Abstract
Obesity leads to inflammation of white adipose tissue involving enhanced secretion of cytokines and acute-phase proteins in response in part to the accumulation of excess lipids in adipocytes. Haptoglobin is an acute-phase reactant secreted by white adipose tissue and induced by inflammatory cytokines such as TNFα. In this study, we investigated the mechanisms regulating haptoglobin expression in adipocytes. Peroxisome proliferator-activated receptor (PPAR)-γ agonists such as thiazolidinediones (TZDs) as well as non-TZD ligands can repress in vitro and in vivo haptoglobin expression in adipocytes and also prevent its induction by TNFα. This action requires direct involvement of PPARγ in regulating haptoglobin gene transcription because mutation of critical amino acids within helix 7 of the ligand-binding domain of PPARγ prevents repression of the haptoglobin gene by the synthetic ligands. Chromatin immunoprecipitation analysis shows active binding of PPARγ to a distal region of the haptoglobin promoter, which contains putative PPARγ binding sites. Additionally, PPARγ induces transcription of a luciferase reporter gene when driven by the distal promoter region of the haptoglobin gene, and TZD treatment significantly reduces the extent of this induction. Furthermore, the mutated PPARγ is incapable of enhancing luciferase activity in these in vitro reporter gene assays. In contrast to other adipokines repressed by TZDs such as resistin and chemerin, repression of haptoglobin does not require either CCAAT/enhancer-binding protein C/EBPα or the corepressors C-terminal binding protein 1 or 2. These data are consistent with a model in which synthetic PPARγ ligands selectively activate PPARγ bound to the haptoglobin gene promoter to arrest haptoglobin gene transcription.
The processes by which the avandia class of insulin sensitizers inhibits the production of haptoglobin, a known contributor to obesity-associated disorders, is identified.
Obesity has now reached pandemic proportions and is a major contributor to several diseases including type 2 diabetes, cardiovascular disease, and inflammation. The increase in fat mass is principally due to a change in lifestyle and eating habits that disrupt energy balance and metabolism. The stressed fat tissue responds by reprogramming its normal functions. This includes changes in the level and nature of the secreted adipokines such as adiponectin and leptin, which regulate overall energy balance by signaling to other tissues, most notably brain, skeletal muscle, and liver. Furthermore, enlarged adipose tissue suffers from but also participates in both systemic and local inflammation by releasing proinflammatory cytokines (TNFα, IL-6) and acute-phase reactants including haptoglobin. Haptoglobin is a tetrachain α2β2-glycoprotein secreted into the plasma (1) and participates in many biological processes including immune regulation (1), angiogenesis (2), and arterial reconstruction (3). As a major positive acute-phase reactant, plasma levels of haptoglobin are increased during inflammation, infection, trauma, or malignancy (4). Haptoglobin has been related to the development of arterial hypertension and to the incidence of myocardial infarction and strokes (5,6). Although the liver is the major source of haptoglobin, studies have highlighted new sites including lung, ovary, testis, arteries, placenta, and white (WAT) and brown adipose tissue (BAT) (7). WAT haptoglobin gene expression is dramatically increased in genetically or experimentally induced obese mice (8). Serum haptoglobin is a marker of adiposity in humans, and adipose tissue likely contributes to the enhanced level of production (9).
Haptoglobin has been shown to be secreted into the culture medium of 3T3-L1 adipocytes using proteomic approaches (8,10). Both transgenic studies and studies in 3T3-L1 adipocytes indicate that TNFα and IL-6 are key regulators in the stimulation of haptoglobin expression. (8,11). In the 3T3-L1 adipocyte model, peroxisome proliferator-activated receptor (PPAR)-γ agonists repress expression of several inflammation-related adipokines (TNFα and leptin), including haptoglobin (11).
Adipocyte function is orchestrated by multiple factors but mainly PPARγ and CCAAT/enhancer-binding protein (C/EBP)-α that regulate expression of hundreds of proteins involved in various functions of the mature fat cell, from lipid metabolism and storage to secretion of adipokines including adiponectin (12,13). Thiazolidinediones (TZDs), known PPARγ ligands, have been shown to down-regulate haptoglobin expression in cultured adipocytes (11). Because higher levels of circulating haptoglobin is an indicator of human obesity and higher inflammation is linked to life-threatening diseases, understanding the mechanism by which haptoglobin expression can be repressed is of significant importance. We recently demonstrated that PPARγ, C/EBPα, and the corepressors carboxy terminal binding protein1/2 are implicated in the down-regulation of adipokine expression including resistin, angiotensinogen, and chemerin, by TZDs (14). Because haptoglobin expression is repressed by TZDs in vitro, we investigated the role of PPARγ, C/EBPα, and CtBP1/2 in this process.
Materials and Methods
Specific reagents were purchased from various vendors as listed: dexamethasone, 3-isobutyl-1-methylxanthine, and insulin from Sigma (St. Louis, MO); leupeptin, aprotin, and puromycin from American Bioanalytical (Natick, MA); DMEM from Mediatech, Inc. (Herndon, VA); fetal bovine serum (FBS) from Gemini Bio-Products (Calabasas, CA); calf serum from Invitrogen (Carlsbad, CA); and troglitazone from Biomol International (Plymouth Meeting, PA).
