Abstract
Today nanotechnology is finding growing applications in industry, biology, and medicine. The clear benefits of using nanosized products in various biological and medical applications are often challenged by concerns about the lack of adequate data regarding their toxicity. One area of interest involves the interactions between nanoparticles and the components of the immune system. Nanoparticles can be engineered to either avoid immune system recognition or specifically inhibit or enhance the immune responses. We review herein reported observations on nanoparticle-mediated immunostimulation and immunosuppression, focusing on possible theories regarding how manipulation of particle physicochemical properties can influence their interaction with immune cells to attain desirable immunomodulation and avoid undesirable immunotoxicity.
More mechanistic studies are required to fully understand particle properties which determine their effects on the immune system.
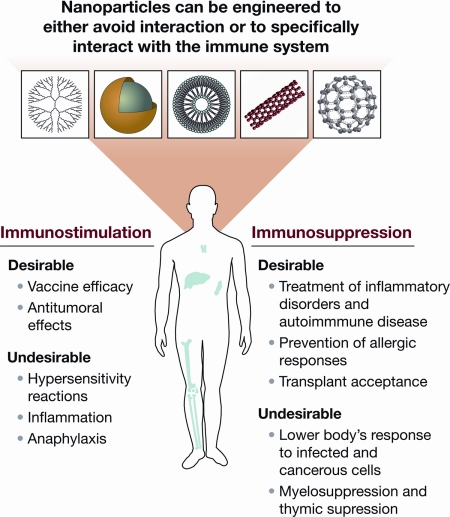
The immune system safeguards the host from infections and malignancies. The immune function is fine-tuned to meet the body’s changing requirements for responding to the internal and external environment. The immune system can be perturbed at different levels, resulting in either its suppression or its overstimulation. Therefore, all new chemical and biological entities require adequate investigations into their interactions with the immune system before their use in industry, biology, and medicine (1,2). Nanoscaled particles can be either engineered or found naturally in the environment (3). Engineered nanoparticles can specifically be designed to either target or avoid interactions with the immune system. An interaction between a nanoparticle and the immune system is considered desirable when it may lead to various beneficial medical applications, such as vaccines or therapeutics for inflammatory and autoimmune disorders (Fig. 1).
Figure 1.
Nanoparticle interactions with the immune system. Nanoparticles’ effects on the immune cells may benefit treatment of disorders mediated by unwanted immune responses and enhance immune response to weak antigens. On the other hand, undesirable immunostimulation or immunosuppression by nanoparticles may result in safety concerns and should be minimized.
Nanoparticle-based delivery systems offer the following potential advantages: 1) site-specific delivery of drugs, peptides, and genes; 2) improved in vitro and in vivo stability; and 3) reduced side effect profile. However, because nanoparticles are often first picked up by the phagocytic cells of the immune system (e.g. macrophages), there may be undesirable interactions between nanoparticles and the immune system, such as immunostimulation or immunosuppression, which may promote inflammatory or autoimmune disorders, or increase the host’s susceptibility to infections and cancer. As mentioned earlier, the main function of the immune system is to protect the host from foreign substances; however, inadvertent recognition of nanoparticles as foreign by the immune cells may result in a multilevel immune response against the nanoparticles and eventually lead to toxicity in the host and/or lack of therapeutic efficacy. For example, granuloma formation was observed in the lungs, skin, and pleural lining of the animals exposed to carbon nanotubes (CNTs) (4,5).
However, when nanoparticles are recognized as self or there is an absence of immune recognition, this represents a major area of interest in the field of drug delivery. It is now well accepted that properties such as nanoparticle size, surface charge, hydrophobicity/hydrophilicity, and the steric effects of particle coating can dictate nanoparticle compatibility with the immune system (6,7,8). For example, nanoparticles can be designed by attaching to poly(ethylene glycol) (PEG) or other types of polymers to provide a hydrophilic environment, thereby shielding them from immune recognition (9). Although these polymers shield the nanoparticles from recognition by the immune system, there are data suggesting the formation of PEG-specific antibodies after administration of PEG-coated liposomes (10,11). Consequently, these antibodies resulted in accelerated clearance of the PEG-liposomes from blood and contributed to the change in pharmacokinetic profile of subsequent injected doses of PEG-liposome (12,13,14). Therefore, it should be noted that generation of particle-specific antibodies may affect the efficacy and the safety of nanoparticle-based therapeutics. Nanoparticles can also be designed to elicit an immune response by either direct immunostimulation of antigen-presenting cells or delivering antigen to specific cellular compartments (15).
The basics of the immune recognition of nanoparticles are reviewed elsewhere (6,7,8) and are omitted from this discussion. The focus of this review was on the most recent advances in understanding nanoparticle interactions with various components of the immune system. This review encompasses the current knowledge about nanoparticle-mediated immunosuppression and immunostimulation, and gives an overview of development strategies of nanoparticles in different preclinical and clinical phases.
