Abstract
Inactivating mutations of PHEX cause X-linked hypophosphatemia and result in increased circulating fibroblast growth factor 23 (FGF23). FGF23 action is dependent upon Klotho, which converts FGF receptor 1 into an FGF23-specific receptor. Disruption of Klotho results in a complex bone phenotype and hyperphosphatemia, the converse phenotype of X-linked hypophosphatemia. We examined effects of disrupting both Klotho and PHEX by creating a double-knockout (Klotho/HYP) mouse. The combined disruption corrected the hypophosphatemia in HYP mice, indicating that Klotho is epistatic to PHEX. FGF23 levels remained elevated in all groups except wild-type, indicating that Klotho is necessary for FGF23-dependent phosphaturic activity. 1,25-Dihydroxyvitamin D levels, reduced in HYP mice, were comparably elevated in Klotho and Klotho/HYP mice, demonstrating that Klotho is necessary for FGF23’s effect on vitamin D metabolism. Serum PTH levels were reduced in both Klotho and Klotho/HYP mice. Moreover, the Klotho null phenotype persisted in Klotho/HYP, maintaining the runty phenotype and decreased life span of Klotho null mice. Notably, microcomputed tomography analysis demonstrated greater trabecular bone volume fraction in Klotho/HYP mice than that in all other groups (Klotho/HYP, 56.2 ± 6.3%; Klotho, 32.5 ± 10.3%; HYP, 8.6 ± 7.7%; and wild type, 21.4 ± 3.4%; P < 0.004). Histomorphometric analysis confirmed the markedly increased trabecular bone density in Klotho/HYP mice and the well-established increase in osteoid volume in HYP mice. These observations suggest that with addition of Klotho loss of function, the overabundant osteoid typically produced in HYP mice (but fails to mineralize) is produced and mineralized in the double knockout, resulting in markedly enhanced trabecular bone density.
Biochemical and skeletal phenotype of the Klotho/HYP mouse (PHEX deletion combined with disruption of the Klotho gene), which is characterized by a markedly elevated bone density and correction of the HYP mouse’s hypophosphatemia, is described.
The HYP mouse, the most completely characterized model of human X-linked hypophosphatemia (XLH), was discovered in 1976 (1). HYP mice were subsequently identified as harboring a large deletion in the PHEX gene, after the human disorder was mapped to loss-of-function mutations in PHEX (2,3), which encodes a protein with homology to zinc metallopeptidases found on the X chromosome (4). As in humans with XLH, HYP mice demonstrate hypophosphatemia secondary to renal phosphate wasting, impaired vitamin D metabolism, and hypomineralization of bone and dentin (1). Recently, elevated circulating levels of fibroblast growth factor 23 (FGF23) have been noted in HYP mice, another characteristic of humans with XLH (5).
FGF23, a novel member of the large fibroblast growth factor family, appears to be central to the pathogenesis of phosphate-wasting disorders. Intact, full-length FGF23 is thought to be required for mediation of the factor’s inhibitory effect on renal tubular phosphate reabsorption (6). In autosomal dominant hypophosphatemic rickets, a specific mutation disrupting an enzyme cleavage site prevents the enzymatic processing of FGF23, so that the intact form persists, engendering hypophosphatemia (6). It remains unclear how loss of function of PHEX results in elevated FGF23 levels. Elevation of FGF23 levels, which may occur due to gain-of-function mutations, as in autosomal dominant hypophosphatemic rickets, or paraneoplastic overproduction of the protein, as in tumor-induced osteomalacia, presumably result in increased FGF receptor (FGFR) signaling and subsequent hypophosphatemia with impaired 1,25-dihydroxyvitamin D [1,25(OH)2D] synthesis (7).
More recently, the novel membrane protein Klotho has been found to be critical for FGF23 signaling (8). Klotho is a type-I membrane protein related to β-glucosidases (9,10). The gene encoding Klotho contains five exons and is expressed in a limited range of tissues (10). Membrane-bound and secreted forms of Klotho have been identified. Circulating Klotho may arise from an alternately spliced gene product in which no transmembrane domain is transcribed or from cleavage of the extracellular domain of membrane-bound Klotho, resulting in shedding and secretion of this fragment into blood and cerebrospinal fluid (10). The Klotho-null mouse, discovered over a decade ago, arose from a randomly mutated mouse that was found to have many features in common with human aging, including infertility, emphysema, and hair loss (11). These mice survive approximately 6–12 wk and have increased serum phosphate and vitamin D levels (11).
Only recently, however, has a relationship between Klotho and FGF23 signaling been identified. FGF23 has a low receptor binding affinity and often requires cofactors such as heparin to activate FGF signaling in cultured cells. Klotho binds to FGFR1 (and other receptors), serving as a coreceptor, and thereby providing the specificity necessary for FGF23 to serve as a ligand that can transduce a signal. In sum, Klotho can convert FGFR1 into a receptor for FGF23, which otherwise would not be capable of efficient signal transduction through this receptor (8,12). The biochemical and skeletal phenotypes of the Klotho-null and HYP mouse are altered in discordant fashion: HYP mouse, with its elevated FGF23 levels, is hypophosphatemic with a hypomineralized skeleton, and the Klotho null mouse, with its impaired ability to transduce an FGF23 signal, is hyperphosphatemic and has a complex bone phenotype, characterized by limited growth and hypermineralization. We sought to discern whether effectively decreasing the FGF23 signal in HYP mouse by crossing with the Klotho-null mouse could rescue the phosphate-wasting and the hypomineralized skeletal phenotype seen in HYP.
