Abstract
Environmental day length drives nocturnal pineal melatonin secretion, which in turn generates or entrains seasonal cycles of physiology, reproduction, and behavior. In mammals, melatonin (MEL) binds to a number of receptor subtypes including high-affinity (MT1 and MT2) and low-affinity (MT3, nuclear orphan receptors) binding sites, which are distributed throughout the central nervous system and periphery. The MEL receptors that mediate photoperiodic reproductive and behavioral responses to MEL have not been identified in a reproductively photoperiodic species. Here I tested the hypothesis that MT1 receptors are necessary and sufficient to engage photoperiodic responses by challenging male Siberian hamsters (Phodopus sungorus), a species that does not express functional MT2 receptors, with ramelteon (RAM), a specific MT1/MT2 receptor agonist. In hamsters housed in a long-day photoperiod, late-afternoon RAM treatment inhibited gonadotropin secretion, induced gonadal regression, and suppressed food intake and body mass, mimicking effects of MEL. In addition, chronic (24 h/d) RAM infusions were sufficient to obscure endogenous MEL signaling, and these treatments attenuated gonadal regression in short days. Together, the outcomes indicate that signaling at the MT1 receptor is sufficient and necessary to mediate the effects of photoperiod-driven changes in MEL on behavior and reproductive function in a reproductively photoperiodic mammal.
In seasonally breeding Siberian hamsters, melatonin signaling at the MT1 receptor is necessary and sufficient for changes in day length to trigger seasonal adaptations in body mass, food intake, gonadotrophin secretion, and reproductive physiology.
Seasonal cycles in physiology and behavior are ubiquitous in nature and contribute to the etiology of psychiatric and infectious disease (1). Changes in day length (photoperiod) and in photoperiod-driven nocturnal melatonin (MEL) secretion play a central role in the transduction of time-of-year information into the central nervous and neuroendocrine systems (2).
Much of what is known about photoperiodic control of the central nervous system (CNS) has been derived from the study of seasonal rhythms in mammalian reproduction. Long- and short-duration MEL signals are generated in winter and summer, respectively (3), and over an interval of many weeks induce seasonal reproductive phenotypes (4,5). Two MEL receptor subtypes, MT1 and MT2, have been identified in mammals (6). Several reports have addressed the role of these receptors in circadian and seasonal biology. MT1 receptors bind MEL in the brain and pituitary of mice (7); however, both MT1 and MT2 receptors have been implicated in the phase-shifting responses of the circadian system to MEL in mice (7,8). Evidence that Siberian hamsters (Phodopus sungorus) exhibit circadian phase shifts and entrainment to MEL points to the existence of species differences in the necessity of MT2 receptors for circadian responses to MEL (9), because Siberian hamsters lack a functional MT2 receptor (10).
Evidence on the role of MT1 and MT2 in the reproductive response to photoperiod and MEL is not consistent. High doses (30 mg/kg) of luzindole, an MT1/MT2 antagonist/inverse agonist, were without effect on hamster reproductive responses to photoperiod or MEL (11), suggesting that disruption of MEL signaling at MT1/MT2 receptors is compatible with normal photoperiodic responses. In contrast, late afternoon injections of MEL elicit changes in ependymal cell layer expression of type II and type III iodothyronine deiodinase mRNA (Dio2 and Dio3, respectively) in wild-type mice but fail to do so in mice with targeted disruption of the MT1 receptor (12). Dio2 and Dio3 enzymes have been implicated as early mediators of MEL-based photoperiod information into the neuroendocrine system of photoperiodic rodents (12,13,14). Although a causal link between Dio2/Dio3 expression and reproductive photoperiodism has not been definitively established in mammals, Dio2/Dio3 expression levels track photoperiod (13) and Dio2/Dio3 protein products control T4 catabolism in a manner consistent with a role in the transduction of photoperiod information into the CNS (15). Additionally, MEL may act at other binding sites (low-affinity MT3 receptors, nuclear orphan receptors) (16,17,18) to mediate gonadal responses to changes in photoperiod.
The recent availability of the MT1/MT2 agonist ramelteon (RAM) may allow insight into the necessity and sufficiency of MT1 MEL receptors in reproductive photoperiodism. RAM acts on MT1 and MT2 receptors, with an affinity three to 16 times higher than that of MEL (19,20), and has no detectable affinity across a wide range of CNS ligand binding sites, including benzodiazepine, monoamine, and opiate receptors, nuclear receptors, ion channels, and transporters, including the MT3 receptor (19,21). Because Siberian hamsters lack functional MT2 MEL receptors, the combination of using a specific MT1/MT2 agonist in conjunction with a natural MT2 knockout species provides a direct examination of the role of MT1 receptors in a reproductively photoperiodic organism.
If MT1 receptors are sufficient to mediate the effects of MEL on the reproductive system, then treatment of Siberian hamsters with RAM should inhibit gonadotropin secretion and induce gonadal regression similar in magnitude and latency to that elicited by MEL. Moreover, if MT1 signaling is necessary to mediate effects of MEL on the reproductive system, then obscuring MT1 signaling via constant-release (24 h) RAM infusions should attenuate gonadotropin and reproductive responses to photoperiod.