Immunosuppression
Immunosuppression may be either inadvertent or desirable. On the one hand, immunosuppression may lower the body’s defense against infection and cancerous cells, and on the other hand, it may enhance the therapeutic benefits of treatments for allergies and autoimmune diseases and prevent rejection of transplanted organs. Although traditional toxicology studies focused on the undesirable consequences of immunosuppression (16), such studies are sparse for nanoparticles. To date, most studies focused on the inflammatory properties of nanoparticles. One of the few studies on immunosuppression has demonstrated that inhalation of CNTs suppresses B cell function and that the TGF-β produced by alveolar macrophages is a key element in the mechanism of the observed immunosuppression (17). Other studies have shown that nanoparticles can be used to deliver immunosuppressive drugs and prevent immunosuppressive properties of small-molecule drugs (18). For example, poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles were administered iv to deliver glucocorticoids to the inflamed joints in the mouse model of arthritis. Complete remission of inflammatory response was achieved with PLGA nanoparticles, and the improved efficacy was due to the targeted and controlled release of steroids from PLGA nanoparticles (19). Another example is a Food and Drug Administration-approved chemotherapeutic, Abraxane, a nanoparticle colloidal suspension in which paclitaxel is bound to human serum albumin nanoparticles. In patients with breast cancer, paclitaxel nanoparticle formulation resulted in a lower incidence of grade 4 neutropenia (a form of myelosuppression that leads to the decreased number of neutrophils) than the first generation of paclitaxel formulation, Taxol, which contained the excipient Cremophor EL (18,20). Yet another example consists of liposomes loaded with clodronate, which were used in a swine model to eliminate macrophages and thus protect animals from lung injury caused by endotoxin (21).
Immunosuppression is often mediated by toxicity of substances to T cells. For example, some immunosuppressive agents (such as tetrachlorodibenzo-p-dioxin, cadmium, corticosteroids, and radiation) have been shown to act against T cells, impairing their development and function (22,23). Whereas cadmium’s suppressive effects on the thymus are well known (23), no studies have been reported regarding the effects of cadmium-containing nanoparticles (e.g. quantum dots) on the thymus. Clearly more studies are necessary in this field.
Induction of immune tolerance by nanoparticles can be considered a form of desirable immunosuppression. For example, a water-soluble fullerene derivative (polyhydroxy C60) has been shown to inhibit type I hypersensitivity reactions to allergens, both in vitro in human primary mast cells and basophils and in vivo in a mouse model of anaphylaxis (24). Similarly, allergen-loaded PLGA, chitosan, poly(lactic acid), poly(methyl vinyl ether-co-maleic anhydride) nanoparticles, and dendrosomes have been reported as effective suppressors of type I and type II allergies to environmental and food allergens in animal models and in vitro (25,26,27,28,29). Recently it has also been shown that synthetic peptide dendrimers may block experimental allergic encephalomyelitis (30).
Whereas the body of knowledge on nanoparticles is still limited, to date, there are no studies linking the incidence of autoimmune diseases to exposure to nanoparticles. However, a few studies have proposed the use of nanoparticles to treat autoimmune diseases (31). For example, collagen type II entrapped in PLGA nanoparticles can suppress inflammation in a mouse model of rheumatoid arthritis. Likewise, delivery of IL-10-encoding DNA by nanoparticles was successful in the suppression of autoimmune diabetes in mice (31,32). Treatment of experimental autoimmune uveoretinitis with betamethasone-poly(lactic acid) nanoparticles, and treatment of experimental autoimmune thyroiditis with cytotoxic T lymphocyte-associated antigen-4Ig-silica-nanoparticles in canine models was shown to ameliorate the course of both diseases (33,34).
Immunostimulation
Nanoparticles are evaluated for their immunostimulatory potential based on their ability to stimulate innate or adaptive immune responses. In the following section, we will describe reported studies on the effects of nanoparticles on the complement system, cytokine secretion, and the induction of antibody response (immunogenicity).
Properties of nanoparticles, which have important interactions with the complement system, have been previously reviewed in detail (7). Briefly, activation of the complement cascade can be harmful if particles inadvertently, or by design, enter the systemic circulation because this may lead to hypersensitivity reactions and anaphylaxis (35,36,37,38). Recent data also suggested that activation of the complement system at tumor sites stimulates tumor-associated immune cells and promotes their conversion into a tumor-supportive phenotype, thereby stimulating cancer progression (39,40). This type of response may impact the therapeutic efficacy of nanoparticle formulations intended for cancer diagnosis or therapy. On the other hand, if particles are intended for sc or intradermal administration, activation of the complement by the particles can benefit vaccine efficacy. Some of these findings have received increased attention among the rapidly growing nanomedicine community (40,41). Whereas many questions still remain, it is important to elucidate how complement activation relates to nanoparticle toxicity and the development of nanotechnology-based formulations for medical applications.