Materials and Methods
Experimental animals
Mice were fed standard chow ad libitum and tap water. All mice were maintained in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals, and procedural protocol was approved by Yale’s Institutional Animal Care and Use Committee.
Generation of Klotho/HYP knockout mice
Klotho mice were generously provided by the Kuro-o laboratory (University of Texas Southwestern Medical Center, Dallas, TX). HYP mice were obtained from colonies maintained by our laboratory. Klotho heterozygous male and female mice were crossed to homozygous or hemizygous null HYP mice to create heterozygous Klotho and homozygous or hemizygous HYP mice (Klotho+/−/HYP). Then the female Klotho+/−/HYP mice were crossed to Klotho heterozygous males (the heterozygous Klotho/HYP hemizygous null mice were slow to breed), the product of which were one 16th Klotho knockout/HYP hemizygous null mice. The Klotho+/−/HYP female mice were then crossed to Klotho+/− HYP hemizygous null males (who, in this generation and later, were more successful breeders), with the production of one quarter Klotho−/−/HYP−/− or −/Y mice. For standardization purposes, only male mice were examined for these studies.
Genotyping
Genomic DNA tissue was extracted and purified from the tail of each mouse using standard protocols. Genotypes were determined by PCR. HYP mice were genotyped using the following primers: PHEX19 forward 5′-GCTTGGGCTAGTTTGCTATCT-3′ and PHEX 19 reverse 5′-TGAGTTGGTGCTATACACGGAG-3′ for PHEX mice. HYP mice were identified by the lack of a PCR product, which indicated that the HYP deletion was present. The wild-type (WT) PCR product was 197 bp.
For Klotho, the following primers were used: JCOM 5′-TGGAGATTGGAAGTGGACG-3′, JMUT 5′-CAAGGACCAGTTCATCATCG-3′, and JWLD 5′-TTAAGGACTCCTGCATCTGC-3′. The expected product size for WT Klotho was 458 bp and for mutant Klotho was 920 bp.
Both HYP and Klotho genotyping protocols used the same PCR program. After an initial denaturation for 2 min at 94 C, amplification cycles consisted of denaturation at 94 C for 30 sec, annealing at 56 C for 30 sec, and 1.5 min extension at 72 C for 30 cycles, followed by a final extension for 10 min at 72 C. For Klotho genotyping, Takara LA Taq and buffer were required.
Serum biochemical measurements
Blood was sampled from the retro-orbital sinus of all mice at 6 weeks of age. Urine was obtained from the bladder by needle puncture at kill. Serum and urinary phosphorus were measured using a commercially available Liqui-UV kit (Stanbio, Boerne, TX). Serum and urinary calcium and creatinine were assessed using Sigma kits employing a plate reader (Titertek Multiscan, Huntsville, AL). Serum FGF23 was measured using a sandwich type ELISA method that detects the intact molecule (Kainos Laboratories, Tokyo, Japan). 1,25(OH)2D was measured using a commercially available RIA kit (DiaSorin, Stillwater MN). The C-terminal telopeptide of type 1 collagen was measured using the RatLaps kit (Immunodiagnostic Systems Limited, Fountain Hills, AZ). Osteocalcin levels were measured using an in-house mouse-specific assay (13). PTH was measured using a two site ELISA that employs antimouse PTH antibodies and intact rat PTH standards (Alpco, Salem, NH). All statistical analyses used SAS software (SAS Institute, Inc., Cary, NC). Two-sided t tests were employed unless otherwise indicated.
Microcomputed tomography (μCT)
Trabecular and cortical compartments of femurs were imaged and quantified using conebeam x-ray computed microtomography (μCT40; Scanco Medical AG, Bassersdorf, Switzerland). Serial tomographic images were acquired at 55 kV and 145 μA, collecting 1000 projections per rotation at 300 msec integration time. Three-dimensional images were reconstructed using standard convolution back-projection algorithms with Shepp and Logan filtering and rendered within a 12.3-mm field of view at a discrete density of 578,704 voxels/mm3 (isometric 12-μm voxels). Segmentation of bone from marrow and soft tissue was performed in conjunction with a constrained Gaussian filter to reduce noise, applying density thresholds of 710 and 470 mg/cm3 for the cortical and trabecular compartments of the femur, respectively. Volumetric regions for trabecular analysis were selected within the endosteal borders of the distal femoral metaphysis to include the secondary spongiosa located 960 μm (∼6% of length) from the growth plate and extending 1 mm proximally. Trabecular morphometry was characterized by measuring the bone volume fraction, trabecular thickness, trabecular number, and trabecular spacing, measured directly and without imposing a structural model (e.g. rod or plate). Cortical morphometry was quantified and averaged for 50 serial cross-sections (600 μm) extending distally from the diaphyseal midpoint between proximal and distal growth plates. Cortical measurements included average cortical thickness, cross-sectional area of cortical bone, sub-periosteal and marrow space cross-sectional area, and intracortical porosity.