Materials and Methods
Animals
Procedures in this experiment conformed to the U.S. Public Health Service Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Chicago Institutional Animal Care and Use Committee. Siberian hamsters (P. sungorus) from my laboratory breeding colony were weaned at 18–21 d of age and raised two to four per cage (28 × 17 × 12 cm) under a 15-h light, 9-h dark photoperiod [long day (LD); lights off at 1800 h Central Standard Time] with ad libitum access to food and filtered water at all times. Ambient temperature was 20 ± 0.5 C, and relative humidity was 53 ± 2% throughout the experiment. Three weeks before the initiation of each experiment, hamsters were housed one per cage.
Drugs and delivery
In experiment 1, MEL (N-acetyl-5-methoxytryptamine; Sigma Chemical Co., St. Louis, MO) and RAM [(S)-N-[2-(1,6,7,8-tetrahyrdo-2H-indeno[5,4-b]furan-8-yl)ethyl]propionamide; Takeda Pharmaceuticals, N.A., Deerfield, IL] were delivered by mouth in a small (100–200 mg) piece of fresh apple, according to methods described by Hiebert et al. (22). The oral route of administration permits drug delivery without the confounds of handling and injection stress on the circadian system. Briefly, MEL and RAM were each dissolved in 70% ethanol at concentrations of 3.5 mg/ml and 7 mg/ml, respectively. Working solutions were protected from light and stored at −20 C until use. Each day, fresh apple cubes were cut and impregnated with 5 μl MEL or RAM solution, and the ethanol solvent was then evaporated at room temperature for 30 min before feeding. Final dosing was 0.5 mg/kg MEL and 1 mg/kg RAM, which lie within the effective range for inducing gonadal regression in Siberian hamsters and inducing phase advances in circadian locomotor activity in rats when given in a single daily oral dose (21,22). Control treatments consisted of apples impregnated with 5 μl 70% ethanol. Before the onset of drug treatments, hamsters were habituated to the feeding protocol for 1 wk with ethanol-treated apples.
In experiment 2, hamsters were administered MEL or RAM via constant release miniosmotic pumps (200 μl vol; 0.25 μl/h delivery rate; Alzet 2004 model; Alza Corp., Mountain View, CA) while concurrently exposed to LD or short day (SD; 9-h light, 15-h dark cycle) photoperiods. MEL and RAM were dissolved in ethanolic saline vehicle to concentrations of 0.24 and 0.48 μg/μl, respectively, and delivered sc in surgically implanted osmotic minipumps according to the manufacturer’s instructions. Final dosing was 1.27 μg MEL/d and 2.53 μg RAM/d. This amount of MEL is sufficient to induce gonadal regression when administered daily to male Siberian hamsters (4); no previous data are available concerning RAM concentrations necessary and sufficient to elicit reproductive responses. Control miniosmotic pumps were loaded with vehicle (14% ethanol in 0.9% saline). Miniosmotic pumps were implanted under 3% isoflurane anesthesia at wk 0 and were replaced at wk 4 with identical pumps. Treatments continued through wk 8.
Somatic, reproductive, and behavioral measures
Estimated testis volumes (ETVs) were determined on wk 0 and 4 in experiment 1, and on wk 0, 1, 2, 3, 5, and 7 in experiment 2. ETVs were obtained by measuring the length and width of the left testis through the abdominal skin with analog calipers (to 0.1 mm) while under light isoflurane anesthesia. In hamsters, ETV is positively correlated with testis weight, circulating testosterone, and spermatogenesis (23,24). In experiment 1, food in the cage hopper was weighed (to 0.1 g) weekly to obtain a measure of food intake. In experiment 2, hamsters were euthanized via CO2 inhalation on wk 8, and paired testes weights were determined (to 0.1 mg). All hamsters were weighed (to 0.1 g) weekly.
Circadian locomotor activity
Circadian locomotor activity was monitored during the final 10–14 d of experiment 1 (wk 3–4). Home cage activity data were collected using passive infrared motion detectors (Coral Plus; Visonic, Bloomfield, CT) positioned 22 cm above the cage floor. Motion detectors registered activity whenever three of 27 zones were crossed. Activity triggered closure of an electronic relay, which was recorded by a PC running ClockLab software (Actimetrics, Evanston, IL). The timing of activity was analyzed using ClockLab software according to methods described by Evans et al. (25). A 24-h histogram was produced for each hamster by averaging activity counts in 5-min bins over a 7- to 10-d window between wk 6 and 8. For each histogram, activity onset was defined as the point in the activity profile after 1400 h with average counts exceeding the daily overall mean level and sustained above the daily mean for at least 30 min. Activity offset was defined as the last time point exceeding this threshold (25). The phase angle of entrainment (ψR,L) to the light-dark cycle was calculated as the difference between the onset of darkness and the onset of activity (positive values indicate activity onset before lights off).
