Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy of reproductive-aged women and is exacerbated by obesity. Exposure of ewes to excess testosterone (T) from d 30–90 of gestation culminates in anovulation, functional hyperandrogenism, LH excess, and polyfollicular ovaries, features similar to those of women with PCOS, with some reproductive defects programmed by androgenic actions of T and others not. Excess weight gain during postnatal life increases the severity of these reproductive defects. Prenatal T-treated ewes also manifest reduced insulin sensitivity, a feature found in more than 70% of PCOS women. We tested the hypotheses that reduced insulin sensitivity of prenatal T-treated ewes is programmed by androgenic actions of T, and excess postnatal weight gain exaggerates this defect. In addition, we tested whether disruptive effects of excess weight gain on insulin sensitivity index are transferred to female offspring. Insulin sensitivity was assessed using iv glucose tolerance tests. Results revealed that disruptive effects of prenatal T excess on insulin sensitivity were programmed by androgenic action of T and postnatal overfeeding-impaired insulin sensitivity in both T-treated and controls and that prenatal T-treated sheep tend to manifest such overfeeding impairments earlier than controls. Importantly, offspring of overweight controls also manifest defects in insulin dynamics supportive of intergenerational transfer of obesity-related traits. The findings are of relevance in the context of developmental programming of insulin resistance by prenatal steroids and excess weight gain.
Fetal exposure to excess androgen and/or postnatal weight gain causes insulin resistance in female lambs with overfeeding-induced insulin resistance carried forward to the next generation.
Polycystic ovary syndrome (PCOS) is one of the most common infertility disorders affecting about 4 million U.S. women. About 70% of women with PCOS manifest insulin resistance (1,2). Insulin-lowering drugs reduce hyperandrogenism (3), implicating a metabolic component in the pathogenesis of the syndrome. Recent research implicates PCOS as a clinical manifestation of metabolic syndrome (4,5,6) with obesity enhancing phenotypic expression (7,8,9,10). Because PCOS mothers are often obese and are at risk for developing gestational diabetes as well as preeclampsia (7), children of PCOS women are more likely to be compromised metabolically. With the prevalence of overweight children increasing substantially in the last 3 decades, childhood obesity has now reached epidemic proportions (8), creating a metabolic platform conducive to adult disorders. In such an environment and considering that PCOS increases the risk of cardiovascular disease, dyslipidemia, hypertension, diabetes mellitus, and endometrial cancer (11), there is particular need to address not only infertility issues but also prevention of type 2 diabetes, heart disease, cancer, and ultimately the transgenerational transfer of unwanted traits to the offspring.
The etiology of PCOS is unknown but appears to have genetic underpinnings (12). In the last decade, enormous strides have been made to document that suboptimal intrauterine conditions, which include exposure to excess native or environmental steroids, program infertility, and numerous metabolic disorders, including insulin resistance, type 2 diabetes, dyslipidemia, and obesity (13,14,15,16). Some believe that androgen excess early in life may provide a hormonal insult that results in the manifestation of PCOS in adulthood (17,18,19,20). For instance, polycystic ovary morphology is highly associated with conditions in which the fetus has been exposed to high amounts of sex steroids before birth (21). Women with classical 21-hydroxylase deficiency, like women with PCOS, exhibit anovulation, ovarian hyperandrogenism, and LH hypersecretion (21). Several animal models have evolved that document developmental exposure to excess testosterone (T), approaching levels found in male fetuses, recreating the PCOS phenotype (22). Interestingly, in humans, fetal serum T levels around midgestation (19–25 weeks) are elevated to those in the normal male fetal range in approximately four of 10 female fetuses sampled as part of a cordiocentesis study (23). Importantly, excess weight gain amplifies the severity of reproductive phenotype in women with PCOS (7,8,9,10) and sheep and monkeys treated prenatally with T (20,24), implicating an interaction between prenatal and postnatal environments in the manifestation of the phenotype. In this regard, it is important to note that administering insulin sensitizing drugs to adult monkeys and sheep treated prenatally with T improves the frequency of ovulatory cycles in the majority of cases (25,26).
Although extensive investigations have been carried out to probe the mechanisms underlying the reproductive disruptions in these animal models, in-depth investigations relative to metabolic input are in their infancy. Studies with rhesus monkeys in their mid- to late reproductive years have found that prenatal T treatment leads to insulin resistance and β-cell defects (27). Studies with prenatal T-treated sheep found that prenatal T treatment causes insulin resistance that can be detected as early as 5 wk of age (28). It still remains to be determined, however, whether developmental programming of insulin resistance is mediated by androgenic actions of T (T can be aromatized to estrogen) and to what extent the postnatal metabolic environment contributes to the manifestation of the defect. The present set of studies was undertaken in sheep to address whether 1) the disruption of insulin homeostasis by prenatal T treatment is programmed by androgenic action, 2) a postnatal overfed state disrupts insulin homeostasis and amplifies the effect of prenatal T, and 3) disrupted insulin homeostasis of the overfed state is transferred to the next generation.