Histology and histomorphometry
The left tibias of 6-wk-old mice were dissected, cleaned, and fixed in 70% ethanol and then further dehydrated through graded ethanols, cleared in toluene, and embedded in methyl methacrylate. After polymerization, methyl methacrylate blocks were removed from the mold, cut to size, sanded, and polished on a Buehler Metaserve grinder to the appropriate level. Longitudinal sections of 5 μm thickness were cut using a Reichert-Jung Polycut microtome, mounted on gelatin-coated slides, and stained with toluidine blue. Measurements were performed in an area located approximately 150–200 μm distal to the growth plate at a final magnification of ×250 using the Osteomeasure program (Osteometrics, Atlanta, GA). Osteoblasts were identified as cuboidal cells lining the trabecular bone. Osteoclasts were identified as multinucleated cells on the trabecular bone surface. Standard histomorphometric parameters as endorsed by the American Society of Bone and Mineral Research Bone Histomorphometry Committee (14) were analyzed.
Results
Gross appearance
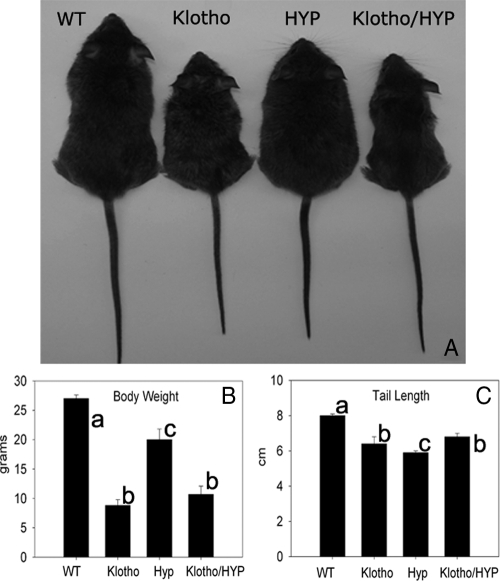
Confirming established literature, Klotho-null mice in these experiments displayed growth retardation and dramatically decreased survival (Fig. 1A). Klotho-null mice also weighed less than WT littermates (P < 0.001) and less than HYP mice of the same age (P = 0.002) (Fig. 1B). HYP mice had lower mean body weight than WT mice, although they weighed more than Klotho-null mice (27.0 ± 0.7 g in WT vs. 20.0 ± 1.8 g in HYP, P < 0.05). HYP mice had a characteristically kyphotic spine consistent with rickets/osteomalacia and shorter mean tail length than WT mice (5.9 ± 0.1 cm in HYP vs. 8.0 ± 0.1 cm in WT, P = 0.05) (Fig. 1C).
Figure 1.
A, Six-week-old WT, Klotho, HYP, and Klotho/HYP mice; B and C, bar graphs indicate average body weight (B) and tail length (C) for the four genotypes. n = 6 for all groups. Differing lowercase letters indicate statistically significant differences (P < 0.05) between the groups. Bars with the same letters are not significantly different. Precise statistical significance values are identified in the accompanying text.
Klotho/HYP double-knockout mice had reduced mean body weight and marked growth retardation of the Klotho-null mouse and retained the kyphosis of the HYP mouse. Mean tail length of the Klotho/HYP mice (6.8 ± 0.2 cm) was of intermediate length, longer than in HYP mice (P = 0.001) but with a reduced tail length compared with normal (P = 0.001) (Fig. 1C).
Life span
Calculation of actual life span of the Klotho/HYP-null mouse was not assessed because ethical guidelines required euthanasia as soon as major signs of morbidity were evident. This occurred between 6 and 8 wk in both the Klotho-null and Klotho/HYP-null mice. HYP mice and WT mice lived a normal life span.
Effect of combined HYP and Klotho deficiency on serum and urine biochemistry
Circulating phosphate and calcium
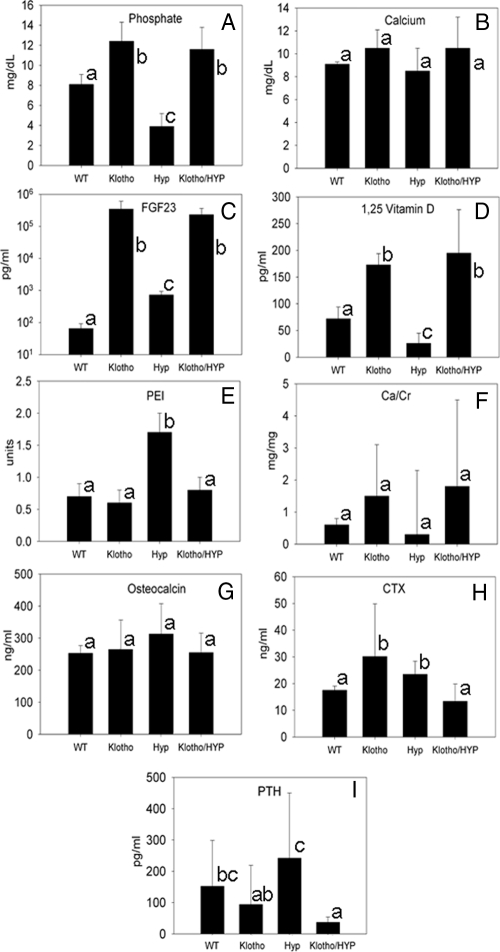
We confirmed the previously described hypophosphatemia in HYP mice used in these experiments as well as hyperphosphatemia in the Klotho-null mice (Fig. 2A) (1,11). Addition of Klotho-null status to the HYP mouse resulted in complete correction of the hypophosphatemia, such that phosphate levels were significantly greater in Klotho/HYP mice than in WT mice (Klotho/HYP, 11.8 ± 2.1 mg/dl; WT, 8.1 ± 1.0 mg/dl; P = 0.0002). Serum phosphate levels in Klotho/HYP mice were indistinguishable from Klotho-null mice (12.4 ± 2.0 mg/dl, P = 0.35) and significantly greater than in HYP mice (3.9 ± 1.3 mg/dl; P < 0.0001). Serum calcium levels between genotypes did not differ significantly in this study, although hypercalcemia has been previously described in Klotho-null mice (Fig. 2B) (11).