Blood sampling
At the end of experiment 1 (wk 4), and 1 wk before the end of experiment 2 (wk 7), blood samples (500 μl) were collected 4–5 h before lights-off under light isoflurane anesthesia from the right retroorbital sinus using heparinized Natelson collection tubes. Blood collections were performed in a room separate from the general animal colonies, and hamsters were separated from the colony until all blood collections for the day were completed. Animal handling during the blood collection was kept to a minimum (<1 min). After collection, blood samples remained on ice less than 1 h and were centrifuged at 300 × g for 30 min at 4 C. Plasma was stored at −80 C until assayed for FSH concentrations.
RIA
Plasma FSH concentrations were determined in duplicate in a single assay with rFSH RP-3 as standard and antirat FSH 11 antibody (National Institute of Diabetes and Digestive and Kidney Diseases, Rockville, MD), as previously described (26,27) and validated for use in this hamster species (26,27). The lower limit of detection was 0.5 ng/ml, and the intraassay coefficient of variation was 5.55%.
Experimental protocols
Experiment 1. Is signaling at the MT1 receptor sufficient? Extension of endogenous MEL signals via late-afternoon MEL and RAM treatment
Adult male Siberian hamsters housed in LD from birth (n = 24) received MEL (0.5 mg/kg; n = 8), RAM (1 mg/kg; n = 8) or ethanolic vehicle (n = 8) twice daily for 4 wk. Treatments were administered by mouth at 4 and 2 h before the onset of darkness.
Experiment 2. Is signaling at the MT1 receptor necessary? Obscuring of endogenous MEL signals via long-term constant-release MEL and RAM treatments
Adult male Siberian hamsters housed in LD from birth (n = 30) were implanted with miniosmotic pumps on wk 0 and received constant-release (0.25 μl/h) MEL (1.27 μg/d; n = 10), RAM (2.53 μg/d; n = 10), or ethanolic vehicle (n = 10) for 8 wk. Half of the hamsters in each treatment group were transferred to SD on wk 0; the other half remained in LD.
Statistical analyses
Animals were randomly assigned to treatment groups (photoperiods and drug treatments). Circadian locomotor activity data were compared across groups using ANOVA followed by t tests. Changes in testis size, food intake, and body mass across treatment weeks were compared using repeated-measures ANOVA. At the conclusion of each experiment, mean values were compared across groups using ANOVA followed by t tests. For all analyses, F statistics, with numerator and denominator degrees of freedom in subscript, are provided. Differences were considered statistically significant if P < 0.05.
Results
Experiment 1
Circadian locomotor activity responses
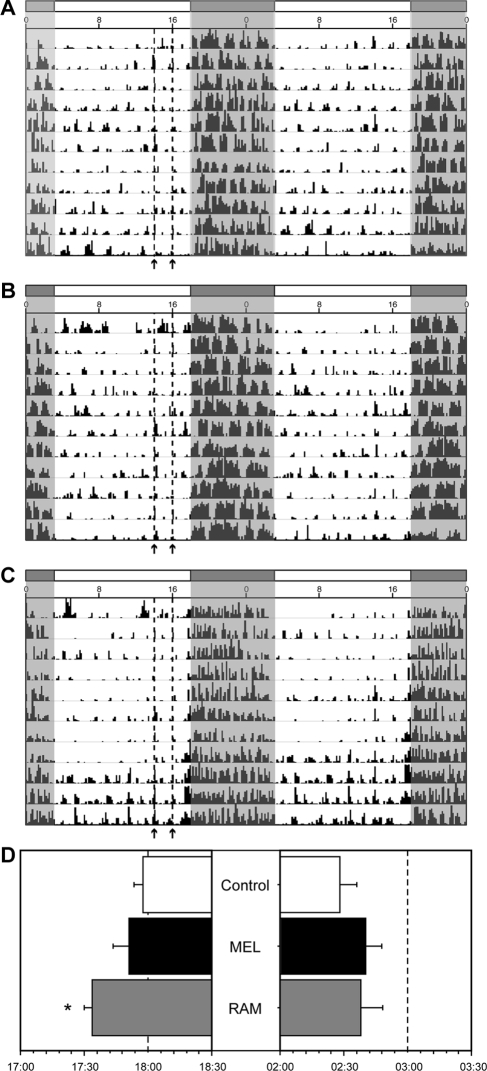
Oral administration of drugs significantly altered phase angles of entrainment across all groups [F(2,21) = 4.9; P < 0.05; Fig. 1, A–C]. Vehicle-treated hamsters entrained with small (2–3 min) positive ψR,L values (Fig. 1, A and D). Daily MEL resulted in slightly more positive and variable ψR,L values, which did not differ significantly from those of controls (P > 0.4; Fig. 1, B and D). RAM treatments induced significant (25–30 min) advances in ψR,L, which differed significantly from those of both MEL (P < 0.05) and vehicle (P < 0.01) treated hamsters (Fig. 1, C and D). Greater variability was evident in activity offsets relative to activity onsets; consequently, neither the duration of the nighttime active phase [F(2,21) = 0.6; P > 0.5; data not shown] nor activity offset [F(2,21) = 2.5; P > 0.1; data not shown] differed significantly across groups.