Materials and Methods
All procedures used in these studies were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with the National Institutes of Health Guide for Use and Care of Animals. All studies were conducted at the University of Michigan Research Facility (Ann Arbor, MI; 42° 18′N) using multiparous Suffolk breed of sheep.
Prenatal treatments
Details of prenatal T and dihydrotestosterone (DHT) treatment, husbandry, and nutrition of maternal sheep and newborn lambs and their impact on reproductive function have been published (29,30). Briefly, generation of prenatal T- and DHT-treated females involved injecting pregnant sheep sc twice weekly from d 30–90 of gestation (except study 2 during which they were injected from d 60–90 of gestation) with either 100 mg of T propionate (∼1.2 mg/kg; Sigma Chemical Co., St. Louis, MO) or DHT propionate (Steraloids, Inc., Newport, RI) suspended in 2 ml cottonseed oil. Prenatal T treatment produces circulating concentrations of T in pregnant sheep and umbilical arterial blood comparable with that seen in intact adult males and 60-d-old male fetuses, respectively (31).
Diet and postnatal treatment
All feeds dispensed to the breeding ewes and offspring were purchased in bulk to prevent potential confounding effects from differences in feeds. Starting 6 wk before lambing and continuing until lambing, pregnant ewes were group fed 0.5 kg shelled corn, 2 kg alfalfa hay, and 250 mg aureomycin crumbles (chlortetracycline)/ewe · d. Lactating ewes were provided a ration of 1 kg shelled corn and 2–2.5 kg of alfalfa hay/ewe · d. Until 3 months of age, all lambs had ad libitum access to commercial feed pellets (Shur-Gain, Elma, NY; contains 18% crude protein) and alfalfa hay. All lambs and ewes were provided with water and minerals ad libitum and were treated regularly with antihelminthics to minimize parasitic infection. Female offspring born from singleton or twin pregnancies were used in the following studies. For all studies, when twins were involved, they were split across treatments such that only one offspring from a given dam was used within a given treatment.
Beginning at 14 wk of age, all offspring except those assigned to be overfed (a subset of control and prenatal T treated females in study 3, below) were fed 0.64 kg corn, 0.64 kg hay/lamb per day and 0.014 kg of supplement (36% crude protein). The overfed groups were maintained and group fed separately and their diet consisted of 0.77 kg corn, 0.014 kg of supplement, and 0.73 kg hay/lamb per day initially and then ad libitum. The diet for all except the overfed females was designed to achieve optimal growth without excess fat deposition, whereas the overfed diet was targeted to achieve a body weight that was about 25–30% above that of maintenance-fed females (24). To compensate for increased energy demands as the lambs grew and to meet additional energy demands during inclement weather, the ration was increased in all groups of females by the same percentage. Offspring resulting from breeding control and overfed controls were fed maintenance ration. Anogenital to anonavel distance of offspring, a bioassay for prenatal T/DHT treatment, was recorded a day after birth and ratios calculated to determine the extent of virilization of the external genitalia.
Experimental design
Study 1: androgenic programming of insulin sensitivity
A comparative approach of studying prenatal T (aromatizable androgen) and DHT (nonaromatizable androgen) treatment was used to determine whether the effects of prenatal T in reducing insulin sensitivity is programmed by its androgenic actions. Control (n = 12) prenatal T- (n = 16) and prenatal DHT-treated (n = 7) females underwent an iv glucose tolerance test (IVGTT) at 11 ± 2 (mean ± sem) wk of age. Basal insulin and glucose levels were measured in samples taken at −15, −10, −5, and −1 min and 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 min after administration of 300 mg/kg glucose (50% dextrose iv sterile solution in bag; Hospira Worldwide Inc., Lake Forest, IL) iv.
Study 2: midgestational programming of insulin sensitivity
To determine whether a shorter and later window of T treatment from d 60 to 90 of gestation (referred to as T60-90 females) would produce insulin resistance, adult control (n = 8) and T60-90 (n = 11) females were subjected to a modified IVGTT at 22 months of age. After fasting overnight, blood samples were collected in sodium fluoride/potassium oxalate tubes (BD Vacutainer, Franklin Lakes, NJ) from both groups of females at −30 and 0 min to establish basal insulin and glucose levels. A bolus injection of glucose (300 mg/kg body weight) was administered via an indwelling jugular catheter and blood samples obtained at 5, 10, 20, 30, 40, 50, 60, 90, and 120 min.