Figure 2.
Biochemical analysis of WT, Klotho, HYP, and Klotho/HYP mice. A, Serum phosphate (n = 8–15 for each genotype); B, serum calcium (n = 8–12 for each genotype); C, serum FGF23 (n = 6–7 for each genotype); D, serum 1,25(OH)2D (n = 6–10 for each genotype); E, urinary phosphate excretion index (PEI) (urine [P]/serum [P] × urine [creatinine]) (n = 5–7 for each genotype); F, urinary calcium excretion as ratio to creatinine (milligrams per milligram) (n = 7 for all genotypes); G, serum osteocalcin levels (n = 5–8 for each genotype); H, serum concentrations of CTX (n = 5–8 for each genotype); I, serum PTH (n = 12–14 for each genotype). Differing lowercase letters indicate statistically significant differences (P < 0.05) between the groups. Bars with the same letters are not significantly different.
FGF23
As previously reported, circulating FGF23 levels in 6-wk-old HYP and homozygous Klotho-null mice were elevated in comparison with WT mice of the same age (HYP, 724 ± 204 pg/ml; Klotho, 236,250 ± 115,089 pg/ml; WT, 65 ± 26 pg/ml; P < 0.05) (Fig, 2C). Circulating FGF23 in Klotho/HYP double-knockout mice was also elevated (231,400 ± 130,367 pg/ml) but at levels indistinguishable from Klotho mice but significantly increased compared with HYP mice (P < 0.05).
PTH
Mean serum PTH levels (Fig. 2I) for both Klotho and HYP were similar to previously reported values (Klotho, 94.5 ± 125.3 pg/ml; HYP, 259.6 ± 205.5 pg/ml; WT, 149.4 ± 148.5 pg/ml) (15,16). HYP and WT levels were significantly elevated in comparison with the Klotho/HYP animals (33.4 ± 9.6 pg/ml, P < 0.01). Also, mean PTH levels in HYP mice were significantly greater than those in Klotho (P = 0.01).
1,25(OH)2D
HYP mice had dramatically reduced levels of 1,25(OH)2D (26.3 ± 19.1 pg/ml in HYP vs. 72.5 ± 22.0 pg/ml in WT, P = 0.0002), confirming previous investigations (Fig. 2D) (17). Klotho/HYP-null mice had significantly increased circulating 1,25(OH)2D levels compared with WT (194.6 ± 81.3 pg/ml, P = 0.05), similar to that observed in Klotho mice (173.1 ± 20.8 pg/ml).
Urinary phosphate and calcium excretion
Urinary phosphate excretion was assessed by the phosphate excretion index (calculated as urine [P]/(serum [P] × urine [creatinine]) (1). Mean urinary phosphate excretion was similar among WT, Klotho-null, and Klotho/HYP mice but reduced in comparison with HYP mice (P < 0.05) (Fig. 2E). Urinary calcium excretion, assessed by the ratio of urine calcium concentration/urine creatinine concentration (milligrams per milligram) were slightly, but not significantly, greater in both Klotho-null and Klotho/HYP mice than in WT and HYP mice (Fig. 2F).
Serum biomarkers of bone turnover
Circulating concentration of osteocalcin, the major noncollagenous protein of bone matrix serves as an indicator of the rate of bone formation. Circulating osteocalcin levels were slightly elevated in HYP mice (313.2 ± 95.8 ng/ml) and Klotho mice (264.7 ± 160.9 ng/ml) but not significantly (Fig. 2G). Osteocalcin levels were restored in the Klotho/HYP mouse to near WT levels (255.7 ± 61.3 ng/ml in Klotho/HYP, 253.0 ± 24.6 ng/ml in WT). Serum levels of the carboxyl-terminal telopeptide of type 1 collagen (CTX), a biomarker of bone resorption activity, were comparable in Klotho/HYP and WT mice but significantly lower (P = 0.05) in these two groups than in Klotho or HYP mice (13.4 ± 6.5 ng/ml in Klotho/HYP, 27.6 ± 2.5 ng/ml in Klotho, 23. 6 ± 5.0 ng/ml in HYP, and 17.5 ± 1.6 ng/ml in WT, Fig. 2H).
Ex vivo skeletal analysis: μCT
Trabecular bone
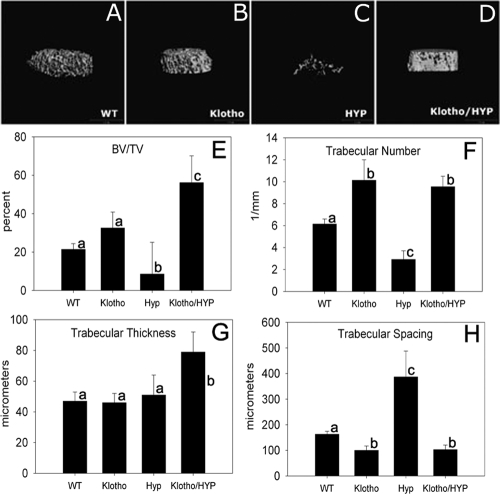
Trabecular bone was assessed by μCT analysis; representative images of distal femoral metaphyses from the four genetic mouse models studied are shown in Fig. 3. Quantification of trabecular bone volume fraction demonstrated substantial increases of this parameter in both Klotho-null and Klotho/HYP mice, and markedly so in Klotho/HYP mice. Fractional trabecular bone volume in Klotho mice (32.5 ± 10.3%) was 1.5-fold greater than that in WT mice (21.4 ± 3.4%). Superimposition of an additional homozygous deletion in PHEX by mating to HYP mice resulted in even greater bone volume fraction in the double-null mouse (56.2 ± 6.3%, or nearly 3-fold greater than that observed in WT mice) and resulted in a substantial increase over the low value in HYP (8.6 ± 7.7%, less than half the fractional trabecular bone volume of WT mice). The increased fractional trabecular bone volume observed in the Klotho mouse was significantly exceeded in the Klotho/HYP mouse (P < 0.004).