Figure 1.
Representative double-plotted locomotor activity records of adult male Siberian hamsters housed in LD and administered oral vehicle (control) (A), MEL (0.5 mg/kg) (B), or RAM (1 mg/kg) (C) twice daily (indicated by arrows and dashed vertical lines) for 4 wk. Data were collected over a 10- to 12-d interval during the last 2 wk of treatments. Clock time (Central Standard Time) is indicated along the horizontal axis along the top of each actogram, and white and shaded regions above and within each actogram illustrate the lighting conditions. D, Mean ± sem activity onset (left) and offset (right) of hamsters in each treatment condition. *, P < 0.05 vs. vehicle-treated control.
Gonadotropin (FSH) responses
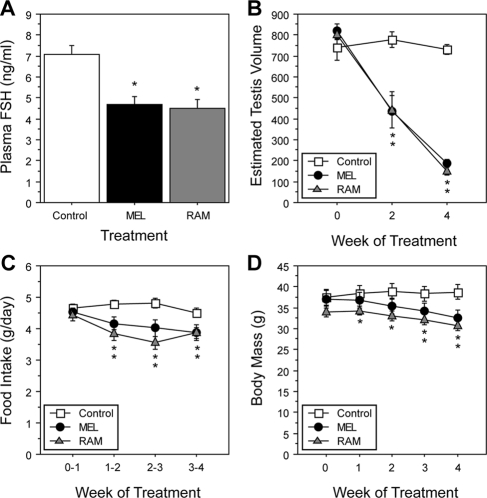
Drug treatments significantly affected plasma FSH concentrations on wk 4 [F(2,21) = 12.7; P < 0.0005; Fig. 2A]. Relative to controls, FSH was significantly inhibited in MEL-treated (P < 0.0005) and RAM-treated (P < 0.0005) hamsters.
Figure 2.
Mean ± sem plasma FSH concentrations (A), ETVs (B), food intake (C), and body mass (D) of hamsters housed in LD and administered oral MEL (0.5 mg/kg), RAM (1 mg/kg), or vehicle (control) twice daily for 4 wk. *, P < 0.05 vs. vehicle control.
Reproductive, behavioral, and somatic responses
Drug treatments significantly affected the pattern of change in testis size [F(4,42) = 22; P < 0.0001; Fig. 2B], food intake [F(6,63) = 5.1; P < 0.0005; Fig. 2C], and body mass [F(8,84) = 8.6; P < 0.0001; Fig. 2D]. Testes of MEL- and RAM-treated hamsters were smaller than those of long-day controls after 2 wk of treatments (P < 0.0001, both comparisons) and remained smaller thereafter (P < 0.0001). Food intake was significantly decreased in both MEL and RAM hamsters by the second week of treatments (P < 0.05, both comparisons). Body mass of MEL-treated hamsters was significantly lower than that of controls by wk 3 (P < 0.05), and RAM-treated hamsters weighed less than controls on wk 1 (P < 0.05). At no time point during treatment did ETV, food intake, or body mass differ significantly between MEL- and RAM-treated hamsters.
Experiment 2
Gonadotropin (FSH) responses
There were no simple main effects of photoperiod [F(1,23) = 0.5; P > 0.4] or implant type [F(2,23) < 0.1; P > 0.9] on plasma FSH, nor was the interaction term significant [F(2,23) = 1.6; P > 0.2; Fig. 3A]. Among controls, FSH concentrations were significantly lower in SD relative to LD hamsters (P = 0.01; Fig. 3A), whereas within each drug (MEL and RAM) treatment, there was no significant effect of photoperiod on mean FSH concentrations (P > 0.7, both comparisons). In all MEL- and RAM-treated hamsters, FSH concentrations were intermediate, differing from neither LD nor SD levels (P > 0.1, all comparisons).
Figure 3.