Cell culture
The cell lines including Swiss fibroblasts and 3T3-L1 preadipocytes were grown in DMEM containing 10%FBS (Swiss fibroblasts) or 10% calf serum (preadipocytes) until confluent and were then maintained in the same medium for an additional 2 d. Differentiation was induced at 2 d after confluence (d 0) by adding fresh DMEM containing 10% FBS, 0.5 mm 3-isobutyl-1-methylxanthine, 1 μm dexamethasone, and 1.67 μm insulin with or without 5 μm troglitazone as indicated. Troglitazone was added for the last 2 d (d 6–8) of 3T3-L1 adipocyte differentiation, and samples were processed (either RNA or protein) on d 8. In experiments investigating effect of Trog on adipocytes gene expression, the adipocytes were treated with the TZD for 2 d between d 8 and 10. Swiss fibroblasts were exposed to troglitazone for the entire period of differentiation. RNA was then harvested from each of the cell lines at d 10 for quantitative PCR (Q-PCR) analysis.
Plasmids and viruses
Expression vectors corresponding to PPARγ and its mutant form were generated as previously described (15). WT-PPARγ pBabe-Puro (WT-PPARγ), EF-PPARγ pBabe-Puro (EF-PPARγ), or pBabe empty vector-Puro (C) were stably introduced into Swiss fibroblasts and 3T3-L1 preadipocytes as follows. Plates (10 cm) of human embryonic kidney 293T cells were transiently transfected with 10 μg of the pBabe vectors and the viral packaging vectors pVPack-VSVG vector and pVPack-GAG-POL vector using TransIT-Express transfection reagent (Mirus Bio Corp., Madison, WI). At 48 h after transfection, virus-containing medium was collected and passed through a 0.45-μm-pore-size syringe filter. Filter-sterilized Polybrene (hexadimethrine bromide; 12 μg/ml) was added to the virus-loaded medium. This medium was then applied to proliferating (40% confluent) 3T3-L1 preadipocytes. At 48 h after infection, cells were treated with trypsin and replated in a medium supplemented with puromycin as the selection antibiotic.
Small interfering (si) RNA transient transfection
Predesigned siRNA against mouse C/EBPα and enhanced green fluorescent protein (EGFP; siEGFP) used as negative control were purchased from Integrated DNA Technology (Coralville, IA). Transfection of siRNA was achieved by using the DeliverX Plus delivery kit (Panomics, Fremont, CA) for 3T3-L1 adipocytes according to the manufacturer’s protocol.
Oil Red O staining
Cells were seeded in 35-mm dishes, and at the specified stage of differentiation they were rinsed with PBS and fixed with formalin in PBS for 15 min. After two washes in PBS, the cells were stained for at least 1 h in a freshly diluted Oil Red O solution (six parts Oil Red O stock solution and four parts H2O; Oil Red O Stock solution is 0.5% Oil Red O in isopropyl alcohol). The stain was the removed, and cells were washed twice with water and then photographed.
Western blot analysis
Monoclonal anti-PPARγ antibody and C/EBPα were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and mouse polyclonal antiadiponectin antibody from Affinity BioReagents (Golden, CO). Polyclonal antiadipocyte P2 (aP2) serum was kindly provided by Dr. David Bernlohr (University of Minnesota, Minneapolis, MN), whereas antiperilipin antibody was kindly provided by Dr. Andrew Greenberg (Tufts University, Boston, MA). Equal amounts of protein extracted from the total cell layer were fractionated on 8% or 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (PerkinElmer Life Sciences, Boston, MA). After transfer, the membranes were blocked with 5% nonfat dry milk in PBS and 0.1% Tween 20 and probed with the antibodies corresponding to the various target proteins indicated in each figure. Horseradish peroxidase-conjugated secondary antibodies (Sigma) and an enhanced chemiluminescence substrate kit (PerkinElmer Life Sciences) were used for detection of specific proteins.
Gene expression analysis
RNA was isolated from cultured cells using TRIzol reagent (Invitrogen) and from frozen fat tissue using TRIzol/ RNeasy tissue kit (QIAGEN, Valencia, CA) both according to the manufacturer’s instructions. cDNAs from cultured cells were made from equivalent amounts of total RNA by using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. cDNA from frozen fat tissue was synthesized from 3 μg isolated RNA using SuperScript II and oligodT (Invitrogen). Analysis of gene expression was done using Maxima Sybr Green Q-PCR master mix (Fermentas Life Sciences, Glen Burnie, MD) in the PRISM 7300 sequence detector (Applied Biosystems) for an initial denaturation at 95 C for 10 min followed by 40 PCR cycles, each cycle consisting of 95 C for 15 sec, 60 C for 20 sec, and 72 C for 30 sec, and SYBR Green fluorescence emissions were monitored after each cycle. For each gene, mRNA expression was calculated relative to TATA binding protein for murine samples. Amplification of specific transcripts was confirmed by melting curve profiles (cooling the sample to 68 C and heating slowly to 95 C with measurement of fluorescence) at the end of each PCR. The specificity of the PCR was further verified by subjecting the amplification products to agarose gel electrophoresis. Primer sequences used for the Q-PCR are previously reported (14).