Study 3: impact of postnatal obesity on insulin sensitivity
To determine whether overfeeding would reduce insulin sensitivity and amplify the disruptive effects of prenatal T treatment on insulin sensitivity, control (n = 8), overfed control (OFC, n = 7), prenatal T (n = 8), and overfed prenatal T (OFT, n = 7) females underwent an IVGTT at 20 ± 2 (prepubertal) and 28 ± 2 wk (pubertal) of age following procedures detailed for study 1. The controls and prenatal T-treated animals used in this study are the same as those used in study 1, barring inclusion of three additional controls (two in control and one in OFC group). Due to their small numbers, prenatal DHT-treated females were not included for postnatal overfeeding studies.
Study 4: insulin dynamics in offspring of overfed females
To determine whether the deleterious effects of excess weight gain on insulin sensitivity is transferred to the next generation, control (n = 6) and OFC (n = 6) females from study 3 were bred and their offspring studied. Only three female offspring from control and OFC groups resulted from this breeding. All animals were weighed at weekly intervals to assess their growth trajectory. All animals were tested prepubertally (11 wk) and as adults (60 wk) following procedures described in study 1. It was not possible to perform similar transgenerational studies with the prenatal T-treated females because their virilized status prevents natural mating.
Hormone measures
Plasma glucose levels were measured by the glucose oxidase method (Pointe Scientific, Inc., Canton, MI). The inter- and intraassay coefficients of variation (CVs) for glucose measures measured at 50 and 200 mg/dl were both less than 5.0% (n = 74 assays). Plasma insulin levels were measured by RIAs (MP Biomedicals, Orangeburg, NY). The sensitivity of the insulin assay was 2.73 ± 0.39 μU/liter (n = 22 assays; mean ± sem). Mean intraassay CVs based on two quality-control pools measuring 38.4 ± 0.8 and 131.2 ± 2.8 μU/liter were 5.1 ± 0.6 and 7.8 ± 0.7%, respectively. The corresponding interassay CVs averaged 9.9 and 10.7%.
Statistical analysis
Basal insulin, glucose, and insulin to glucose ratio (I to G ratio) were calculated by averaging the values obtained before glucose administration. Acute insulin response was calculated as the increment of insulin within 2 min of glucose administration (28). Cumulative insulin and cumulative insulin to glucose ratio were calculated from measures during the first 20 min of the IVGTT. The incremental area under the curve (AUC) of insulin, calculated as the difference between the basal AUC and the AUC of insulin secreted in response to the glucose administration during the first 20 min of the IVGTT were calculated with the trapezoidal formula, as reported earlier (28,32). Insulin sensitivity index was calculated using the formula adapted from Matsuda and De Fronzo (33) in which insulin sensitivity index = 10,000/square root of [(fasting glucose × fasting insulin) × (mean glucose × mean insulin during the first 20 min of the IVGTT)]. Plasma glucose and insulin pharmacokinetic responses to the IVGTT were evaluated by calculating the fractional turnover rate or clearance rate (k) (34) using the incremental plasma glucose and insulin concentrations relative to baseline values between 8 and 19 min. k between time1 and time2 (percent min−1) = [ln(x1) − ln(x2)/(time2 − time1)] × 100, where x is glucose or insulin level at a given time point.
Differences in basal glucose, insulin, and insulin to glucose ratio as well as cumulative insulin and insulin to glucose ratio, acute insulin response, AUC, and insulin sensitivity index were compared using ANOVA (studies 1 and 3) or independent-samples t test (studies 2 and 4) after appropriate transformations to account for heterogeneity of variances. Multiple comparisons were tested using least significant differences or Bonferroni post hoc tests. Univariate general linear model was used to test differences in the homogeneity of the regression slopes between cumulative insulin and weight.
Linear random-effect mixed model was used to capture the weight growth curves and test difference among groups. It was assumed that the growth curve of each group followed a linear, quadratic, or cubic function. Random effects were modeled to account for correlations between weight measurements from the same animal. Significance was defined as P < 0.05. All results are presented as mean ± sem. All analyses were carried out using SPSS for Windows release 16.0.0 or using SAS for Windows release 9.1.3 (SAS Institute Inc., Cary, NC).