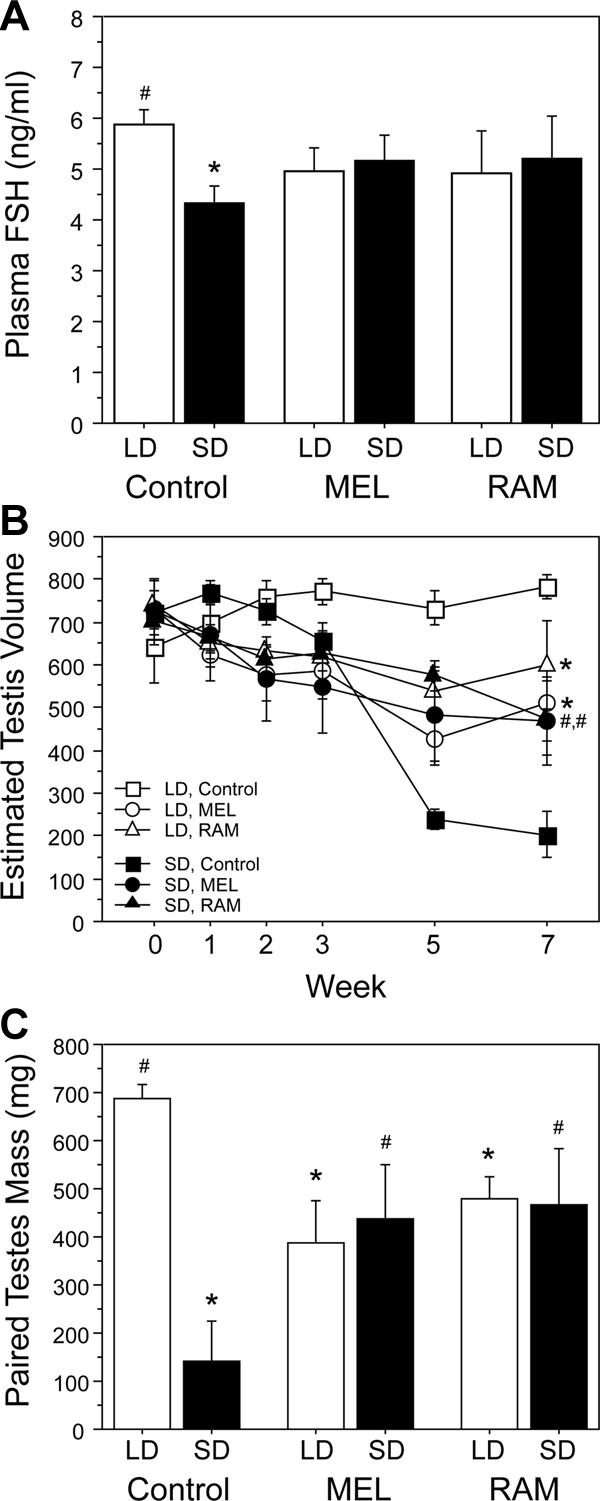
Mean ± sem plasma FSH concentrations (A), ETVs (B), and testis masses (C) of hamsters receiving chronic (24 h/d, 8 wk) infusions of MEL (1.27 μg/d), RAM (2.53 μg/d), or ethanolic (14% vol/vol) saline vehicle (control). *, P < 0.05 vs. LD-control; #, P < 0.05 vs. SD-control.
Reproductive responses
Drug treatments significantly affected the pattern of change in testis size [F10,120 = 3.5, P < 0.0005; Fig. 3B] between wk 0 and 7. Hamsters implanted with osmotic pumps containing the ethanolic saline vehicle maintained fully developed testes in LD and exhibited gonadal regression after transfer to SD (Fig. 3B). MEL treatment induced moderate gonadal regression in both LD and SD hamsters, as did treatment with RAM; on wk 7, ETVs of MEL and RAM hamsters in both LD and SD were significantly lower than those of LD-vehicle hamsters (P < 0.05, all comparisons), and significantly greater than those of SD-vehicle hamsters (P < 0.05, all comparisons). Effects of MEL and RAM on reproduction were comparable in long and short photoperiods; within a given photoperiod, ETVs of MEL- and RAM-treated hamsters did not differ at any time point between wk 0 and 7 (P > 0.2, all comparisons), and within a given drug treatment, there was no effect of photoperiod (MEL, P > 0.4, all comparisons; RAM, P > 0.3, all comparisons).
Paired testis weights yielded a comparable pattern of results (Fig. 3C). There was no significant main effect of implant type [F(2,23) = 0.3; P > 0.7] but a significant main effect of photoperiod [F(1,23) = 5.5; P < 0.05] and a significant interaction between photoperiod and implant type [F(2,23) = 6.9; P < 0.005]. In LD, both MEL and RAM treatment induced significant gonadal regression relative to controls (P < 0.01, both comparisons); final testis weights of MEL and RAM hamsters in LD were also significantly greater than those of SD controls (P < 0.05, both comparisons). In SD, MEL and RAM treatments yielded testis weights that were lower than those of LD hamsters (MEL, P < 0.05; RAM, P < 0.06) and that were greater than those of SD controls (P < 0.05, both comparisons). Testis weights of MEL-treated hamsters did not differ from those of RAM-treated hamsters in either photoperiod (P > 0.4, both comparisons).
Discussion
In experiment 1, 4 wk of late-afternoon MEL treatments inhibited FSH secretion, induced gonadal regression, and suppressed food intake and body mass, confirming and extending other work in this species (22). These effects can be attributed to an extension of the endogenous pattern of short-duration nocturnal MEL secretion into a longer MEL signal, which mimics that generated under a SD photoperiod (5). In experiment 2, 8 wk of treatment with 24-h/d MEL attenuated the effects of SD on FSH secretion and reproductive condition. Discrete, long-duration MEL signals are substantially more inhibitory than chronic, 24-h MEL signals; the latter lack a daily MEL-free interval (28) and obscure the endogenous short-day pattern of MEL secretion (29,30). This masking of endogenous MEL signaling was responsible for the attenuated reproductive responses to SD in hamsters bearing MEL implants (31). In both experiments 1 and 2, treatment with the MT1/MT2 agonist RAM mimicked the effects of MEL on reproductive physiology and hormone secretion, food intake, and body mass. In light of the functional inadequacy of the MT2 receptor subtype in this species (10), the data indicate that photoperiod signal transduction through the MT1 MEL receptor is sufficient to induce seasonal reproductive, behavioral, and somatic responses to photoperiod.
