 software (Oberkochen).
Immunohistochemistry (IHC)
IHC procedures were modified from techniques previously reported (33). IHC using polyclonal antiserum to green fluorescent protein (GFP; Molecular Probes/Invitrogen, Eugene, OR) was performed on free-floating brain sections from male F40: TRH-Cre, F40: TRH-Cre x R26-GFP, and F5: TRH-Cre x R26-GFP mice. IHC using polyclonal antiserum to Cre (Novagen/Merck, Darmstadt, Germany) was performed on free-floating brain sections from male hypothyroid WT, euthyroid F40: TRH-Cre, hypothyroid F40: TRH-Cre, and hyperthyroid F40: TRH-Cre mice.
Free-floating sections were rinsed six times in 1× PBS, then washed in 0.3% H2O2 in PBS to block endogenous peroxide activity. Sections were washed in PBS, blocked for 2 h with 3% normal goat serum in PBT-Azide (0.25% Triton X-100 in 1× PBS with 0.2 mg/ml sodium azide), and then incubated overnight with 1:7500 dilution of primary antibody in 3% normal goat serum in PBT-Azide. After PBS washes the next day, sections were incubated in antirabbit biotinylated secondary antibody (Vectastain Elite ABC kit; Vector Labs, Burlingame, CA) at 1:1000 in 3% normal goat serum in PBT for 1 h. Tissue was rinsed in PBS and incubated in Avidin: biotinylated enzyme complex (Vector Labs) at 1:500 in PBS for 1 h. Sections were washed again in PBS then developed in 0.04% 3,3′-diaminobenzidine and 0.01% H2O2 in PBS. Tissue was mounted on Superfrost Plus (Thermo Fisher Scientific) slides, dried, cleared in xylenes, and coverslipped with Cytoseal 280 (Richard-Allan Scientific). Light-field images were captured using the Zeiss Axioimager.Z1 with Axiovision 4.5 software (Oberkochen).
Dual-label ISH/IHC
Dual-label ISH/IHC was performed to confirm colocalization of GFP immunoreactivity with TRH mRNA expressing neurons. Brain sections from F40: TRH-Cre and F40: TRH-Cre x R26-GFP mice were mounted onto Superfrost Plus slides (Thermo Fisher Scientific). Prehybridization and hybridization steps used DEPC-treated solutions. Prehybridization steps were similar to those performed for radioactive ISH. Tissue was fixed for 30 min at 4 C in 4% formaldehyde. After washes with PBS, slides were incubated in 0.25% acetic anhydride in 0.1 m TEA and washed in PBS again. Hybridization buffer (same as radioactive ISH) was applied with 120 μl of S35 radiolabed antisense TRH cRNA riboprobe diluted to 106 cpm/ml. Coverslips were added to each slide and the tissue was incubated at 57 C for 12–16 h. Posthybridization washes were similar to radioactive ISH. Coverslips were removed in 1× SSC, washed four times in 1× SSC, and incubated at 37 C for 30 min in RNase wash buffer with 100 μg/ml RNase A (Sigma). Sections were rinsed in decreasing concentrations of SSC (two washes of 5× SSC for 10 min at 59 C, two washes of 1× SSC for 10 min at 59 C, two washes of 0.2× SSC for 10 min at 59 C, two washes of 0.2× SSC for 10 min at room temperature, and two washes of 1× PBS for 10 min at room temperature). Slides were then incubated with 0.3% H2O2 in PBS to block endogenous peroxide activity. Sections were washed in PBS, blocked for 2 h with 3% normal goat serum in PBT-Azide, and incubated overnight at 4 C with gasketed coverslips (Molecular Probes/Invitrogen) with 1:1000 dilution of anti-GFP (Molecular Probes/Invitrogen) antibody in 3% normal goat serum in PBT-Azide. After PBS washes the next day, slides were incubated in antirabbit biotinylated secondary antibody (Vector Labs) at 1:1000 in 3% normal goat serum in PBT for 1 h. Tissue was rinsed in PBS and incubated in ABC (Vector Labs) at 1:500 in PBS for 1 h. Slides were washed again in PBS and developed in 0.04% 3,3′-diaminobenzidine and 0.01% H2O2 in PBS. Slides were placed in x-ray film cassettes with BioMax film (Eastman Kodak) for 16–18 h and then dipped in K5 Ilford photographic emulsion (Polysciences, Inc.), dried, and stored at 4 C with desiccant in light-blocking boxes for 7 d. Slides were developed with Dekol developer and Fixer (Eastman Kodak), cleared with xylenes, and coverslipped with Cytoseal 280 (Richard-Allan Scientific). Light-field images were captured using the Zeiss Axioimager.Z1 with Axiovision 4.5 software.