Mouse studies
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center at Dallas. All in vivo experiments were performed in littermate controlled male FVB mice. Mice were housed in groups of two to five in filter-top cages. The colony was maintained in a pathogen-free Assessment and Accreditation of Laboratory Animal Care-accredited facility at the University of Texas Southwestern Medical Center at Dallas under controlled environment settings (22–25 C, 40–50% humidity). Mice were maintained on 12-h light, 12-h dark cycles with ad libitum access to water and standard chow diet (5058; LabDiet) as indicated. The high-fat diet-fed cohort was maintained on this diet for 12 wk. The PPARγ agonist 2-(2-(4-phenoxy-2-propylphenoxy)ethyl)indole-5-acetic acid (COOH) was a kind gift from Merck (Whitehouse Station, NJ) (16). The COOH and vehicle were gavaged daily at 12 noon for 14 days at 10 mg/kg body weight at wk 10 of age. Mice were killed within 6 h after the last gavage, and adipose tissues were collected.
Plasmid constructs and luciferase reporter gene assays
The mouse haptoglobin promoter constructs −3874/−2848, −2848/+1 were generated by PCR using C57BL/6NCrl mouse genomic DNA and the following oligonucleotides: −3874 forward, 5′-AAGGTACCAGACTTGTTCCATGG-3′; −2848 reverse, 5′-ACAGGAACACTAGTCAGAAGAAAGCTAGC-3′; −2848 forward, 5′-GCTAGCACATCCTGAGAGACTT-3′; and +1 reverse, 5′-GAGAGGCAAGAGAGGTCCACGATGAGATCT-3′. The PCR-amplified fragments were cloned into KpnI/NheI sites and into NheI/BglII sites, respectively, of the luciferase reporter plasmid pGL3 promoter. For transfection assays, the Swiss fibroblasts (control expressing a pBabe-puro empty vector) or cells expressing a WT-PPARγ or EF-PPARγ were seeded in 24-well plates in triplicate for 24 h, at which time 500 ng of the haptoglobin-promoter plasmids plus 20 ng of Renilla luciferase plasmid were transfected into each well using Fugene 6 (DNA/Fugene 6 ratio, 1:6). Eight hours later, when appropriate, the cells were treated for 48 h with 5 μm troglitazone and were then washed twice with PBS and lysed with 70 μl of passive lysis buffer. Luciferase/Renilla assays were performed using the dual-luciferase reporter assay System kit (Promega, Madison, WI) and a Luminoskan Ascent luminometer (Thermo Labsystems, Franklin, MA). The average ratio (from three wells) of luciferase activity (relative light units) to Renilla activity was calculated. The same experiment was repeated at least three times. The final value with sd was calculated based on all repeats.
Chromatin immunoprecipitation (ChIP) assay
Adipocytes derived from 3T3-L1 preadipocytes treated for the last 2 d with either dimethylsulfoxide (DMSO) or troglitazone were fixed by addition of 37% formaldehyde to a final concentration of 1% formaldehyde and incubation at room temperature for 10 min. Cross-linking was stopped by addition of glycine to a final concentration of 0.125 m. Cells were then scraped and samples were prepared with EZ-Magna ChIP G kit (Millipore, Bedford, MA) according to the manufacturer’s protocol. The chromatin fractions were incubated in each case with 2 μg of one of the following antibodies: anti-PPARγ (Santa Cruz), anti-C/EBPα (Santa Cruz), anti-CtBP (Santa Cruz), antiacetylated histone H3 (provided by Millipore kit), and IgG mouse and rabbit (Millipore) at 4 C overnight with magnetic protein G beads. After extensive washing and final elution, the product was treated for 4 h at 65 C to reverse cross-linking. Input DNA and immunoprecipitated DNA were purified using kit column and analyzed by Q-PCR using Maxima Sybr Green Q-PCR master mix (Fermentas Life Sciences) with different primers. Proximal haptoglobin promoter primers were: forward, 5′-TAACACAACGCAGAGGGCCAAGTA-3′, reverse, 5′-AACAGCCAGTGACCTTAGAG ACGT-3′, and distal haptoglobin promoter primers forward, 5′-TGACTACCAAGGTTG TCCTCCACA-3′, reverse, 5′-TTCAAACAGGTGTG GTGGCTCATG-3′. Data are normalized by input values (for each promoter and ± troglitazone), and binding is expressed relative to the nonspecific binding of IgG immunoprecipitated DNA content. Each bar represents mean ± se (n = 3) (*, P < 0.01).