Daily oral administration of RAM yielded significant phase advances in the circadian locomotor activity rhythm, consistent with previous reports in rats (21) and humans (32) indicating efficacy at concentrations of 1 mg/kg and lower (33). When administered daily, the concentration of MEL required to entrain the circadian clock greatly exceeds that required to induce gonadal regression (micrograms vs. nanograms) in Siberian hamsters (4,34) and Syrian hamsters (29,35). If a similar relation obtains for all MEL receptor ligands, then the identification of a RAM concentration sufficient to entrain/phase-advance the circadian system increased the likelihood that such a concentration was above a value minimally sufficient for engaging the MEL-sensitive hypothalamic-pituitary-gonadal axis. Indeed, this appeared to be the case.
In experiment 2, any differential reproductive responses to LD vs. SD in hamsters bearing constant-release RAM implants would have constituted evidence in support of non-MT1-mediated MEL signaling in the photoperiodic control of reproduction. This was not observed, however. Instead, against a background of mild reproductive inhibition (induced by the constant-release implant), obscuring MEL signaling at the MT1 receptor only, via RAM treatment, abolished the effects of photoperiod, preventing both SD-induced gonadal regression and LD-induced gonadal maintenance. Together, this pattern of outcomes suggests that any non-MT1 receptor-mediated signaling by endogenous MEL is functionally irrelevant to the photoperiodic control of reproduction in this species.
Recent work in a reproductively non-photoperiodic mammalian model [wild-type and MEL receptor-deficient (MT1−/− and MT2−/−) C3H mice] also implicate MT1 receptors in the transduction of MEL signals into changes in expression of T4 catabolism enzymes (Dio2 and Dio3) at the hypothalamo-hypophyseal level (12). Photoperiodic changes in Dio3 expression were evident in wild-type and MT2−/− C3H mice but were abolished in MT1−/− mice; both Dio2 and Dio3 responses to MEL treatments were absent in MT1−/− mice (12). Several parallels exist between photic regulation of Dio in reproductively photoperiodic mammalian species and those evident in reproductively non-photoperiodic species (e.g. inbred mice) after manipulations of photoperiod (36,37). However, no evidence of photic or melatonergic regulation of seasonal behavior, body mass, gonadotropin secretion, or reproductive physiology exists in inbred mouse models of seasonality. Moreover, it is not known whether photoperiodic or MEL-driven changes in Dio2 and Dio3 expression in mice would exceed a threshold sufficient to engage seasonal phenotypic changes in behavioral or gonadotropic effector pathways in the brain and pituitary. Lastly, clear species differences in the MEL receptor subtypes sufficient to mediate effects of MEL on the circadian system do not encourage a priori confidence that MEL engages the seasonal photoperiodic system via similar MEL receptor subtypes in mice and hamsters. Although work in models with targeted deletion of specific receptor subtypes provides important insights into photic regulation of Dio expression, the present report provides evidence, in a reproductively photoperiodic mammalian species, that photoperiodic changes in gonadotropin secretion, reproductive condition, behavior (food intake), and body mass are mediated by the MT1 MEL receptor.
Although the present report cannot categorically exclude a role for non-MT1 receptors in seasonal biology, any such MEL signaling seems unlikely to play a major role in the induction of the seasonal reproductive and energetic phenotype in this species. In experiment 2, hamsters were housed in LD or SD and received a 24-h RAM signal that occluded only the MT1/MT2 receptors. Hamsters were pineal intact; therefore, any endogenously generated MEL signals should have been free to communicate photoperiod-specific (i.e. short or long) MEL signals at putative extra-MT1/MT2 targets. If any such signaling impacted the hypothalamic-pituitary-gonadal axis, then reproductive responses in LD-RAM and SD-RAM hamsters would have been expected to diverge. However, gonadotropin and reproductive responses in these two groups were indistinguishable, further supporting the conclusion that MEL signaling at receptors other than MT1 appears to be functionally irrelevant. Whether data obtained in this species extrapolates to other photoperiodic seasonal breeders (e.g. sheep and Syrian hamsters) cannot be determined from the present data set, but the available data suggest that the data may be generalizable. In sheep, MT2 is undetectable in the majority of breeds (38,39,40), and among breeds in which MT2 is detectable (Han sheep), a disrupted sequence likely renders it incapable of binding MEL (41). Moreover, a number of functional polymorphisms in the structure of the MT1 receptor have been associated with the timing of seasonal breeding in sheep (42,43,44,45), suggesting that MT1 is the major mediator of ovine reproductive responses to MEL. It is unknown whether the MT2 receptor is functional in Syrian hamsters.