Nanoparticle immunogenicity is drawing interest because nanoparticles have been shown to improve antigenicity of conjugated weak antigens and thus serve as adjuvants and because some nanoparticles have been shown to be antigenic themselves. The former property has been shown to depend on particle size and surface charge (42,43,44) and can significantly contribute to the development of improved vaccine formulations. Particle size has been reported as a major factor in determining whether antigens loaded into nanoparticles induce type I (interferon-γ) or type II (IL-4) cytokines, thereby contributing to the type of immune response (45). A leading hypothesis on why nanotechnology-driven formulations are effective in vaccine development is that nonsoluble nanoparticles provide controlled, slow release of antigens, creating a depot at the site of injection and providing protection in the destabilizing in vivo environment (46,47).
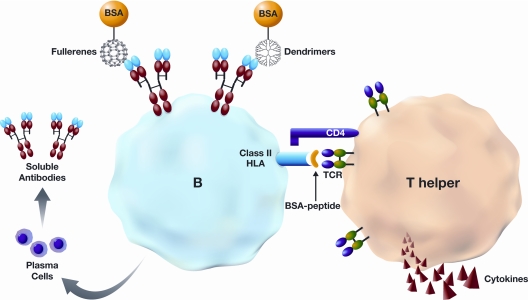
Antigenicity of the nanoparticles per se is less well understood. Two studies demonstrated the generation of particle-specific antibodies when C60 fullerene derivatives conjugated to a protein carrier, BSA, were used for immunization (48,49). However, other studies using different fullerene derivatives, gold colloids, and cationic polyamidoamine and polypropyleneimine dendrimers have reported no particle-specific immune response, even in the presence of strong adjuvants (50,51,52,53,54,55). However, polyamidoamine dendrimers conjugated to BSA showed increased antigenic properties and as result dendrimer-specific antibody was observed in vivo (56). Common features of all of these studies in which nanoparticle-specific antibodies were generated is nanoparticle conjugation a protein carrier BSA. Therefore, these limited data may suggest that some water-soluble nanoparticles may behave as haptens, i.e. they are not antigenic until they bind to protein carrier (Fig. 2) possibly as a result of their small size.
Figure 2.
Hypothetical model of nanoparticle antigenicity. Nanoparticles (dendrimers and fullerenes) may not be antigenic unless they are bound to a protein carrier such as BSA. BSA-nanoparticle conjugate is endocytosed by B cells, and the protein is digested inside the cell. B cells then may present BSA peptides on their human leukocyte antigen-class II molecules to T-helper cells. Both cells become activated, resulting in production of cytokines by T lymphocytes and antibodies directed to nanoparticles by plasma B cells. The protein carrier is indispensable for T cell activation. Antibodies to fullerenes and dendrimers are produced by different B cells. For simplicity of the figure, only one B cell is shown. TCR, T cell receptor.
Another area of nanoparticle-mediated immunostimulation is the elicitation of allergic reactions. A few studies linked exposure to nanoparticles to allergic reactions in test animals and humans (57,58). For example, both single-wall and multiwall CNTs enhanced the allergenicity of egg albumin when administered via intranasal or sc routes in mice. The mechanism of this enhanced allergenicity is thought to be due to the CNT-mediated induction of the acute inflammatory response (57). Occupational exposure during the manufacture of nanomaterials has been linked to allergic reactions. For example, toxic epidermal necrolysis-like dermatitis was observed in a chemist exposed to high levels of intermediate or final products of dendrimers while performing dendrimer synthesis (58). However, it is unclear whether the dermatitis was caused by nanoparticles or reactive species used in their synthesis. It should be noted that these studies dealt with raw nanomaterials, which were not intended for biological or medicinal use. More studies involving well-characterized nanoparticles, relevant animal models, and routes of nanoparticle administration are required to understand whether nanoparticles can cause allergy in humans.
In some cases, reformulation of an approved drug in nanoformulation may circumvent allergic reactions associated with the previously approved formulations. Abraxane is an example of such a reformulation. In this case, reformulated paclitaxel-bound albumin nanoparticles did not exhibit any allergic reaction, whereas the first-generation formulation of paclitaxel in the nonionic surfactant Cremophor EL caused severe hypersensitivity reaction, often requiring premedication with a histamine blocker and steroids (59).