Figure 3.
A–D, Representative μCT generated images of trabecular bone from femur distal metaphyses of WT (A), Klotho (B), HYP (C) and Klotho/HYP (D) mice; E–H, bar graphs show the derived values from μCT analysis for bone volume/total volume (E), trabecular number (F), trabecular thickness (G), and trabecular spacing (H). n = 6 for all groups. Differing lowercase letters indicate statistically significant differences (P < 0.05) between the groups. Bars with the same letters are not significantly different.
Trabecular number in Klotho and Klotho/HYP mice was significantly increased compared with both WT and HYP mice (P = 0.001) (Fig. 3F). Likewise, trabecular spacing (Fig. 3H) was decreased in Klotho and Klotho/HYP (P < 0.001), all consistent with the increased bone volume and overall appearance of increased skeletal density in Klotho and Klotho/HYP mice, Finally, trabecular thickness in femurs (Fig. 3G) from Klotho/HYP mice was significantly greater than all groups (P < 0.01), including Klotho (which had trabecular thickness equivalent to WT). Thus, in aggregate, the defects seen as a consequence of PHEX loss of function, when occurring in the setting of Klotho loss of function, result in an exaggeration of the hyperdense phenotype seen in the Klotho mice.
Cortical bone
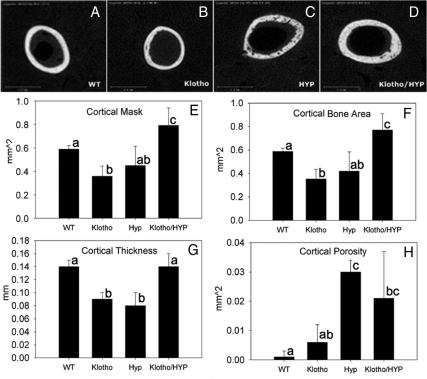
In addition, μCT analysis of femoral cortex was performed in these four strains of mice (Fig. 4). Cross-sectional area of the femoral cortex (inclusive of intracortical porosity) or cortical mask in the Klotho/HYP mouse was significantly increased in comparison with all other groups (P < 0.05) (Fig. 4E). Cortical thickness (Fig. 4G), however, was reduced in both HYP and Klotho mice (0.08 ± 0.02 and 0.09 ± 0.01 mm, respectively, vs. 0.14 ± 0.01 mm in the WT) (P = 0.001). With the double-mutant Klotho/HYP mice, however, cortical thickness was restored to values identical to that of WT mice (0.14 ± 0.02 mm). Cortical porosity (Fig. 4H), easily identifiable in the images, was increased in the HYP and Klotho/HYP mice compared with WT mice (P < 0.05). The Klotho mouse had cortical porosity similar to the WT mouse (0.006 ± 0.006 and 0.001 ± 0.002 mm2, respectively) and less than that of the HYP (0.030 ± 0.004 mm2, P < 0.001) and Klotho/HYP mice, although levels were not significantly reduced (0.021 ± 0.016 mm2). The cortical bone area (cross-sectional area, exclusive of porosity) compared among the groups in a similar manner as did the cortical cross-sectional area (Fig. 4F).
Figure 4.
A–D, Representative cross-sectional μCT generated images of the midshaft cortical bone from femurs of WT (A), Klotho (B), HYP (C), and Klotho/HYP (D) mice; E–H, bar graphs show the derived values for the μCT analysis for cortical mask (cross-sectional area inclusive of porosity) (E), cortical bone area (exclusive of porosity) (F), cortical thickness (G), and cortical porosity (H). Different letters indicate P < 0.05 between the two groups. n = 6 for all groups. Differing lowercase letters indicate statistically significant differences (P < 0.05) between the groups. Bars with the same letters are not significantly different.