In summary, the present study demonstrated that MT1/MT2 agonist treatments that were timed so as to summate with endogenous MEL induced gonadal regression and the short-day phenotypes in several photoperiodic traits in LD hamsters; in addition, RAM infusions that obscured endogenous MEL signals attenuated gonadal regression in SD. Respectively, these outcomes indicate that signaling at the MT1 receptor is sufficient and is necessary to mediate effects of photoperiod-driven changes in MEL on seasonal changes in behavior and reproductive function in a reproductively photoperiodic mammal.
Acknowledgments
I thank Jerome Galang, Sean Bradley, August Kampf-Lassin, Jenny Wei, and Priyesh Patel for expert technical assistance.
Footnotes
This work was supported by Grant AI-67406 from the National Institute of Allergy and Infectious Diseases, the Social Sciences Divisional Research Fund of the University of Chicago, and Grant ISR 06-048R from Takeda Pharmaceuticals.
Disclosure Summary: The author has nothing to disclose.
First Published Online December 4, 2009
Abbreviations: CNS, Central nervous system; ETV, estimated testis volume; LD, long day; MEL, melatonin; RAM, ramelteon; SD, short day.
References
- Prendergast BJ, Zucker I, Nelson RJ 2009 Seasonal rhythms of mammalian behavioral neuroendocrinology. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, eds. Hormones, brain, and behavior. 2nd ed. San Diego: Academic Press [Google Scholar]
- Goldman BD 2001 Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms 16:283–301 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Tamarkin L, Pratt BL, Goldman BD 1982 Pineal melatonin in the Djungarian hamster: photoperiodic regulation of a circadian rhythm. Endocrinology 111:488–492 [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD 1983 Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology 113:1261–1267 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD 1993 The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res 15:161–190 [DOI] [PubMed] [Google Scholar]
- von Gall C, Stehle JH, Weaver DR 2002 Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 309:151–162 [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM 1997 Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19:91–102 [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI 1998 Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J 12:1211–1220 [DOI] [PubMed] [Google Scholar]
- Butler MP, Paul MJ, Turner KW, Park JH, Driscoll JR, Kriegsfeld LJ, Zucker I 2008 Circadian rhythms of photorefractory Siberian hamsters remain responsive to melatonin. J Biol Rhythms 23:160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Liu C, Reppert SM 1996 Nature’s knockout: the Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol Endocrinol 10:1478–1487 [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Fang JM, Dubocovich ML 1990 Effects of melatonin agonists and antagonists on reproduction and body weight in the Siberian hamster. J Pineal Res 9:231–242 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf HW 2009 Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J Neurosci 29:2885–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, Yoshimura T 2004 Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology 145:1546–1549 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf HW 2007 Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology 148:4385–4392 [DOI] [PubMed] [Google Scholar]
- Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, Archer ZA, Mercer JG, Morgan PJ 2007 Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology 148:3608–3617 [DOI] [PubMed] [Google Scholar]
- Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F, Fauchere JL, Delagrange P, Canet E, Boutin JA 2000 Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem 275:31311–31317 [DOI] [PubMed] [Google Scholar]
- Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA 2001 Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem Pharmacol 61:1369–1379 [DOI] [PubMed] [Google Scholar]
- Boutin JA, Audinot V, Ferry G, Delagrange P 2005 Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci 26:412–419 [DOI] [PubMed] [Google Scholar]
- Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S, Kawamata Y, Hinuma S, Miyamoto M 2005 Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48:301–310 [DOI] [PubMed] [Google Scholar]
- Uchikawa O, Fukatsu K, Tokunoh R, Kawada M, Matsumoto K, Imai Y, Hinuma S, Kato K, Nishikawa H, Hirai K, Miyamoto M, Ohkawa S 2002 Synthesis of a novel series of tricyclic indan derivatives as melatonin receptor agonists. J Med Chem 45:4222–4239 [DOI] [PubMed] [Google Scholar]
- Hirai K, Kita M, Ohta H, Nishikawa H, Fujiwara Y, Ohkawa S, Miyamoto M 2005 Ramelteon (TAK-375) accelerates reentrainment of circadian rhythm after a phase advance of the light-dark cycle in rats. J Biol Rhythms 20:27–37 [DOI] [PubMed] [Google Scholar]