Many immunostimulatory reactions initiated by nanoparticles are mediated by the production of inflammatory cytokines. Several studies have reported cytokine induction by different types of nanomaterials (gold colloids, dendrimers, polymers, lipid nanoparticles, etc.) (44,45,60,61,62,63). Nanoparticle size has been suggested as a leading parameter that determines a nanoparticle’s potential to induce cytokine responses (44,45,60). For example, the length of CNTs was shown to correlate with sc inflammation induced by CNT in vivo (64). However, a few studies have shown that cytokines were induced not by nanoparticles per se but by surfactants or bacterial endotoxins present in the formulation (61,63). Therefore, it is important to quantify the presence of chemical (formation of byproducts) and biological (endotoxin) contaminants before analysis of inflammatory properties of nanoparticles.
Examples of Known Parameters for Interaction with the Immune System
In this part of the review, examples are provided for various nanoparticle platforms currently being developed for drug applications. A brief discussion is provided for each nanoplatform’s key components and their interaction with the immune system.
Polymeric nanoparticles
Polymeric nanoparticles are defined as nanoscale drug delivery platforms assembled by, for example, biodegradable polymers, dendrimers, and micelles. Polymeric biodegradable nanoparticles have been explored as vaccine formulations, in which antigen is encapsulated in polymers such as PLGA or polylactide. The use of PLGA or polylactide in vaccine development is beneficial because of their biodegradability and biocompatibility (65). Similar to other drug delivery systems, biodegradable polymeric-based delivery systems such as PLGA may offer the following advantages: 1) they provide sustained release; 2) they protect encapsulated antigen from harsh environment and enzymatic degradation; 3) they provide targeted delivery with attachment of ligands; and 4) they may have adjuvant effects. One of the design strategies to increase the functionality of the nanoparticles is to change their physicochemical properties, such as particle size. For example, it has been reported that smaller nanoparticles (∼25 nm) travel through the lymphatic more readily than the larger particles (∼100 nm) and accumulate in lymph node resident dendritic cells (43). In addition to size, other physicochemical properties, such as charge, can be manipulated for desired therapeutic benefit.
Nanoliposomes
Nanoliposomes are defined as liposomes in nanoscale assemblies that are composed of one or more bilayers of amphipathic lipid molecules enclosing one or more aqueous compartments (66). From immunological prospective, two types of nanoliposomes have been reported: 1) liposomes designed to elicit immune response to an antigen that is encapsulated in the liposome; and 2) liposomes with a polymer coat to prevent immune recognition. Several liposomal formulations have already been approved for treatment of cancer, infections, and meningitis (67). Many other liposome-based formulations are being developed as therapeutic vaccines against malaria, HIV, hepatitis A, influenza, prostate cancer, and colorectal cancer (68). For example, Stimuvax, a cancer vaccine that targets cancer cells expressing mucin-1 protein, is undergoing phase 3 trials in more than 30 countries including the United States in patients with unresectable stage III non-small-cell lung cancer (http://www.oncothyreon.com/clinical/stimuvax.html). It has been hypothesized that the inherent ability of antigen-presenting cells to sequester nanoscale liposomes more efficiently than larger-sized liposome counterparts may be the key to the enhanced immune response observed with nanoliposome formulation. Additionally, the surface charge on nanoliposomes can be manipulated to improve antigen delivery; for example, cationic liposomes are much more potent in generating immune response than anionic or neutral liposomes (69). Targeting moieties such as antibodies can also be attached to liposomes to improve antigen delivery to dendritic cells (70).
Nanoemulsions
Nanoemulsions are emulsions with droplet size in the nanometer scale. An emulsion is a thermodynamically unstable system, unless stabilized by the presence of emulsifying agent, consisting of at least two immiscible liquid phases, one of which is dispersed as globules (the dispersed phase) in the other liquid phase (the continued phase) (71).
Emulsions are traditionally used in vaccines. It has been reported that the nanoscale-sized emulsions are able to permeate the nasal mucosa and carry the antigen to the antigen-presenting cells more efficiently than larger-sized emulsions. Nanoscale emulsion-based intranasal vaccines have been investigated for hepatitis B, HIV, influenza, and anthrax (72). One example of such an application is a nanoemulsion-based seasonal influenza vaccine NB-1008, currently undergoing phase 1 clinical trials in the United States (http://www.nanobio.com/nanobio_phase1_flu.html). Another application of the nanoemulsion platform is a hepatitis vaccine herein referred to as nano-HBsAg. This vaccine is composed of a 400-nm oil-in-water emulsion containing surface antigen (HBsAg) of recombinant hepatitis B virus. In vivo studies showed a robust and systemic IgG, mucosal IgA, and strong antigen-specific immune response after an intranasal administration of the nano-HBsAg formulation (72). Although the serum IgG titers induced by this formulation were comparable with those of an alum-based traditional vaccine, the nanoformulation was more efficient and required fewer administrations. Other advantages of the nanoformulation are the ease of administration, via the nasal route, and a longer shelf life at elevated temperatures, which allows this formulation to be stored at room temperature, overcoming common limitations in developing countries, such as access to a refrigerator (72).