Results
T3 regulates the TRH gene at the level of transcription in vivo
To determine if the TRH gene is regulated at the level of transcription by T3, we developed an ISH probe directed against the first intron of the TRH gene that is able to detect TRH heteronuclear RNA (hnRNA). Previously, this technique has been used to examine in vivo transcription of other hypothalamic genes (34,35,36,37). We used this probe in concert with a TRH cRNA probe, which detects mature TRH mRNA. We studied three groups of mice that were either euthyroid, made hypothyroid by 21 d of a PTU/LI diet or hypothyroid followed by transient hyperthyroidism.
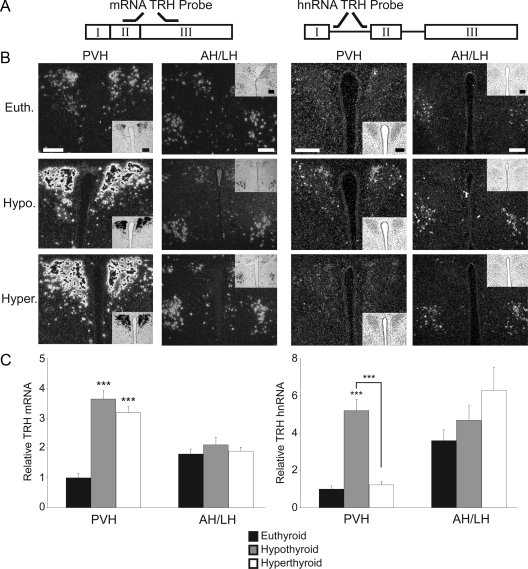
TRH mRNA is expressed in many areas of the hypothalamus, including the PVH (Fig. 1), the anterior and lateral hypothalamus (Fig. 1), and the DMH (Fig. 2). As has been previously shown by many groups, the hypothyroid state leads to the induction of TRH mRNA expression in the PVH only (8,38,39). In our experiments, there is a 3.5-fold increase in TRH mRNA expression in the PVH compared with euthyroid controls. Under these conditions, TRH mRNA expression is not regulated in the anterior/lateral hypothalamus or DMH. Although T3 administration over a number of days is known to repress TRH mRNA expression in the PVH, there is no change in TRH mRNA expression 5 h after the administration of a large dose of T3, suggesting that either transcriptional events or mRNA stability mediated by T3-action require a longer period of time.
Figure 1.
TRH is regulated by T3 at the level of transcription in the PVH. ISH using S35 radiolabeled probes. A, Representation of the two probes used for ISH. The cRNA probe labels nucleotides 307–572 of the murine prepro-TRH gene (TRH mRNA, left); the heteronuclear TRH probe labels the first intron in the TRH RNA message (TRH hnRNA, right). B, ISH was performed on brain slices from euthyroid (Euth.), hypothyroid (Hypo.), and hyperthyroid (Hyper.) WT mice. Images detail the paraventricular hypothalamus (PVH) and anterior hypothalamus (AH). n = 4 mice for each probe under each condition. Magnification, ×10; scale bars per column, 100 μm. C, Quantification of ISH via pixel density. Data are presented as sample means relative to control euthyroid mice ± sem. Significance was tested within each brain region and within each probe via one-way ANOVA. ***, P < 0.001.