Results
WAT is known to be the largest secretory organ in the body. In parallel to adipocyte formation and the induction of adipocyte markers (PPARγ and aP2), adipokine levels are increased during adipocyte differentiation of 3T3-L1 cells in vitro. Haptoglobin and angiotensinogen are expressed at confluence (d 0), and their level of expression is significantly increased during adipocyte differentiation (Fig. 1A). Chemerin and resistin are expressed after PPARγ expression and coincident with expression of the fatty acid binding protein 4 (FABP4) and accumulation of lipids (Fig. 1A). In Fig. 1B, we treated fully differentiated 3T3-L1 adipocytes from d 6 to d 8 with troglitazone (a TZD), TNFα, or both and analyzed gene expression by Q-PCR. As previously described by others (11), haptoglobin expression is dramatically down-regulated by troglitazone (85%) and induced by TNFα (7-fold) with no effect of these effectors on FABP4/aP2 and perilipin gene expression. Importantly, troglitazone is capable of preventing induction of haptoglobin by TNFα (Fig. 1B), strongly suggesting that PPARγ plays a key role in the repression mechanism. Interestingly, TNFα represses the normal expression of C/EBPα and adiponectin in the adipocytes, and this repression is not reversed by treatment with troglitazone (Fig. 1B). We then investigated the capacity of PPARγ agonists to down-regulate haptoglobin expression in mice adipose depots. Figure 1C shows that in vivo, haptoglobin is expressed at a higher level in white adipose depots (mesenteric, gonadal, and sc) than in BAT. After 2 wk of treatment of mice with a potent PPARγ agonist (COOH), a decrease in haptoglobin expression is observed in all white depots but not BAT (Fig 1C).
Figure 1.
Haptoglobin expression is up-regulated during adipocyte differentiation, activated by TNFα treatment, and actively down-regulated by PPARγ ligands in vitro and in vivo. A, RT-PCR analysis of genes encoding adipokines [chemerin, Wdnm1-like protein, resistin (retn), angiotensinogen (agt), and pank3] and classic adipocyte markers (PPARγ and Fabp4/aP2) during adipocyte differentiation of 3T3-L1. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. B, Q-PCR analysis of genes expressed in 3T3-L1 adipocytes exposed to 0.1% DMSO, 5 ng/ml TNFα, 5 μm troglitazone (Trog), or TNFα +Trog for 24 h. Data are represented as fold change in mRNA expression compared with mRNA expressed in DMSO-treated cells. Each bar represents mean ± se (n = 3). *, P < 0.01 relative to control (DMSO). C, Mice were given a PPARγ agonist (COOH) and vehicle at 10 wk of age for 14 d at 10 mg/kg. Total RNA was extracted from WAT of different depots mesenteric (Mes), gonadal (Gon), sc (Sub), and brown. Expression of haptoglobin was analyzed by Q-PCR. Data are represented as relative amount of mRNA expression (five mice for each group). Each bar represents mean ± se (n = 5). *, P < 0.01 relative to vehicle treated.
The expression of other adipokines (chemerin, resistin, and angiotensinogen) is also repressed by TZDs in vitro and in vivo in white adipocytes by a mechanism that involves both C/EBPα and the corepressors CtBP1/2 (14). Because C/EBPα is also known to regulate haptoglobin expression in response to inflammation in the liver (17), we addressed the role of C/EBPα and CtBP1/2 in regulating haptoglobin gene expression in adipocytes. We first knocked down C/EBPα transiently in fully differentiated adipocytes using siRNA technology. C/EBPα protein level is extensively decreased (90%) by the siRNA in 3T3-L1 adipocytes treated or not with troglitazone as shown in Fig. 2A. PPARγ and aP2 protein levels are unaffected by the absence of C/EBPα when compared with cells that were transfected with the control virus (siEGFP cells), which abundantly express the transcription factor. In contrast, adiponectin expression, which is known to be regulated by C/EBPα (18,19), is reduced in the knockdown cells. Troglitazone treatment of fully differentiated control adipocytes (siEGFP) decreases the level of haptoglobin, chemerin, resistin, and angiotensinogen gene expression as assessed by Q-PCR analysis (Fig. 2B). Importantly, the absence of C/EBPα prevents the repression of chemerin and angiotensinogen by troglitazone as reported earlier (14), but it has no effect on the ability of the TZD to repress haptoglobin mRNA expression (Fig. 2B). Interestingly, resistin expression is reduced in adipocytes lacking C/EBPα in the absence as well as the presence of troglitazone. These data are consistent with those of Lazar and coworkers (20) showing that C/EBPα regulates the normal expression of resistin in adipocytes.
Figure 2.
C/EBPα and the corepressors CtBP1 and CtBP2 are not required for TZD-associated repression of haptoglobin expression. C/EBPα was knocked down transiently by using specific siRNA (siC/EBPα) in fully differentiated adipocytes at d 6 of differentiation. Two days later, in the presence or absence of troglitazone (Trog), gene expression was analyzed by Western blot (A) and Q-PCR (B). C, 3T3-L1 cell lines in which either CtBP1 (shCtBP1) or CtBP2 (shCtBP2) were knocked down and control cells (shEGFP) were induced to differentiate with a classic adipogenic cocktail. At d 6, 5 μm of Trog or 0.1% DMSO was added to the medium. Two days later, RNA was harvested and gene expression analyzed by Q-PCR. The results are expressed as relative amount of mRNA. Each bar represents mean ± se (n = 3). *, P < 0.01 results is a mean. D, Association of particular nuclear factors with the haptoglobin promoter was determined by ChIP technology. ChIP assays were performed by immunoprecipitating protein-associated DNA in fully differentiated 3T3-L1 adipocytes with antibodies against PPARγ, C/EBPα, CtBP1/2, nuclear receptor corepressor (NCoR), Ace-H3, and IgG (negative control). DNA was amplified by primer sets designed for haptoglobin promoter distal promoter and analyzed by Q-PCR. Each bar represents mean ± se (n = 3). *, P < 0.01. Binding is expressed relative to the nonspecific binding of IgG immunoprecipitated DNA content.