Solid lipid nanoparticles (SLNs)
SLNs are defined as colloidal particles of a lipid matrix solid at physiological temperature (73). Similar to the aforementioned drug delivery systems, SLNs have been used to encapsulate either hydrophilic or hydrophobic molecular entities and generally exhibit particle size of up to 400 nm. When compared with free (not encapsulated) antisense oligodeoxyribonucleotide G3139, SLN-encapsulated G3139 demonstrated greater immunostimulatory property and antitumor activity. It is hypothesized that the increased activity of nanoparticles is due to their small particle size, leading to the more efficient uptake of SLNs by tumor-resident macrophages and dendritic cells (74). Differences in the pharmacokinetic profile of the free vs. encapsulated G3139 may also be responsible for the enhanced immunostimulatory effect observed.
Targeted delivery systems
Targeted delivery systems include any delivery platform with a targeting moiety such as antibodies, peptides, and glycoprotein to improve the efficiency and specificity of drug delivery. A targeted nanoparticle-based gene delivery concept was translated from bench to bedside for the first time with Rexin-G and Reximmune-C formulations. Rexin-G and Reximmune-C are targeted multilamellar vesicle-based nanoparticles approximately 100 nm in size. The former encapsulates a construct encoding a dominant-negative mutant of human cyclin G1 protein; the latter carries a cytokine, granulocyte-macrophage colony-stimulating factor (75,76,77). After receiving orphan drug status from the Food and Drug Administration in 2003, the Rexin-G formulation entered phase 1/2 clinical oncology trials in 2007 (www.epeiusbiotech.com). The clinical trials of Reximmune-C formulation started in February 2009 in the Philippines and included a limited number of patients who benefited from previous Rexin-G therapy. These two drug therapies exemplify the two-tier complementary approach aimed at both tumor eradication and cancer vaccination. The first step involves administration of Rexin-G to target and kill the tumor cell in a programmed fashion, whereas the second step employs Reximmune-C to induce a localized cancer autoimmunization (75,76,77).
Conclusion and Lessons Learned
In summary, existing studies have demonstrated that nanotechnology offers many advantages, such as improved stability, favorable biodistribution profiles, slower drug release kinetics, lower immunotoxicity, and targeting to specific cell populations. Lessons learned from previous studies include the importance of detection and prevention of potential particle contamination with such things as bacterial endotoxins and/or toxic synthesis by-products, and the importance of understanding how route of administration and particle biodistribution in the body may result in either desirable and undesirable immunomodulation (e.g. complement activation on iv administration is not desirable, whereas on sc administration, it is beneficial for vaccinations).
Nanotechnology platforms are being investigated as vaccine carriers, adjuvants, and drug delivery systems to target inflammatory and inflammation-associated disorders. Some formulations are already in clinical trials, whereas many others are in various phases of preclinical development. Although in recent years, our understanding of nanoparticle interaction with components of the immune system has improved, many questions still require more thorough investigation and deeper understanding. Further mechanistic studies investigating particle immunomodulatory effects (immunostimulatory and immunosuppression) are required to improve our understanding of the physicochemical parameters of nanoparticles that define their effects on the immune system.
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This paper reflects the current thinking and experience of the authors (B.S.Z. and N.S.). However, this is not a policy document and should not be used in lieu of regulations, published Food and Drug Administration guidance documents, or direct discussions with the agency. We are grateful to Dr. Scott McNeil for critique and helpful discussion during manuscript preparation.
Footnotes
This work was supported in whole or part by federal funds from the National Cancer Institute, National Institutes of Health, under Contract HHSN261200800001E (to M.A.D.) and the Ministerio de Ciencia y Tecnología under Contract Consolider Ingenio 2010 (CSD2006-12, to A.G.-F.) and the Xunta de Galicia under Contract PGIDIT06TMT31402PR (to A.G.-F.).
Disclosure Summary: B.S.Z, Á.G.-F., N.S., and M.A.D. have nothing to disclose.
First Published Online December 16, 2009
Abbreviations: CNT, Carbon nanotube ; PEG, poly(ethylene glycol); PLGA, poly(D,L-lactide-co-glycolide); SLN, solid lipid nanoparticle.