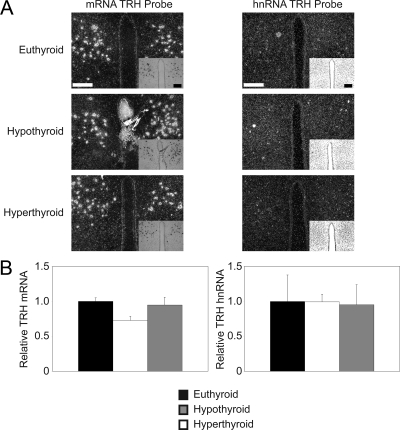
Figure 2.
TRH mRNA is expressed in the DMH. ISH using S35 radiolabeled probes. A, ISH was performed on brain slices from euthyroid, hypothyroid, and hyperthyroid WT mice using the TRH mRNA (left column) and TRH hnRNA (right column) probes. Magnification, ×10; scale bars per column, 100 μm. n = 4 mice for each probe under each condition. B, Quantification of ISH via pixel density. Data are presented as sample means relative to control euthyroid mice ± sem. Significance was tested within each probe via one-way ANOVA.
Next, we analyzed hypothalamic sections from these same animals with the TRH hnRNA probe to confirm whether the TRH gene was transcriptionally regulated. In the euthyroid state (Fig. 1B), there is low expression of TRH hnRNA in the PVH. However, TRH hnRNA is 3-fold higher in the lateral hypothalamus in euthyroid animals, suggesting a much higher transcriptional rate of TRH in this region of the hypothalamus. In hypothyroid animals, there is a 5-fold induction of TRH hnRNA in the PVH consistent with transcriptional activation of the gene in the absence of T3. Remarkably, 5 h after the administration of T3, TRH hnRNA is completely suppressed in the PVH, demonstrating that T3 negatively regulates this gene at the level of transcription. Similar to expression of TRH mRNA, TRH hnRNA was not regulated in the anterior or lateral hypothalamus in either the hypothyroid or transiently hyperthyroid state.
Previously, TRH mRNA has been detected in the DMH (38). Although there is little data regarding the function of TRH in the DMH, we examined hypothalamic sections containing the DMH from the same animals as above to ascertain whether TRH mRNA or hnRNA expression was altered with changes in thyroid status. As shown in Fig. 2, TRH mRNA expression in the DMH is not regulated under conditions of hypothyroidism or with administration of T3. Additionally, in the euthyroid, hypothyroid, and transient hyperthyroid state, TRH hnRNA expression remained consistently low.
The TRH gene contains a negative TRE that functions in vivo
Given that TRH is a transcriptional target in vivo of T3, we next sought to use a model to identify the molecular mechanism by which the T3/TRβ2 signaling system mediates negative regulation. Although a negative TRE has been identified in cell culture models in the TRH promoter, its function in vivo has never been tested. To use an in vivo model to begin to identify the TRH negative TRE, we developed transgenic mice using a TRH BAC that contains at least 30 kb of genomic flanking sequence 5′ and 40 KB 3′ to the TRH gene (Fig. 3). Within this BAC, we inserted the Cre recombinase cDNA into exon 2 adjacent to the endogenous translation start site of the TRH gene.
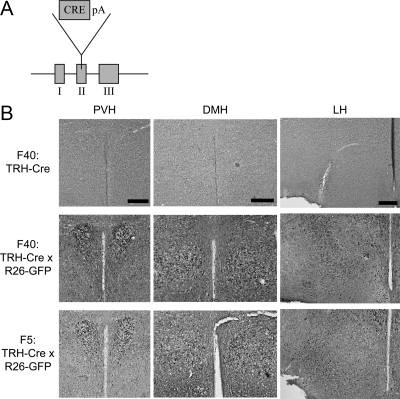
Figure 3.
Cre recombinase is biologically active in transgenic TRH-Cre mice. A, Representation of the TRH BAC in TRH-Cre mice. The Cre is inserted into the second exon of TRH. B, To show that the Cre recombinase in TRH-Cre mice is biologically active, TRH-Cre mice were crossed with Rosa-26 GFP mice. IHC staining was performed for GFP on TRH-Cre mice from the Founder 40 line (F40: TRH-Cre) and TRH-Cre x Rosa-26 GFP mice from both the Founder 40 and founder 5 lines (F40: TRH-Cre x R26-GFP and F5: TRH-Cre x R26-GFP). Magnification, ×10; scale bars per column, 100 μm.