CtBP1/2 corepressors bind to both the resistin and angiotensinogen promoters through a mechanism that appears to include their association with C/EBPα (14). To analyze the potential role of CtBP1/2 in regulating haptoglobin repression, we used 3T3-L1 cell lines that we previously established (14) in which expression of CtBP1 or CtBP2 is significantly reduced using shRNA technology (shCtBP1 and shCtBP2). Figure 2C shows, as observed previously (14), that CtBP1 or CtBP2 mRNA expression is efficiently down-regulated by the specific shRNA (80 and 60%, respectively), and this down-regulation does not affect other adipocyte differentiation markers (PPARγ and aP2) in the presence or absence of troglitazone. Importantly, the repression of haptoglobin expression by troglitazone still occurs in the absence of CtBP1 (shCtBP1) or CtBP2 (shCtBP2). Taken together, the above data show that repression of haptoglobin is independent of C/EBPα and CtBP1/2 corepressors in the adipocyte.
To investigate further the role of PPARγ in regulating haptoglobin gene expression, we used ChIP analysis of the haptoglobin promoter. Using ChIP assays, we found that PPARγ binds to haptoglobin promoter in 3T3-L1 adipocytes, but the extent of binding is slightly lower in cells treated with troglitazone (Fig. 2D). Interestingly, there is a significant decrease in acetylation of histone H3 in response to troglitazone consistent with the expected lower activity of the promoter in the presence of TZD. Additionally, neither C/EBPα nor CtBP1/2 are recruited to the haptoglobin promoter in fully differentiated adipocytes, confirming that they have no role to play in regulating basal transcription or repressing haptoglobin expression in the presence of troglitazone. Nuclear receptor corepressor (NCoR), a known repressor of PPARγ transcriptional activity, also does not interact with haptoglobin promoter in cells treated with or without troglitazone.
We recently showed that mutation of critical amino acids within helix 7 of PPARγ modifies its ability to induce (15) or repress (14) specific sets of genes in response to TZDs. To analyze the effect of the mutations on haptoglobin expression, Swiss fibroblasts overexpressing wild-type (Swiss WT-PPARγ) or the mutant molecule (Swiss EF-PPARγ) as well as the empty vector (Swiss Control) were induced to differentiate using standard 3T3-L1 cell conditions. Control cells express negligible amounts of PPARγ and, consequently, do not differentiate into adipocytes shown by the low expression of FABP4/aP2 (Fig. 3A, right panel) and as previously reported elsewhere (15). Overexpression of WT-PPARγ causes the fibroblasts to differentiate into adipocytes in the presence or absence of troglitazone, whereas EF-PPARγ is capable only of inducing differentiation (FABP4/aP2 expression) in the presence of the TZD (Fig. 3A, right panel). Haptoglobin expression is induced manyfold by WT-PPARγ in the absence of troglitazone, and this induction is inhibited by TZD (Fig. 3A, left panel). As expected EF-PPARγ is incapable of inducing haptoglobin expression without treatment with troglitazone, but interestingly, addition of troglitazone does not further reduce haptoglobin mRNA expression; instead it significantly enhances its production (Fig. 3A, left panel). We also investigated EF-PPARγ in 3T3-L1 preadipocytes to assess whether factors not present in Swiss fibroblasts could influence its activity. This involved using 3T3-L1 cells overexpressing WT-PPARγ, EF-PPARγ, or the empty vector (Cont) (Fig. 3B). 3T3-L1 preadipocytes expressing WT-PPARγ (3T3-L1 WT-PPARγ) differentiate to a greater extent than control cells as shown by significantly enhanced FABP4/aP2 mRNA expression level (Fig. 3C, right panel) in the presence or absence of troglitazone. EF-PPARγ appears to be capable of enhancing differentiation to a modest extent, which is significantly less than WT-PPARγ (Fig 3C, right panel). As observed in Swiss fibroblasts (Fig. 3A), WT-PPARγ induces haptoglobin expression during differentiation of 3T3-L1 preadipocytes and addition of troglitazone significantly reduces the extent of the induction (Fig. 3C, left panel). In contrast, EF-PPARγ modestly enhances haptoglobin expression above the normal levels in control preadipocytes, but addition of troglitazone significantly induces haptoglobin expression (Fig. 3C, left panel).
Figure 3.