References
- 1997 S6: preclinical safety evaluation of biotechnology-derived pharmaceuticalsin. Fed Regist 62:61515 [PubMed] [Google Scholar]
- 2006 S8: immunotoxicity studies for human pharmaceuticals. Fed Regist 71:19193–19194 [PubMed] [Google Scholar]
- Stern ST, McNeil SE 2008 Nanotechnology safety concerns revisited. Toxicol Sci 101:4–21 [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K 2008 Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3:423–428 [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Monteiro-Riviere NA 2006 Multi-walled carbon nanotube exposure alters protein expression in human keratinocytes. Nanomedicine 2:158–168 [DOI] [PubMed] [Google Scholar]
- Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE 2009 Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev 61:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE 2008 Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm 5:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, McNeil SE 2007 Immunological properties of engineered nanomaterials. Nat Nanotechnol 2:469–478 [DOI] [PubMed] [Google Scholar]
- Moghimi SM 2002 Chemical camouflage of nanospheres with a poorly reactive surface: towards development of stealth and target-specific nanocarriers. Biochim Biophys Acta 1590:131–139 [DOI] [PubMed] [Google Scholar]
- Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H 2007 PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release 122:349–355 [DOI] [PubMed] [Google Scholar]
- Wang X, Ishida T, Kiwada H 2007 Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release 119:236–244 [DOI] [PubMed] [Google Scholar]
- Ishida T, Atobe K, Wang X, Kiwada H 2006 Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release 115:251–258 [DOI] [PubMed] [Google Scholar]
- Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H 2006 Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release 112:15–25 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kiwada H 2008 Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharm 354:56–62 [DOI] [PubMed] [Google Scholar]
- Kalkanidis M, Pietersz GA, Xiang SD, Mottram PL, Crimeen-Irwin B, Ardipradja K, Plebanski M 2006 Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods 40:20–29 [DOI] [PubMed] [Google Scholar]
- Descotes J 2004 Immunotoxicology of drugs and chemicals: an experimental and clinical approach. Amsterdam: Elsevier [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, McDonald JD 2009 Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol 4:451–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe TE, Socinski MA, Walko CM, O'Neil BH, Collichio FA, Ivanova A, Mu H, Hawkins MJ, Goldberg RM, Lindley C, Claire Dees E 2007 Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors. Cancer Chemother Pharmacol 60:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki M, Ishihara T, Izumo N, Takatsu M, Mizushima Y 2005 Treatment of experimental arthritis with poly(D, L-lactic/glycolic acid) nanoparticles encapsulating betamethasone sodium phosphate. Ann Rheum Dis 64:1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JL, Savin MA, Edelman G, Pippen JE, Robert NJ, Geister BV, Kirby RL, Clawson A, O'Shaughnessy JA 2007 Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer 7:850–856 [DOI] [PubMed] [Google Scholar]
- Gaca JG, Palestrant D, Lukes DJ, Olausson M, Parker W, Davis Jr RD 2003 Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res 112:19–25 [DOI] [PubMed] [Google Scholar]
- Wiedmeier SE, Samlowski WE, Rasmussen CJ, Huang K, Daynes RA 1988 Effect of ionizing radiation on thymic epithelial cell function. I. Radiation-spared thymic epithelial grafts expedite the recovery of T cell function in lethally irradiated and fetal liver reconstituted mice. J Immunol 140:21–29 [PubMed] [Google Scholar]
- Lafuente A, González-Carracedo A, Romero A, Esquifino AI 2003 Effect of cadmium on lymphocyte subsets distribution in thymus and spleen. J Physiol Biochem 59:43–48 [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, Schwartz LB, Lenk R, Kepley CL 2007 Fullerene nanomaterials inhibit the allergic response. J Immunol 179:665–672 [DOI] [PubMed] [Google Scholar]
- Balenga NA, Zahedifard F, Weiss R, Sarbolouki MN, Thalhamer J, Rafati S 2006 Protective efficiency of dendrosomes as novel nano-sized adjuvants for DNA vaccination against birch pollen allergy. J Biotechnol 124:602–614 [DOI] [PubMed] [Google Scholar]
- Gómez S, Gamazo C, San Roman B, Ferrer M, Sanz ML, Espuelas S, Irache JM 2008 Allergen immunotherapy with nanoparticles containing lipopolysaccharide from Brucella ovis. Eur J Pharm Biopharm 70:711–717 [DOI] [PubMed] [Google Scholar]
- Roy K, Mao HQ, Huang SK, Leong KW 1999 Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med 5:387–391 [DOI] [PubMed] [Google Scholar]