To test whether this BAC was expressed in mice in an analogous fashion to endogenous TRH, we crossed the TRH-Cre mice with Rosa26-GFP reporter mice that express GFP only if a transcriptional stop cassette is removed by the Cre recombinase. As shown in Fig. 3B, crossing the TRH-Cre mice with Rosa26-GFP animals results in GFP staining in the PVH. In addition, GFP is seen in other regions of the hypothalamus that express TRH such as the lateral hypothalamus and DMH. However, GFP staining is also seen in regions of the hypothalamus and brain such as the arcuate and ventromedial nuclei where expression of TRH is not seen in the mouse by ISH. Interestingly, expression of TRH has been reported in some of these regions in the rat (38,40). This suggests that early developmental expression of TRH in the mouse, which would lead to transient Cre expression in these regions, could yield GFP staining in the adult (41).
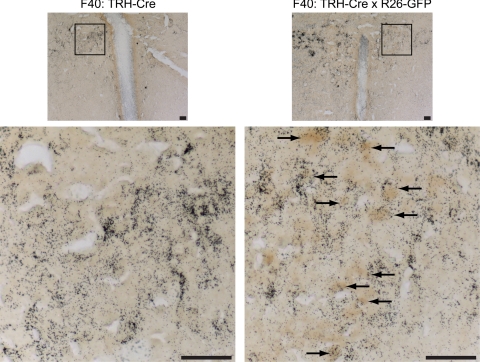
To establish whether GFP-positive neurons in the PVH also expressed TRH, we performed double-label studies with IHC for GFP and ISH for TRH mRNA. As shown in Fig. 4, TRH-expressing neurons in the PVH are also GFP-positive, indicating that the TRH BAC has appropriately targeted TRH neurons in the PVH. However, there is also GFP-staining seen in PVH neurons that do not express TRH, indicating that the transgene may target other PVH neuronal groups. Importantly, control animals show no evidence of GFP staining.
Figure 4.
GFP-positive neurons colocalize with neurons expressing TRH mRNA. Dual-label ISH/IHC for TRH mRNA using a S35 radiolabeled riboprobe (black grains) and immunostaining for GFP (dark brown) was performed in Founder 40 TRH-Cre (F40: TRH-Cre) and F40: TRH-Cre crossed to Rosa-26-GFP reporter (F40: TRH-Cre x R26-GFP) mice. Magnifications, ×10 (top) and ×40 (bottom) of the highlighted area of the PVH are shown; scale bars, 100 μm. Arrows identify neurons positive for both TRH mRNA and GFP immunostaining.
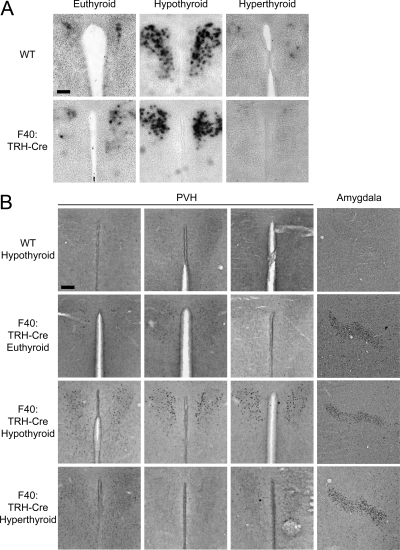
Because TRH-Cre was expressed in TRH neurons in the PVH of transgenic animals, we next asked whether expression of the transgene could be regulated by T3. Groups of transgenic animals and WT controls were either placed on chow or PTU/LI diet for 3 wk. After 3 wk, half of the PTU/LI-treated animals were given ip injections of T3 to induce hyperthyroidism. To validate the treatment paradigm and also to evaluate whether the transgene affected endogenous TRH expression, we also examined TRH mRNA expression in TRH-Cre transgenic mice using a digoxigenin-labeled TRH cRNA probe. As expected in both WT and transgenic animals (Fig. 5A), TRH mRNA was up-regulated after 3 wk on the PTU/LI diet and then was suppressed after 3 d of T3 administration consistent with appropriate regulation of the endogenous TRH gene.