PPARγ mediates the troglitazone (Trog)-associated inhibition of haptoglobin expression in adipocytes. A, Swiss control, Swiss WT-PPARγ, and Swiss EF-PPARγ fibroblasts were induced to differentiate into adipocytes in the presence or absence of 5 μm Trog. Haptoglobin, PPARγ, and aP2 gene expression was analyzed by Q-PCR and expressed as relative amount of RNA. Each bar represents mean (n = 3). *, P < 0.01 relative to DMSO-treated Swiss control. B, Q-PCR analysis of PPARγ expression in adipocytes derived from 3T3-L1 cell lines ectopically expressing WT-PPARγ (3T3-L1 WT-PPARγ), EF-PPARγ (3T3-L1 EF-PPARγ), or a control vector (3T3-L1 cont). Each bar represents mean ± se (n = 3). *, P < 0.01 relative to control (DMSO). C, Fully differentiated adipocytes derived from 3T3-L1 WT-PPARγ, 3T3-L1 EF-PPARγ, or control preadipocytes were treated for 2 d with DMSO or troglitazone and expression of haptoglobin and aP2 mRNA measured using Q-PCR. Data are represented as relative mRNA number and each bar represents mean (n = 3). *, P < 0.01 relative to DMSO-treated 3T3-L1 control cell line.
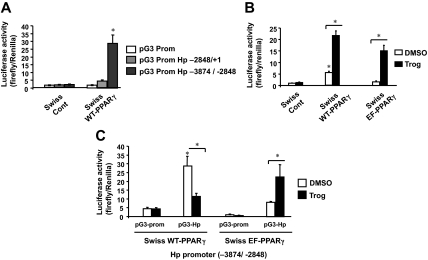
After analyzing the haptoglobin gene promoter sequence, we identified putative PPARγ response elements (TGTTCCATGGATCC and AGGGCTAGGGTCA) in the region −3874/−2848. We therefore subcloned two different parts of the promoter into pG3 prom-luciferase (+1/−2848 and −3874/−2848) and transiently transfected these constructs into control Swiss 3T3 fibroblasts or cells expressing WT-PPARγ (Swiss WT-PPARγ). The data in Fig. 4A shows that PPARγ stimulates the region of the haptoglobin gene containing the PPAR response elements (PPREs) (−3874/−2848) to promote transcription of the luciferase reporter gene. The proximal region of the gene (+1/−2848), however, appears not to respond to PPARγ activity (Fig. 4A).
Figure 4.
Mutation within helix 7 of the ligand-binding domain of PPARγ abolishes troglitazone repression of haptoglobin expression. A, Different haptoglobin promoter-luciferase reporter constructs were transiently cotransfected with pef-Renilla in Swiss control or Swiss WT-PPARγ fibroblasts. Results are represented as luciferase activity corresponding to different haptoglobin promoter regions normalized to the renilla signal. B, The consensus PPRE (DR1)-luciferase reporter construct was cotransfected with control pef-Renilla plasmid into Swiss control, Swiss WT-PPARγ, and Swiss EF-PPARγ cell lines and cells treated for 2 d with 5 μm troglitazone (Trog) or DMSO. Each bar represents mean ± se (n = 3). *, P < 0.01. C, The distal haptoglobin promoter-luciferase plasmid (pG3-Hp) and a control luciferase reporter (pG3-prom) were cotransfected with the control pef-Renilla plasmid into Swiss control, Swiss WT-PPARγ, and Swiss EF-PPARγ cell lines, which were then treated for 2 d with 5 μm troglitazone or DMSO. Each bar represents mean ± se (n = 3). *, P < 0.01.
These results led us to determine the capacity of EF-PPARγ to induce luciferase transcription directed by a known PPARγ element (DR1-luciferase) as well as the distal region of the haptoglobin gene promoter using a transient transfection assay. Figure 4B shows that WT-PPARγ induces basal luciferase activity from the DR1 element without treatment with troglitazone, whereas EF-PPARγ possesses negligible activity without the TZD. In contrast, the activity of EF-PPARγ as well as WT-PPARγ is stimulated by troglitazone (Fig. 4B), supporting the data shown in Fig. 3 that the transcriptional activity of EF-PPARγ depends on association with synthetic as opposed to endogenous ligands. Figure 4C shows that the distal region (−3874/−2848 = pG3-Hp) of the haptoglobin gene promoter is activated to direct transcription of the luciferase gene by WT-PPARγ but not EF-PPARγ in the absence of troglitazone when compared with the level of background showed by transfecting a control luciferase reporter (pG3-prom). Treatment with troglitazone, however, attenuates the activity of WT-PPARγ but significantly enhances EF-PPARγ activity (Fig. 4C). This result shows that PPARγ is an important regulator of haptoglobin expression and that mutations within helix 7 eliminate the repressive activity of troglitazone but retain the activation properties.