- Schöll I, Weissenböck A, Förster-Waldl E, Untersmayr E, Walter F, Willheim M, Boltz-Nitulescu G, Scheiner O, Gabor F, Jensen-Jarolim E 2004 Allergen-loaded biodegradable poly(D,L-lactic-co-glycolic) acid nanoparticles down-regulate an ongoing Th2 response in the BALB/c mouse model. Clin Exp Allergy 34:315–321 [DOI] [PubMed] [Google Scholar]
- Gómez S, Gamazo C, Roman BS, Ferrer M, Sanz ML, Irache JM 2007 Gantrez AN nanoparticles as an adjuvant for oral immunotherapy with allergens. Vaccine 25:5263–5271 [DOI] [PubMed] [Google Scholar]
- Wegmann KW, Wagner CR, Whitham RH, Hinrichs DJ 2008 Synthetic peptide dendrimers block the development and expression of experimental allergic encephalomyelitis. J Immunol 181:3301– 3309 [DOI] [PubMed] [Google Scholar]
- Basarkar A, Singh J 2009 Poly(lactide-co-glycolide)-polymethacrylate nanoparticles for intramuscular delivery of plasmid encoding interleukin-10 to prevent autoimmune diabetes in mice. Pharm Res 26:72–81 [DOI] [PubMed] [Google Scholar]
- Kim WU, Lee WK, Ryoo JW, Kim SH, Kim J, Youn J, Min SY, Bae EY, Hwang SY, Park SH, Cho CS, Park JS, Kim HY 2002 Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum 46:1109–1120 [DOI] [PubMed] [Google Scholar]
- Choi EW, Shin IS, Lee CW, Youn HY 2008 The effect of gene therapy using CTLA4Ig/silica-nanoparticles on canine experimental autoimmune thyroiditis. J Gene Med 10:795–804 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kohno H, Ishihara T, Higaki M, Saito S, Matsushima M, Mizushima Y, Kitahara K 2006 Treatment of experimental autoimmune uveoretinitis with poly(lactic acid) nanoparticles encapsulating betamethasone phosphate. Exp Eye Res 82:657–663 [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR, Muggia FM 2003 Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol 14:1430–1437 [DOI] [PubMed] [Google Scholar]
- Szebeni J 2005 Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology 216:106–121 [DOI] [PubMed] [Google Scholar]
- Szebeni J, Alving CR, Rosivall L, Bünger R, Baranyi L, Bedöcs P, Tóth M, Barenholz Y 2007 Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res 17:107–117 [DOI] [PubMed] [Google Scholar]
- Szebeni J, Baranyi L, Savay S, Milosevits J, Bunger R, Laverman P, Metselaar JM, Storm G, Chanan-Khan A, Liebes L, Muggia FM, Cohen R, Barenholz Y, Alving CR 2002 Role of complement activation in hypersensitivity reactions to Doxil and hypnic PEG liposomes: experimental and clinical studies. J Liposome Res 12:165–172 [DOI] [PubMed] [Google Scholar]
- Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD 2008 Modulation of the antitumor immune response by complement. Nat Immunol 9:1225–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, Lambris JD 2009 Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol 30:286–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi SM, Andresen TL 2009 Complement-mediated tumour growth: implications for cancer nanotechnology and nanomedicines. Mol Immunol 46:1571–1572 [DOI] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF 2008 Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol 38:1404–1413 [DOI] [PubMed] [Google Scholar]
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA 2007 Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 25:1159–1164 [DOI] [PubMed] [Google Scholar]
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M 2004 Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol 173:3148–3154 [DOI] [PubMed] [Google Scholar]
- Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M 2007 Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm 4:73–84 [DOI] [PubMed] [Google Scholar]
- O'Hagan DT, De Gregorio E 2009 The path to a successful vaccine adjuvant—‘the long and winding road.’ Drug Discov Today 14:541–551 [DOI] [PubMed] [Google Scholar]
- Panyam J, Labhasetwar V 2003 Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 55:329–347 [DOI] [PubMed] [Google Scholar]
- Braden BC, Goldbaum FA, Chen BX, Kirschner AN, Wilson SR, Erlanger BF 2000 X-ray crystal structure of an anti-Buckminsterfullerene antibody fab fragment: biomolecular recognition of C(60). Proc Natl Acad Sci USA 97:12193–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BX, Wilson SR, Das M, Coughlin DJ, Erlanger BF 1998 Antigenicity of fullerenes: antibodies specific for fullerenes and their characteristics. Proc Natl Acad Sci USA 95:10809–10813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agashe HB, Dutta T, Garg M, Jain NK 2006 Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J Pharm Pharmacol 58:1491–1498 [DOI] [PubMed] [Google Scholar]
- Andreev SM, Babakhin AA, Petrukhina AO, Romanova VS, Parnes ZN, Petrov RV 2000 Immunogenic and allergenic properties of fullerene conjugates with amino acids and proteins. Dokl Biochem 370:4–7 [PubMed] [Google Scholar]
- Kreuter J 1995 Nanoparticles as adjuvants for vaccines. Pharm Biotechnol 6:463–472 [DOI] [PubMed] [Google Scholar]