Figure 5.
Cre recombinase expression is regulated by TH. A, ISH for TRH mRNA using a digoxigenin-labeled probe in WT and F40: TRH-Cre mice under euthyroid, hypothyroid, and hyperthyroid conditions. For the PVH: magnification, ×10; scale bar, 100 μm. B, IHC staining was performed for Cre on hypothyroid WT and euthyroid, hypothyroid, and hyperthyroid F40: TRH-Cre mice. The PVH of each genetic background is shown under different thyroid conditions (n = 3). In the fourth column, images of the amygdala are representative of each thyroid condition and genetic background. Images of the PVH and the amygdala: magnification, ×10; scale bar per region, 100 μm.
To assess the regulation of the transgene, we used IHC to visualize the level of Cre expression in each of the treatment groups. As shown in Fig. 5B, Cre immunostaining is only faintly detectable in the PVH of TRH-Cre mice in the euthyroid state. We also detected Cre immunostaining in the amygdala of transgenic mice (Fig. 5B). Similar to the expression of TRH mRNA, Cre staining in the hypothyroid transgenic mice was induced in the PVH. Importantly, Cre immunostaining was not seen in hypothyroid WT control animals, verifying the specificity of the antibody. In the amygdala, Cre immunostaining remained unchanged in the hypothyroid state of transgenic animals, suggesting a lack of regulation by T3 in this region. After T3 administration for 3 d, Cre immunostaining was strongly suppressed in the transgenic animals with no change seen in the amygdala.
Discussion
Seminal experiments by Tata in the 1960s demonstrated that T3 mediates many of its effects by regulating transcription (42,43). Since that time, much has been learned about how T3 regulates positive gene expression. Although T3 also mediates negative regulation, the molecular mechanisms of such are still unknown, including the identification of in vivo negative regulatory elements and the role of coactivators and corepressors. Work by other laboratories has established that the negative regulation of TRH by T3 occurs through the TRβ2 isoform and DNA-binding (8,23,44). Mice lacking TRβ2 have higher basal expression of TRH mRNA, similar to a WT hypothyroid mouse. Furthermore, hypothyroidism or treatment with T3 in the TRβ2 null mouse failed to significantly change TRH mRNA levels. In vivo siRNA experiments have confirmed these studies, outlining the explicit role of the TR in mediating negative regulation of TRH (45). However, before the work presented here, little evidence existed substantiating that the TRH gene is regulated by T3 at the level of transcription in vivo and that genomic negative response elements are present near to the TRH gene.
Using an ISH technique that measures heteronuclear RNA, whose production is the best measure of transcription in vivo, we have definitively confirmed that T3 regulates the transcription of the TRH gene in an anatomically precise manner. Strikingly, TRH hnRNA levels were very high in the euthyroid state in the anterior and lateral hypothalamus, an area where TRH mRNA is expressed but not regulated by T3 (38,39). The reason for the anatomic specificity of T3-mediated TRH regulation is unknown. The data presented here strongly suggest that the anterior/lateral hypothalamus is functionally hypothyroid and cannot sense T3 levels. A number of reasons for this may exist. This region may not actively receive T3 via the third ventricle and projecting tanycytes (46). Also, these neurons potentially may not import T3 through the lack of an appropriate transporter or alternatively may inactivate T3 through the expression of the type 3 deiodinase (47,48,49,50). Finally, it remains possible that these neurons lack the molecular machinery to respond to T3. In contrast, TRH mRNA levels are robust in the DMH, whereas TRH hnRNA levels are very low. Although this is similar to the euthyroid PVH of a WT mouse, TRH mRNA and hnRNA expression remain unchanged in the DMH with hypothyroidism or administration of T3. Clearly further experiments will be required to define the anatomic specificity of T3 action and TRH expression in the hypothalamus, but analysis of heteronuclear RNA expression suggests that multiple mechanisms may exist.