Discussion
Haptoglobin is an acute-phase reactant synthesized mainly by liver with other acute phase proteins. In rat, haptoglobin liver expression is regulated by increased binding of signal transducer and activator of transcription-3 and the 35-kDa isoform of C/EBPβ (liver-enriched activating protein) to the hormone-responsive element in the haptoglobin gene during the turpentine-induced acute-phase response (21). Haptoglobin synthesis is additively induced by IL-6 and glucocorticoids. Signal transducer and activator of transcription-3 serves as the mediator of the IL-6 receptor signal and appears to contribute to the transcriptional induction of acute-phase protein genes (22). In addition, ectopic expression of either C/EBPβ or C/EBPα in the B13 pancreatic cell line induces haptoglobin expression and oncostatin M is sufficient to enhance the expression of haptoglobin in C/EBPβ transfected cells from 18 to 43% (17). Haptoglobin gene expression in WAT as well as serum levels is significantly higher in genetically or experimentally induced obese mice compared with lean animals (8,9). In vitro, IL-6 and TNFα induce haptoglobin expression in 3T3-L1 adipocytes (23,24) and TZDs repress it, but very little is known about the molecular mechanism involved in its regulation. Because C/EBPα, as in liver, plays a major role in adipocyte function, we assessed its role in regulating haptoglobin expression in adipocytes cultured with or without troglitazone. We transiently knocked down C/EBPα in fully differentiated adipocytes and observed no significant changes in haptoglobin expression under basal as well as TZD-treated conditions. We also showed by ChIP that C/EBPα appears not to bind to the haptoglobin promoter in adipocytes treated with or without troglitazone, in contrast to its binding to the promoter when regulating haptoglobin gene expression in human monocytic cells in response to all-trans retinoic acid (25). It appears therefore that C/EBPα plays a minimal role in regulating haptoglobin expression in adipocytes in contrast to its prominent role along with C/EBPβ in mediating the induction of haptoglobin expression by inflammatory cytokines (TNFα, IL-6) in hepatocytes (26).
We previously demonstrated that TZDs can also down-regulate expression of an additional set of specific adipokines (chemerin, resistin, angiotensinogen, and westmead DMBA8 nonmetastatic 1-like protein) that we referred to as visceral white genes because they were more abundant in visceral WAT depots than sc depots or BAT (14). The data in Fig. 1C suggest that expression of haptoglobin differs from that of the other adipokines because it is abundantly expressed in sc as well as visceral WAT depots. Additionally, the visceral white genes are responsive to the corepressors CtBP1/2 as well as C/EBPα, whereas these factors appear not to influence haptoglobin expression in adipocytes.
Using ChIP assays, we demonstrate that PPARγ binds to the haptoglobin promoter in fully differentiated adipocytes in culture as was previously shown by others using ChIP-on-ChIP technology (27). The haptoglobin upstream promoter contains atypical PPREs that are likely to be functional regulatory elements because the corresponding region of the gene drives transcription of a luciferase reporter gene in response to WT-PPARγ activity. Moreover, activation of WT-PPARγ by TZDs represses its ability to up-regulate transcription from the region of the gene containing the PPREs. Interestingly, mutation of PPARγ within helix 7 of the ligand-binding domain prevents it from activating the PPRE region of the haptoglobin promoter, demonstrating therefore a direct role of PPARγ in regulating haptoglobin gene expression. Additionally, the mutations appear to eliminate the repressive activity of PPARγ in response to the TZDs, without affecting the inductive activity.
Identifying the mechanisms and nuclear factors regulating haptoglobin as well as other adipokines will hopefully provide information for the development of strategies to reduce the degree of inflammation associated with obesity, which leads to debilitating disorders including type 2 diabetes and cardiovascular disease.
Acknowledgments
We thank Meghan MacDonald for generating 3T3-L1 cell lines (shEGFP, shCtBP1, and shCtBP2) and suggestions with writing the manuscript.
Footnotes
This work was supported by U.S. Public Health Service Grants DK51586 and DK58825 (to S.R.F.) and DK55758 and the University of Texas Southwestern (UTSW) Taskforce for Obesity Research (TORS) Consortium Grant 1PL1DK081182 (to P.E.S.). K.E.D. was supported by UTSW TORS/Interdisciplinary Research Training Program Grant T90-DK081181 and a National Research Service Award fellowship F32 DK081279-01.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 1, 2009
Abbreviations: aP2, Adipocyte P2; BAT, brown adipose tissue; C/EBP, CCAAT/enhancer-binding protein; ChIP, chromatin immunoprecipitation; COOH, 2-(2-(4-phenoxy-2-propylphenoxy)ethyl)indole-5-acetic acid; DMSO, dimethylsulfoxide; EGFP, enhanced green fluorescent protein; FABP4, fatty acid binding protein 4; FBS, fetal bovine serum; PPAR, peroxisome proliferator-activated receptor; PPRE, PPAR response element; Q-PCR, quantitative PCR; sh, short hairpin; si, small interfering; TZD, thiazolidinedione; WAT, white adipose tissue.