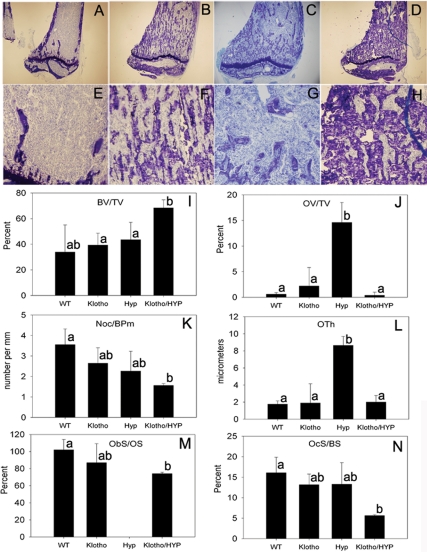
Skeletal histology and histomorphometry
Representative histological sections from tibias of normal, Klotho, HYP, and Klotho/HYP mice are shown in Fig. 5, A–H. Compared with WT tibias, HYP mice tibias demonstrated morphological abnormalities including widened epiphysis, disruption of the growth plates, and large fissures occupied by unmineralized osteoid. Although total bone volume in HYP tibias was comparable to that of WT tibias as determined by standard histomorphometric analysis (Fig. 5I), bone volume in HYP tibia was comprised of an extensive osteoid component, significantly greater than that observed in all other strains (P < 0.01) (Fig. 5J). Thus, the volume of mineralized tibial trabecular bone in HYP was consistent with that demonstrated in HYP femur by μCT analysis (Fig. 3E). In contrast, Klotho mice tibias appeared to have slightly greater fractional bone volume compared with WT mice; although this finding was confirmed by both μCT (Fig. 3E) and histomorphometric analysis (Fig. 5I), these differences did not reach statistical significance. Finally, as also observed in the μCT analysis of femoral trabecular bone, bone volume observed in Klotho/HYP was dramatically increased in comparison with tibias from either HYP or Klotho-null mice (P < 0.02). Compared with HYP mice, Klotho/HYP animals demonstrated both an increase in mineralized bone volume and a reduction in the unmineralized osteoid, as assessed by either osteoid volume or osteoid thickness (Fig. 5, J and L). Thus, with the additional deletion of the Klotho gene in the HYP mouse, with documented correction of ambient mineral concentrations, the osteoid matrix becomes appropriately mineralized, unlike in HYP mice. Ultimately, the double knockout resulted in a very dense mineralization phenotype, with far greater fractional bone volume than that observed in either WT or Klotho-null mice. The deletion of Klotho also appeared to correct the growth plate defect seen in HYP (Fig. 5, C and D).
Figure 5.
Histomorphometric analysis. A–H, Undecalcified tibial sections stained with toluidine blue; A–D, ×4 magnification of WT, Klotho, HYP, and Klotho/HYP; E–H, ×10 magnifications of WT, Klotho, HYP, and Klotho/HYP bone. I–N, Bar graphs showing the total bone volume (BV/TV; I), osteoid volume/bone volume (OV/TV; J), number of osteoclasts/bone perimeter (Noc/BPm; K), osteoid thickness (OTh; L), osteoblast surface/osteoid surface (ObS/OS; M), and osteoclast surface/bone surface (OcS/BS; N) determined by histomorphometric analysis of sections from three to four tibias from each group. Differing lowercase letters indicate statistically significant differences (P < 0.05) between the groups. Bars with the same letters are not significantly different.
Osteoblastic surface (expressed per total osteoid surface) was slightly lower in Klotho/HYP mice than in WT (Fig. 5M) (P = 0.05); osteoclastic surface (expressed per bone surface, Fig. 5N) and the number of osteoclasts (expressed per bone perimeter, Fig. 5K) were significantly reduced in the Klotho/ HYP mouse compared with WT (P < 0.05). These cellular parameters suggest that, at least by 6 wk of age, increased bone formation is not ongoing in the Klotho/HYP mouse and that the extremely dense phenotype may be maintained at this age by decreased bone resorption.
Discussion
FGF23 is a hormone inextricably linked to the regulation of phosphate and vitamin D metabolism. FGF23 is predominantly produced in bone by osteocytes, is secreted into the circulation, and acts at a distant site, the kidney (18). Thus, FGF23 acts in endocrine fashion, in contrast to other members of the FGF family that function in paracrine or autocrine fashion (19). Furthermore, this endocrine action of circulating FGF23, together with the widespread distribution of FGFR, suggests that a renal-specific mediator of FGF23 activity is necessary for this unique system. Thus, the concept of a coreceptor has been raised as a facilitator of FGF23 action upon the kidney, which would serve to potentiate an interaction between FGF23 and FGFR. To this end, it has recently been demonstrated that the Klotho gene product, expressed in kidney, binds to FGF23 and that FGF23 requires Klotho for its activity. That is, Klotho converts FGFR from low-affinity to high-affinity FGF23 receptors (8,12), thereby serving as the necessary cofactor for the renal specificity of FGF23’s endocrine action.
HYP mice are a murine homolog of human XLH; the genetic disruption in both HYP mice and humans with XLH are loss-of-function mutations in the PHEX gene. HYP mice have reduced body weight, a shortened tail, rickets, kyphosis, and histological features of osteomalacia, all in concert with decreased serum phosphate levels (1). The defect in renal phosphate reabsorption is due to a decrease in abundance of the Npt2 class of transport proteins on the apical membranes of proximal renal tubular cells (20). In addition, there is disordered regulation of renal mitochondrial enzymes that regulate the synthesis and catabolism of 1,25(OH)2D. However, the mechanism by which the loss of PHEX results in these clinical and biochemical features is not understood. The observation that serum FGF23 levels are increased in both XLH and the HYP mouse has implicated FGF23 as an important mediator of hypophosphatemic syndromes; however, FGF23 is not thought to be a physiological substrate for PHEX (21). Moreover, it has recently been demonstrated that the HYP phenotype is dependent on FGF23, and the HYP/FGF23 double-null mouse has phenotypic, biochemical, and mineralization features resembling the FGF23-null mouse, including hyperphosphatemia and increased circulating 1,25(OH)2D levels, the converse of findings in the HYP mouse (22).