- Masalova OV, Shepelev AV, Atanadze SN, Parnes ZN, Romanova VS, Vol'pina OM, Semiletov IuA, Kushch AA 1999 [Immunostimulating effect of water-soluble fullerene derivatives—perspective adjuvants for a new generation of vaccine]. Dokl Akad Nauk 369:411–413 [PubMed] [Google Scholar]
- Roberts JC, Bhalgat MK, Zera RT 1996 Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J Biomed Mater Res 30:53–65 [DOI] [PubMed] [Google Scholar]
- Tomii A, Masugi F 1991 Production of anti-platelet-activating factor antibodies by the use of colloidal gold as carrier. Jpn J Med Sci Biol 44:75–80 [DOI] [PubMed] [Google Scholar]
- Lee SC, Parthasarathy R, Botwin K, Kunneman D, Rowold E, Lange G, Klover J, Abegg A, Zobel J, Beck T, Miller T, Hood W, Monahan J, McKearn JP, Jansson R, Voliva CF 2004 Biochemical and immunological properties of cytokines conjugated to dendritic polymers. Biomed Microdevices 6:191–202 [DOI] [PubMed] [Google Scholar]
- Nygaard UC, Hansen JS, Samuelsen M, Alberg T, Marioara CD, Løvik M 2009 Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol Sci 109:113–123 [DOI] [PubMed] [Google Scholar]
- Toyama T, Matsuda H, Ishida I, Tani M, Kitaba S, Sano S, Katayama I 2008 A case of toxic epidermal necrolysis-like dermatitis evolving from contact dermatitis of the hands associated with exposure to dendrimers. Contact Dermatitis 59:122–123 [DOI] [PubMed] [Google Scholar]
- Hawkins MJ, Soon-Shiong P, Desai N 2008 Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev 60:876–885 [DOI] [PubMed] [Google Scholar]
- Schöler N, Hahn H, Müller RH, Liesenfeld O 2002 Effect of lipid matrix and size of solid lipid nanoparticles (SLN) on the viability and cytokine production of macrophages. Int J Pharm 231:167–176 [DOI] [PubMed] [Google Scholar]
- Schöler N, Olbrich C, Tabatt K, Müller RH, Hahn H, Liesenfeld O 2001 Surfactant, but not the size of solid lipid nanoparticles (SLN) influences viability and cytokine production of macrophages. Int J Pharm 221:57–67 [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P 2005 Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol 289:L698–L708 [DOI] [PubMed] [Google Scholar]
- Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S 2006 The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett 6:1682–1686 [DOI] [PubMed] [Google Scholar]
- Sato Y, Yokoyama A, Shibata K, Akimoto Y, Ogino S, Nodasaka Y, Kohgo T, Tamura K, Akasaka T, Uo M, Motomiya K, Jeyadevan B, Ishiguro M, Hatakeyama R, Watari F, Tohji K 2005 Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol Biosyst 1:176–182 [DOI] [PubMed] [Google Scholar]
- Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM 2008 Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release 125:193–209 [DOI] [PubMed] [Google Scholar]
- 2002 Guidance for industry: liposome drug products. Rockville, MD: U.S. Food and Drug Administration [Google Scholar]
- Zolnik BS, Sadrieh N 2009 Regulatory perspective on the importance of ADME assessment of nanoscale material containing drugs. Adv Drug Deliv Rev 61:422–427 [DOI] [PubMed] [Google Scholar]
- Peek LJ, Middaugh CR, Berkland C 2008 Nanotechnology in vaccine delivery. Adv Drug Deliv Rev 60:915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tanaka K, Mayumi T 1999 Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release 61:233–240 [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM 2001 Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255–258 [DOI] [PubMed] [Google Scholar]
- Matrin A 1993 Physical pharmacy: physical chemical principles in the pharmaceutical sciences. 4th ed. Baltimore, MD: Williams, Wilkins [Google Scholar]
- Makidon PE, Bielinska AU, Nigavekar SS, Janczak KW, Knowlton J, Scott AJ, Mank N, Cao Z, Rathinavelu S, Beer MR, Wilkinson JE, Blanco LP, Landers JJ, Baker Jr JR 2008 Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS One 3:e2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi MD, Müller RH 2009 Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm 71:161–172 [DOI] [PubMed] [Google Scholar]
- Pan X, Chen L, Liu S, Yang X, Gao JX, Lee RJ 2009 Antitumor activity of G3139 lipid nanoparticles (LNPs). Mol Pharm 6:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Chan MT, Geraldino N, Lopez FF, Cornelio GH, Lorenzo 3rd CC, Levy JP, Reed RA, Liu L, Hall FL 2007 Le morte du tumour: histological features of tumor destruction in chemo-resistant cancers following intravenous infusions of pathotropic nanoparticles bearing therapeutic genes. Int J Oncol 30:1297–1307 [PubMed] [Google Scholar]
- Gordon EM, Hall FL 2009 The ‘timely’ development of Rexin-G: first targeted injectable gene vector. Int J Oncol 35:229–238 (Review) [PubMed] [Google Scholar]
- Gordon EM, Lopez FF, Cornelio GH, Lorenzo 3rd CC, Levy JP, Reed RA, Liu L, Bruckner HW, Hall FL 2006 Pathotropic nanoparticles for cancer gene therapy Rexin-G IV: three-year clinical experience. Int J Oncol 29:1053–1064 [PubMed] [Google Scholar]