- Hiebert SM, Green SA, Yellon SM 2006 Daily timed melatonin feedings mimic effects of short days on testis regression and cortisol in circulation in Siberian hamsters. Gen Comp Endocrinol 146:211–216 [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I 1995 Seasonal adaptations of Siberian hamsters. II. Pattern of change in daylength controls annual testicular and body weight rhythms. Biol Reprod 53:116–125 [DOI] [PubMed] [Google Scholar]
- Schlatt S, De Geyter M, Kliesch S, Nieschlag E, Bergmann M 1995 Spontaneous recrudescence of spermatogenesis in the photoinhibited male Djungarian hamster, Phodopus sungorus. Biol Reprod 53:1169–1177 [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR 2004 Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am J Physiol Regul Integr Comp Physiol 286:R539–R546 [DOI] [PubMed] [Google Scholar]
- Wolfe AM, Turek FW, Levine JE 1995 Blockade of singular follicle-stimulating hormone secretion and testicular development in photostimulated Djungarian hamsters (Phodopus sungorus) by a gonadotropin-releasing hormone antagonist. Biol Reprod 53:724–731 [DOI] [PubMed] [Google Scholar]
- Meredith JM, Turek FW, Levine JE 1998 Effects of gonadotropin-releasing hormone pulse frequency modulation on the reproductive axis of photoinhibited male Siberian hamsters. Biol Reprod 59:813–819 [DOI] [PubMed] [Google Scholar]
- Freeman DA, Larkin JE, Seliby L 2002 Testicular and somatic growth in Siberian hamsters depend on the melatonin-free interval between twice daily melatonin signals. J Neuroendocrinol 14:228–233 [DOI] [PubMed] [Google Scholar]
- Maywood ES, Lindsay JO, Karp J, Powers JB, Williams LM, Titchener L, Ebling FJ, Herbert J, Hastings MH 1991 Occlusion of the melatonin-free interval blocks the short day gonadal response of the male Syrian hamster to programmed melatonin infusions of necessary duration and amplitude. J Neuroendocrinol 3:331–337 [DOI] [PubMed] [Google Scholar]
- Gorman MR 2003 Melatonin implants disrupt developmental synchrony regulated by flexible interval timers. J Neuroendocrinol 15:1084–1094 [DOI] [PubMed] [Google Scholar]
- Gorman MR 2003 Independence of circadian entrainment state and responses to melatonin in male Siberian hamsters. BMC Physiol 3:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GS, Zee PC, Wang-Weigand S, Rodriguez L, Peng X 2008 Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med 4:456–461 [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Poeggeler B, Hardeland R, Cardinali DP 2007 Drug insight: the use of melatonergic agonists for the treatment of insomnia-focus on ramelteon. Nat Clin Pract Neurol 3:221–228 [DOI] [PubMed] [Google Scholar]
- Duffield GE, Hastings MH, Ebling FJ 1998 Investigation into the regulation of the circadian system by dopamine and melatonin in the adult Siberian hamster (Phodopus sungorus). J Neuroendocrinol 10:871–884 [DOI] [PubMed] [Google Scholar]
- Schuhler S, Pitrosky B, Kirsch R, Pévet P 2002 Entrainment of locomotor activity rhythm in pinealectomized adult Syrian hamsters by daily melatonin infusion. Behav Brain Res 133:343–350 [DOI] [PubMed] [Google Scholar]
- Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T 2008 Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA 105:18238–18242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PJ, Hazlerigg DG 2008 Photoperiodic signaling through the melatonin receptor turns full circle. J Neuroendocrinol 20:820–826 [DOI] [PubMed] [Google Scholar]
- Barrett P, Conway S, Jockers R, Strosberg AD, Guardiola-Lemaitre B, Delagrange P, Morgan PJ 1997 Cloning and functional analysis of a polymorphic variant of the ovine Mel1a melatonin receptor. Biochim Biophys Acta 1356:299–307 [DOI] [PubMed] [Google Scholar]
- Drew JE, Barrett P, Williams LM, Conway S, Morgan PJ 1998 The ovine melatonin-related receptor: cloning and preliminary distribution and binding studies. J Neuroendocrinol 10:651–661 [DOI] [PubMed] [Google Scholar]
- Migaud M, Daveau A, Malpaux B 2005 MTNR1A melatonin receptors in the ovine premammillary hypothalamus: day-night variation in the expression of the transcripts. Biol Reprod 72:393–398 [DOI] [PubMed] [Google Scholar]
- Xiao CT, Chu MX, Fu Y, Fang L, Ye SC 2007 Analysis of polymorphism, structure and function of exon 2 of ovine melatonin receptor 1b gene: a clue as to why it lacks expression in sheep. J Pineal Res 42:97–104 [DOI] [PubMed] [Google Scholar]
- Brydon L, Petit L, de Coppet P, Barrett P, Morgan PJ, Strosberg AD, Jockers R 1999 Polymorphism and signalling of melatonin receptors. Reprod Nutr Dev 39:315–324 [DOI] [PubMed] [Google Scholar]
- Chu MX, He YQ, Cheng DX, Ye SC, Fang L, Wang JY 2007 Association between expression of reproductive seasonality and alleles of melatonin receptor 1A in goats. Anim Reprod Sci 101:276–284 [DOI] [PubMed] [Google Scholar]
- Mateescu RG, Lunsford AK, Thonney ML 2009 Association between melatonin receptor 1A gene polymorphism and reproductive performance in Dorset ewes. J Anim Sci 87:2485–2488 [DOI] [PubMed] [Google Scholar]
- Carcangiu V, Mura MC, Vacca GM, Pazzola M, Dettori ML, Luridiana S, Bini PP 2009 Polymorphism of the melatonin receptor MT1 gene and its relationship with seasonal reproductive activity in the Sarda sheep breed. Anim Reprod Sci 116:65–72 [DOI] [PubMed] [Google Scholar]