The Klotho gene plays a critical role in regulating aging, perhaps, in part, via its effects on calcium and phosphate metabolism (23). As noted above, Klotho functions as a coreceptor for FGF23 in FGF23 signaling, which down-regulates the production of 1,25(OH)2D and of renal tubular phosphate reabsorption (8,12). The Klotho-null mouse is short-lived and displays atherosclerosis, skin atrophy, and pulmonary emphysema with a complex bone phenotype, and the Klotho overexpression mouse has an increased life span (11,24,25).
Mineral homeostasis in HYP, Klotho, and Klotho/HYP mice
Previous studies have documented increases in circulating levels of calcium, phosphate, and 1,25(OH)2D in Klotho mutant mice (11). This biochemical phenotype is similar to the FGF23-null mouse phenotype, yet serum FGF23 is extremely elevated in the Klotho-null mouse. Thus, these data provide evidence that Klotho is downstream of FGF23 in the pathway regulating these biochemical aberrations.
Due to the similarities between Klotho-null and the FGF23-null mice, and the contrasting natures of Klotho and HYP mice, we performed the Klotho/HYP cross to determine the relationship between Klotho, FGF23, and PHEX. The biochemical profile of serum and urine from Klotho/HYP mice was similar to what we observed (and previously reported) in Klotho mice and indicate a rescue of the HYP biochemical phenotype (or, in fact, an overcorrection of that observed in HYP mice). Therefore, regarding biochemical mineral homeostasis, our results are consistent with Klotho serving as a necessary mediator of the characteristic hypophosphatemia and abnormal vitamin D metabolism in HYP mice.
FGF23, together with Klotho as a critical mediator, inhibits expression of renal 1α-hydroxylase (Cyp27b1), the rate-limiting enzyme in the production of the metabolically active form of vitamin D, 1,25(OH)2D (26,27). In the absence of Klotho, Cyp27b1 expression is increased as are circulating levels of 1,25(OH)2D. It is possible that the premature aging phenotype of Klotho and the Klotho/HYP mice detailed here is in large part due to the combination of hypercalcemia and hyperphosphatemia, generating a calcium × phosphate ion product that would be favorable for soft-tissue mineral precipitation. Increased circulating calcium occurs as a consequence of increased circulating 1,25(OH)2D levels directly generated by loss of Klotho. Increased phosphate levels occur as a consequence of enhanced expression of the Npt2 class of phosphate transporters, resulting in an increased abundance of NaPi2a and NaPi2c transporters on the apical membranes of proximal renal tubular epithelium, and via enhanced intestinal absorption, mediated by increased circulating 1,25(OH)2D, both direct effects of loss of Klotho. Supporting this consideration, the reduced life span observed in FGF23-null mice occurs in the setting of hypercalcemia, hyperphosphatemia, and resultant renal calcification. It would therefore follow that the deletion of Klotho from the HYP mouse results in the severe disruption of calcium and phosphate metabolism described here, with subsequent premature death probably attributable to this effect. In contrast, it has become of great interest that overexpression of Klotho in a murine model does extend the life span (25); however, no studies have been reported regarding the effect of Klotho overexpression in mice on alterations in biochemical phenotype or changes in FGF23 activity. We have previously described an individual with increased circulating Klotho levels resulting from a chromosomal translocation with a breakpoint in the regulatory region of the Klotho gene who came to clinical attention because of phosphate-wasting rickets (analogous to that seen in XLH and HYP mice) and marked parathyroid hyperplasia (28).
The low PTH levels observed in Klotho-null mice and in Klotho/HYP mice raise the possibility of a direct regulatory effect of Klotho on the parathyroid glands, but the hypercalcemia in concert with the significantly greater circulating 1,25(OH)2D observed in these models are considered principal mediators of PTH suppression. Decreased PTH levels in the Klotho/HYP mouse may also play a role in decreased bone resorption suggested by the histological features and the low serum CTX values. Indeed, changes in the markers of bone turnover in the Klotho/HYP mouse were consistent with the histological findings; circulating osteocalcin, comparable to that in WT animals, and the associated decreased osteoblastic surface argue against a role for increased bone anabolic activity in the dense structure of bone in Klotho/HYP. On the other hand, serum CTX was lower in Klotho/HYP mice than in the other models and, consistent with the finding of reduced osteoclasts, support the consideration that decreased bone resorption may play a role in the high bone mass phenotype observed in this model, or at least in the maintenance of this phenotype at 6 wk of age, when these animals were studied.
The biochemical studies described in this report support the notion that, with respect to mineral metabolism, Klotho is epistatic to PHEX. Furthermore, Klotho is a critical regulator of phosphate homeostasis and, directly or indirectly, of bone mineralization. Deletion of Klotho eliminated phosphate wasting in HYP mice, and concomitantly, the mineralization defect was rescued in these severely osteomalacic animals. With respect to mineral homeostasis, the Klotho-null phenotype appears to be dominant over the HYP-related abnormalities, yet the skeletal phenotype of Klotho/HYP mice is not a simple phenocopy of the Klotho-null mice.
Skeletal phenotype in HYP, Klotho, and Klotho/HYP mice
Indeed, the skeletal phenotype in Klotho/HYP mice is particularly striking, demonstrating restricted linear growth but intense trabecular mineralization with increased bone volume, trabecular thickness, trabecular number, and decreased trabecular spacing. Klotho/HYP mice do not maintain the excess of undermineralized osteoid characteristic of HYP mice, and osteomalacia is not apparent histologically. These observations in aggregate suggest that loss of Klotho does not result in a substantial overproduction of osteoid but that this feature is PHEX dependent, typical of the skeletal lesion in HYP mice. However, restoration of supraphysiological levels of phosphate in HYP mice, achieved by crossing with Klotho mice, results in an apparent overproduction of matrix, which subsequently appropriately mineralizes. This phenomenon occurs despite the persistence of elevated circulating levels of FGF23 observed in both HYP mice and in Klotho/HYP mice and is a more extreme phenotype than that observed in the FGF23−/−/HYP mouse (22). The disruption of FGF23 in the HYP background partially restores the mineralization defect seen in HYP but does not completely correct the defect (22).