References
- Langlois MR, Delanghe JR 1996 Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 42:1589– 1600 [PubMed] [Google Scholar]
- Cid MC, Grant DS, Hoffman GS, Auerbach R, Fauci AS, Kleinman HK 1993 Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J Clin Invest 91:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleijn DP, Smeets MB, Kemmeren PP, Lim SK, Van Middelaar BJ, Velema E, Schoneveld A, Pasterkamp G, Borst C 2002 Acute-phase protein haptoglobin is a cell migration factor involved in arterial restructuring. FASEB J 16:1123–1125 [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J 1994 The acute phase response. Immunol Today 15:74–80 [DOI] [PubMed] [Google Scholar]
- Suleiman M, Aronson D, Asleh R, Kapeliovich MR, Roguin A, Meisel SR, Shochat M, Sulieman A, Reisner SA, Markiewicz W, Hammerman H, Lotan R, Levy NS, Levy AP 2005 Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes 54:2802–2806 [DOI] [PubMed] [Google Scholar]
- Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G 2009 Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Ann Med 4:1–11 [DOI] [PubMed] [Google Scholar]
- Quaye IK 2008 Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg 102:735–742 [DOI] [PubMed] [Google Scholar]
- Chiellini C, Bertacca A, Novelli SE, Görgün CZ, Ciccarone A, Giordano A, Xu H, Soukas A, Costa M, Gandini D, Dimitri R, Bottone P, Cecchetti P, Pardini E, Perego L, Navalesi R, Folli F, Benzi L, Cinti S, Friedman JM, Hotamisligil GS, Maffei M 2002 Obesity modulates the expression of haptoglobin in the white adipose tissue via TNFα. J Cell Physiol 190:251–258 [DOI] [PubMed] [Google Scholar]
- Chiellini C, Santini F, Marsili A, Berti P, Bertacca A, Pelosini C, Scartabelli G, Pardini E, López-Soriano J, Centoni R, Ciccarone AM, Benzi L, Vitti P, Del Prato S, Pinchera A, Maffei M 2004 Serum haptoglobin: a novel marker of adiposity in humans. J Clin Endocrinol Metab 89:2678–2683 [DOI] [PubMed] [Google Scholar]
- Kratchmarova I, Kalume DE, Blagoev B, Scherer PE, Podtelejnikov AV, Molina H, Bickel PE, Andersen JS, Fernandez MM, Bunkenborg J, Roepstorff P, Kristiansen K, Lodish HF, Mann M, Pandey A 2002 A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics 1:213–222 [DOI] [PubMed] [Google Scholar]
- do Nascimento CO, Hunter L, Trayhurn P 2004 Regulation of haptoglobin gene expression in 3T3-L1 adipocytes by cytokines, catecholamines, and PPARγ. Biochem Biophys Res Commun 313:702–708 [DOI] [PubMed] [Google Scholar]
- Farmer SR 2006 Transcriptional control of adipocyte formation. Cell Metab 4:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA 2006 Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896 [DOI] [PubMed] [Google Scholar]
- Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR 2009 C/EBPα and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor γ agonists. Mol Cell Biol 29:4714–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qiang L, Farmer SR 2008 Identification of a domain within peroxisome proliferator-activated receptor γ regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 28:188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley AN, Semeniuk LM, Shimoni Y, Aasum E, Larsen TS, Berger JP, Severson DL 2004 Treatment of type 2 diabetic db/db mice with a novel PPARγ agonist improves cardiac metabolism but not contractile function. Am J Physiol Endocrinol Metab 286:E449–E455 [DOI] [PubMed] [Google Scholar]
- Kurash JK, Shen CN, Tosh D 2004 Induction and regulation of acute phase proteins in transdifferentiated hepatocytes. Exp Cell Res 292:342–358 [DOI] [PubMed] [Google Scholar]
- Park BH, Qiang L, Farmer SR 2004 Phosphorylation of C/EBPβ at a consensus ERK/GSK3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol 24:8671–8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, Friedman JE, Shao J 2005 C/EBPα regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 54:1744–1754 [DOI] [PubMed] [Google Scholar]
- Hartman HB, Hu X, Tyler KX, Dalal CK, Lazar MA 2002 Mechanisms regulating adipocyte expression of resistin. J Biol Chem 277:19754–19761 [DOI] [PubMed] [Google Scholar]
- Uskokovic A, Arambasic J, Bogojevic D, Ivanovic-Matic S, Mihailovic M, Dinic S, Grigorov I 2009 Differences between molecular mechanisms involved in the regulation of haptoglobin gene expression during the acute phase response and dietary restriction. Folia Biol (Praha) 55:107–115 [PubMed] [Google Scholar]
- Kim H, Baumann H 1997 The carboxyl-terminal region of STAT3 controls gene induction by the mouse haptoglobin promoter. J Biol Chem 272:14571–14579 [DOI] [PubMed] [Google Scholar]
- Andersson CX, Sopasakis VR, Wallerstedt E, Smith U 2007 Insulin antagonizes interleukin-6 signaling and is anti-inflammatory in 3T3-L1 adipocytes. J Biol Chem 282:9430–9435 [DOI] [PubMed] [Google Scholar]
- Wang B, Jenkins JR, Trayhurn P 2005 Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-α. Am J Physiol Endocrinol Metab 288:E731–E740 [DOI] [PubMed] [Google Scholar]
- Lee IH, Lee JH, Lee MJ, Lee SY, Kim IS 2002 Involvement of CCAAT/enhancer-binding protein α in haptoglobin gene expression by all-trans-retinoic acid. Biochem Biophys Res Commun 294:956–961 [DOI] [PubMed] [Google Scholar]
- Dinić S, Bogojević D, Petrović M, Poznanović G, Ivanovic-Matić S, Mihailović M 2005 C/EBPα and C/EBPβ regulate haptoglobin gene expression during rat liver development and the acute-phase response. Mol Biol Rep 32:141–147 [DOI] [PubMed] [Google Scholar]
- Nakachi Y, Yagi K, Nikaido I, Bono H, Tonouchi M, Schönbach C, Okazaki Y 2008 Identification of novel PPARγ target genes by integrated analysis of ChIP-on-chip and microarray expression data during adipocyte differentiation. Biochem Biophys Res Commun 372:362–366 [DOI] [PubMed] [Google Scholar]