As outlined previously, significant insight into cis-acting elements that bind the TR isoforms has come from positively regulated genes such as the growth hormone gene in the pituitary or the type I deiodinase gene in the liver (51,52,53). In contrast, cis-acting elements that mediate negative regulation in vivo have not been identified, although a variety of potential elements have been described in the TSHα and TSHβ subunit genes in cell culture models (18,54,55). Although the transgenic model presented here may not be useful for conditional knockout experiments in TRH neurons because of the transient or ectopic expression of the Cre recombinase in other neurons, it distinctly demonstrates that genomic elements surrounding the TRH gene mediate its regulation by T3 in the PVH only. Thus, further modification of the genomic sites such as Site 4 in separate transgenic models should allow for the identification of the cis-acting element that mediates the negative regulation of TRH by T3 and the understanding of how the TRs are able to both induce and repress transcription in the presence of T3. The specific identification of such elements is paramount because only then can the specific recruitment of coregulators including corepressors and coactivators be examined and the mechanism of negative regulation understood.
Acknowledgments
We thank Drs. Inna Astapova, Preeti Ramadoss, and Jill M. Paulson for critical reading of the manuscript, Francis Lam for technical assistance, and Dr. Franck Chiappini for helpful discussion.
Footnotes
This work was supported by National Institutes of Health Grants DK056123 and DK078090 (to A.N.H.) and T32 DK07516 (to K.R.V.).
M.L.S. and K.R.V. contributed equally to the study.
Present address for M.E.L.: Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin-Madison, 1656 Linden Drive, Madison, Wisconsin 53706.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2009
Abbreviations: BAC, Bacterial artificial chromosome; DEPC, diethylpyrocarbonate; DMH, dorsomedial hypothalamus; GFP, green fluorescent protein; HPT, hypothalamic-pituitary-thyroid; IHC, immunohistochemistry; ISH, in situ hybridization; LI, low iodine; PTU, propylthiouracil; PVH, paraventricular nucleus; SSC, standard saline citrate; TEA, tetraethylammonium chloride; TH, thyroid hormone; TR, TH receptor; TRE, TH response element; TRH hnRNA, TRH heteronuclear RNA; WT, wild type.
References
- Hollenberg AN 2008 The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid 18:131–139 [DOI] [PubMed] [Google Scholar]
- Hollenberg AN 2005 Regulation of thyrotropin secretion. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 197–214 [Google Scholar]
- Lechan RM, Hollenberg AN 2003 Thyrotropin-releasing hormone (TRH). In: Henry HL, Norman AW, eds. Encyclopedia of hormones. New York: Elsevier Science; 510–524 [Google Scholar]
- Yamada M, Saga Y, Shibusawa N, Hirato J, Murakami M, Iwasaki T, Hashimoto K, Satoh T, Wakabayashi K, Taketo MM, Mori M 1997 Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc Natl Acad Sci USA 94:10862–10867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collu R, Tang J, Castagné J, Lagacé G, Masson N, Huot C, Deal C, Delvin E, Faccenda E, Eidne KA, Van Vliet G 1997 A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. J Clin Endocrinol Metab 82:1561–1565 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Wu P, Jackson IM, Wolf H, Cooperman S, Mandel G, Goodman RH 1986 Thyrotropin-releasing hormone precursor: characterization in rat brain. Science 231:159–161 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Abel ED, Ahima RS, Boers ME, Elmquist JK, Wondisford FE 2001 Critical role for thyroid hormone receptor β2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J Clin Invest 107:1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alland L, Muhle R, Hou Jr H, Potes J, Chin L, Schreiber-Agus N, DePinho RA 1997 Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49–55 [DOI] [PubMed] [Google Scholar]
- Guenther MG, Barak O, Lazar MA 2001 The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21:6091–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen TM, Söderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG 1997 A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387:43–48 [DOI] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM 1997 Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89:373–380 [DOI] [PubMed] [Google Scholar]
- Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J 2003 Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J 22:1336–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN 2008 The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci USA 105:19544–19549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Love JD, Li C, Banayo E, Gooch JT, Krishna V, Chatterjee K, Evans RM, Schwabe JW 1999 Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev 13:3209–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG 2000 The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- Feng X, Jiang Y, Meltzer P, Yen PM 2001 Transgenic targeting of a dominant negative corepressor to liver blocks basal repression by thyroid hormone receptor and increases cell proliferation. J Biol Chem 276:15066–15072 [DOI] [PubMed] [Google Scholar]
- Wang D, Xia X, Liu Y, Oetting A, Walker RL, Zhu Y, Meltzer P, Cole PA, Shi YB, Yen PM 2009 Negative regulation of TSHα target gene by thyroid hormone involves histone acetylation and corepressor complex dissociation. Mol Endocrinol 23:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T, Park Y, Jameson JL 1999 Mechanisms that mediate negative regulation of the thyroid-stimulating hormone α gene by the thyroid hormone receptor. J Biol Chem 274:22345–22353 [DOI] [PubMed] [Google Scholar]
- Satoh T, Yamada M, Iwasaki T, Mori M 1996 Negative regulation of the gene for the preprothyrotropin-releasing hormone from the mouse by thyroid hormone requires additional factors in conjunction with thyroid hormone receptors. J Biol Chem 271:27919- 27926 [DOI] [PubMed] [Google Scholar]
- Satoh T, Monden T, Ishizuka T, Mitsuhashi T, Yamada M, Mori M 1999 DNA binding and interaction with the nuclear receptor corepressor of thyroid hormone receptor are required for ligand-independent stimulation of the mouse preprothyrotropin-releasing hormone gene. Mol Cell Endocrinol 154:137–149 [DOI] [PubMed] [Google Scholar]
- Hollenberg AN, Monden T, Flynn TR, Boers ME, Cohen O, Wondisford FE 1995 The human thyrotropin-releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements. Mol Endocrinol 9:540–550 [DOI] [PubMed] [Google Scholar]
- Shibusawa N, Hashimoto K, Nikrodhanond AA, Liberman MC, Applebury ML, Liao XH, Robbins JT, Refetoff S, Cohen RN, Wondisford FE 2003 Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo. J Clin Invest 112:588–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S 1999 Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J 18:1900–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RE, Gehin M, Xu J, Sadow PM, O'Malley BW, Chambon P, Refetoff S 2002 Thyroid function in mice with compound heterozygous and homozygous disruptions of SRC-1 and TIF-2 coactivators: evidence for haploinsufficiency. Endocrinology 143:1554–1557 [DOI] [PubMed] [Google Scholar]
- Muyrers JP, Zhang Y, Testa G, Stewart AF 1999 Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Res 27:1555–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG 2001 A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56–65 [DOI] [PubMed] [Google Scholar]
- Satoh T, Yamada M, Monden T, Iizuka M, Mori M 1992 Cloning of the mouse hypothalamic preprothyrotropin-releasing hormone (TRH) cDNA and tissue distribution of its mRNA. Brain Res Mol Brain Res 14:131–135 [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjøorbaek C, Elmquist JK, Flier JS, Hollenberg AN 2001 Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Kabeer N, Albers HE 1990 Cold exposure elevates cellular levels of messenger ribonucleic acid encoding thyrotropin-releasing hormone in paraventricular nucleus despite elevated levels of thyroid hormones. Endocrinology 127:2955–2962 [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK 2001 Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25 [DOI] [PubMed] [Google Scholar]
- Vella KR, Burnside AS, Brennan KM, Good DJ 2007 Expression of the hypothalamic transcription factor Nhlh2 is dependent on energy availability. J Neuroendocrinol 19:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK 1998 Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402:442–459 [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK 2002 Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Schäfer MK, Watson SJ, Sherman TG 1991 In situ hybridization analysis of arginine vasopressin gene transcription using intron-specific probes. Mol Endocrinol 5:1447–1456 [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Thompson RC, Watson SJ 1992 Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol 6:1061–1069 [DOI] [PubMed] [Google Scholar]