Such an interpretation implies that PHEX loss of function may result in phosphate-independent regulation of osteoblastic activity. Furthermore, it is possible that FGF23 may act directly on the skeleton, playing a role in the osteomalacic phenotype; if this is the case, these experiments would indicate that such a function does not require Klotho. However, serum FGF23 in Klotho-deficient mice and Klotho/HYP mice are similar (Fig. 2C), and FGF23−/−/HYP mice have a less severe mineralization defect than Klotho/HYP mice, which would argue against a direct role for Klotho in bone mineralization. On the other hand, PHEX may be required to prevent overproduction of bone matrix. If so, increased bone in Klotho/HYP mice may be due to the combination of overproduction of bone matrix and high circulating phosphate levels, which are in turn, regulated by Klotho-mediated FGF23 effects on the kidney. These considerations warrant further investigation.
Acknowledgments
We acknowledge the expert technical assistance of Carol Nelson-Williams, Mary Clough, and Sherril Nieman.
Footnotes
This work was supported by Howard Hughes Medical Institute, National Institutes of Health (NIH) Center for Research Translation Award P50-AR054086 (T.O.C.), NIH AR38460 (C.M.G.), and the Yale Core Center for Musculoskeletal Disorders (NIH P30-AR46032).
Disclosure Summary: T.O.C. consults for Kyowa Hakko Kirin. C.A.B., J.Z., A.S., B.E., N.T., D.J.A., C.M.G., and R.P.L. have nothing to declare.
First Published Online December 1, 2009
Abbreviations: μCT, Microcomputed tomography; CTX, carboxyl-terminal telopeptide of type 1 collagen; FGF23, fibroblast growth factor 23; FGFR, FGF receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; WT, wild-type; XLH, X-linked hypophosphatemia.
References
- Eicher EM, Southard JL, Scriver CR, Glorieux FH 1976 Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci USA 73:4667–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom TM, Francis F, Lorenz B, Böddrich A, Econs MJ, Lehrach H, Meitinger T 1997 Pex gene deletions in Gy and Hyp mice provide mouse models for X-linked hypophosphatemia. Hum Mol Genet 6:165–171 [DOI] [PubMed] [Google Scholar]
- Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS 1997 Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest 99:1200–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The HYP Consortium 1995 A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11:130–136 [DOI] [PubMed] [Google Scholar]
- Strewler GJ 2001 FGF23, hypophosphatemia, and rickets: has phosphatonin been found? Proc Natl Acad Sci USA 98:5945–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai XY, Miao D, Goltzman D, Karaplis AC 2003 The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 278:9843–9849 [DOI] [PubMed] [Google Scholar]
- Kumar R 2000 Tumor-induced osteomalacia and the regulation of phosphate homeostasis. Bone 27:333–338 [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T 2006 Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774 [Google Scholar]
- Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y 1998 Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424:6–10 [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y 1998 Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242:626–630 [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI 1997 Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51 [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M 2006 Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundberg CM, Clough ME, Carpenter TO 1992 Development and validation of a radioimmunoassay for mouse osteocalcin: paradoxical response in the Hyp mouse. Endocrinology 130:1909–1915 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujimori T, Nabeshima Y 2002 Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1α-hydroxylase gene. Endocrinology 143:683–689 [DOI] [PubMed] [Google Scholar]
- Posillico JT, Lobaugh B, Muhlbaier LH, Drezner MK 1985 Abnormal parathyroid function in the X-linked hypophosphatemic mouse. Calcif Tissue Int 37:418–422 [DOI] [PubMed] [Google Scholar]
- Meyer Jr RA, Meyer MH, Gray RW, Bruns ME 1987 Evidence that low plasma 1,25-dihydroxyvitamin D causes intestinal malabsorption of calcium and phosphate in juvenile X-linked hypophosphatemic mice. J Bone Miner Res 2:67–82 [DOI] [PubMed] [Google Scholar]
- Strom TM, Jüppner H 2008 PHEX, FGF23, DMP1 and beyond. Curr Opin Nephrol Hypertens 17:357–362 [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamashita T 2007 FGF23 is a hormone-regulating phosphate metabolism: unique biological characteristics of FGF23. Bone 40:1190–1195 [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K 2003 Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol 285:F1271–F1278 [DOI] [PubMed] [Google Scholar]
- Benet-Pagès A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM 2004 FGF23 is processed by proprotein convertases but not by PHEX. Bone 35:455–462 [DOI] [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD 2006 Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291:E38–E49 [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Lanske B 2007 The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol 194:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M 1999 Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest 104:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M 2005 Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perwad F, Zhang MY, Tenenhouse HS, Portale AA 2007 Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293:F1577–F1583 [DOI] [PubMed] [Google Scholar]
- Imai M, Ishikawa K, Matsukawa N, Kida I, Ohta J, Ikushima M, Chihara Y, Rui X, Rakugi H, Ogihara T 2004 Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1α-hydroxylase gene expression. Endocrine 25:229–234 [DOI] [PubMed] [Google Scholar]
- Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP 2008 A translocation causing increased α-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA 105:3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]