- Kovács KJ, Sawchenko PE 1996 Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci 16:262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerson TP, Kauer J, Wolfe HC, Mobtaker H, Wu P, Jackson IM, Lechan RM 1987 Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science 238:78–80 [DOI] [PubMed] [Google Scholar]
- Dyess EM, Segerson TP, Liposits Z, Paull WK, Kaplan MM, Wu P, Jackson IM, Lechan RM 1988 Triiodothyronine exerts direct cell-specific regulation of thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus. Endocrinology 123:2291–2297 [DOI] [PubMed] [Google Scholar]
- Espinosa VP, Ferrini M, Shen X, Lutfy K, Nillni EA, Friedman TC 2007 Cellular colocalization and coregulation between hypothalamic pro-TRH and prohormone convertases in hypothyroidism. Am J Physiol Endocrinol Metab 292:E175–E186 [DOI] [PubMed] [Google Scholar]
- McKnight KD, Hou J, Hoodless PA 2007 Dynamic expression of thyrotropin-releasing hormone in the mouse definitive endoderm. Dev Dyn 236:2909–2917 [DOI] [PubMed] [Google Scholar]
- Tata JR, Ernster L, Lindberg O, Arrhenius E, Pedersen S, Hedman R 1963 The action of thyroid hormones at the cell level. Biochem J 86:408–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata JR, Widnell CC 1966 Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J 98:604–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibusawa N, Hollenberg AN, Wondisford FE 2003 Thyroid hormone receptor DNA binding is required for both positive and negative gene regulation. J Biol Chem 278:732–738 [DOI] [PubMed] [Google Scholar]
- Guissouma H, Froidevaux MS, Hassani Z, Demeneix BA 2006 In vivo siRNA delivery to the mouse hypothalamus confirms distinct roles of TR β isoforms in regulating TRH transcription. Neurosci Lett 406:240–243 [DOI] [PubMed] [Google Scholar]
- Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM 1997 Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology 138:3359–3368 [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S 2004 A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Unmehopa UA, Alkemade A 2006 Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol Cell Endocrinol 251:1–8 [DOI] [PubMed] [Google Scholar]
- Hernández A, Lyon GJ, Schneider MJ, St Germain DL 1999 Isolation and characterization of the mouse gene for the type 3 iodothyronine deiodinase. Endocrinology 140:124–130 [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St Germain DL 2007 Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology 148:5680–5687 [DOI] [PubMed] [Google Scholar]
- Brent GA, Larsen PR, Harney JW, Koenig RJ, Moore DD 1989 Functional characterization of the rat growth hormone promoter elements required for induction by thyroid hormone with and without a co-transfected β type thyroid hormone receptor. J Biol Chem 264:178–182 [PubMed] [Google Scholar]
- Brent GA, Harney JW, Chen Y, Warne RL, Moore DD, Larsen PR 1989 Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol Endocrinol 3:1996–2004 [DOI] [PubMed] [Google Scholar]
- Ohguchi H, Tanaka T, Uchida A, Magoori K, Kudo H, Kim I, Daigo K, Sakakibara I, Okamura M, Harigae H, Sasaki T, Osborne TF, Gonzalez FJ, Hamakubo T, Kodama T, Sakai J 2008 Hepatocyte nuclear factor 4α contributes to thyroid hormone homeostasis by cooperatively regulating the type 1 iodothyronine deiodinase gene with GATA4 and Kruppel-like transcription factor 9. Mol Cell Biol 28:3917–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita A, Sasaki S, Kashiwabara Y, Nagayama K, Ohba K, Iwaki H, Misawa H, Ishizuka K, Nakamura H 2007 Essential role of GATA2 in the negative regulation of thyrotropin β gene by thyroid hormone and its receptors. Mol Endocrinol 21:865–884 [DOI] [PubMed] [Google Scholar]
- Bodenner DL, Mroczynski MA, Weintraub BD, Radovick S, Wondisford FE 1991 A detailed functional and structural analysis of a major thyroid hormone inhibitory element in the human thyrotropin β-subunit gene. J Biol Chem 266:21666–21673 [PubMed] [Google Scholar]