Results
Study 1: androgenic programming of insulin sensitivity
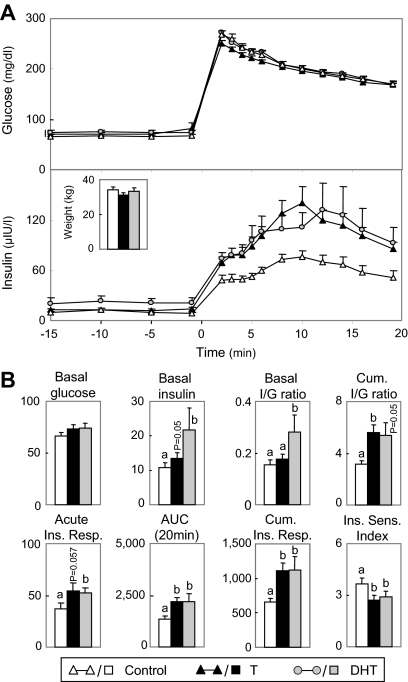
Birth weights of prenatal T-treated (4.5 ± 0.2 kg), but not DHT-treated (6.1 ± 0.3 kg), female offspring were reduced (P < 0.05) relative to controls (6.2 ± 0.3 kg). The anogenital to anonavel distance ratio was increased (P < 0.05) in prenatal T- (0.88 ± 0.01) and DHT-treated (0.79 ± 0.03) females compared with controls (0.09 ± 0.01). Body weights of control and prenatal T- and DHT-treated females were similar at the time of IVGTT (Fig. 1A, insert). Basal glucose levels did not differ between treatment groups (Fig. 1A and summary statistics in Fig. 1B). Basal insulin (tendency, P = 0.05), acute (tendency, P = 0.057), and cumulative insulin response, area under the insulin response curve, cumulative I to G ratio were all higher in prenatal T-treated females. Basal insulin, basal I to G ratio, acute and cumulative insulin response, area under the insulin response curve, cumulative I to G ratio (tendency, P = 0.05) were all higher in prenatal DHT-treated females compared with controls. Insulin sensitivity index was significantly lower in both prenatal T- and DHT-treated females.
Figure 1.
Comparison of insulin dynamics of control females (n = 12) and females treated prenatally from d 30 to 90 of gestation with T propionate (n = 16) or DHT propionate (n = 7) at 11 wk of age. A, Circulating patterns of glucose and insulin before and after administration of 300 mg/kg glucose via an indwelling catheter. Insert (bar graph), Body weight of control, prenatal T- and DHT-treated females at the time of the IVGTT. B, Summary bar graphs for the different variables: basal glucose (milligrams per deciliter), basal insulin (microunits per liter), basal I to G (I/G) ratio, cumulative I to G ratio, acute insulin response (microunits per liter), AUC of insulin response (AUC at 20 min) (microunits per liter), cumulative insulin response (Cum. Ins. Resp.;/Ins. Resp; microunits per liter), and insulin sensitivity index. Differing letters (a vs. b) indicate significant differences (P < 0.05).
Study 2: midgestational programming of insulin sensitivity
At the time of study, the T60-90 females were marginally heavier (P < 0.05) than controls (Fig. 2A, inset). In previous studies, T treatment from d 60 to 90 of gestation was found not to affect anogenital ratio (35). Circulating patterns of glucose and insulin from control and T60-90 females, before and after the glucose challenge, are shown in Fig. 2A (left panel). For comparison, the responses (comparable time points during the first 15 min) of females from a different cohort treated with T from d 30 to 90 of gestation are shown on the right. Basal insulin and fasting I to G ratio were higher in T60-90 females compared with controls (P < 0.05) (Fig. 2B, left panels). Cumulative insulin response and cumulative I to G ratio (tendency, P < 0.08) during the first 20 min after glucose challenge were higher and insulin sensitivity index was reduced (P < 0.05) in T60-90 females compared with controls. Cross-correlation between body weight and insulin sensitivity indexes revealed that the prenatal T-induced changes were independent of weight gained. Basal I to G ratio, cumulative insulin response, and cumulative I to G ratio were also higher and insulin sensitivity index lower (tendency, P < 0.07) in animals treated from d 30 to 90 of gestation (Fig. 2B, right panels), despite body weights being similar.
Figure 2.
Comparison of insulin dynamics of adult controls (n = 8) and females treated prenatally from d 60 to 90 of gestation with T (n = 11) are shown on the left. For comparison responses of adult sheep treated prenatally from d 30 to 90 of gestation with T and corresponding controls to a truncated IVGTT are shown on the right (note the 60–90 and 30–90 gestational T treated animals were from different breeding cohorts). A, Circulating patterns of glucose and insulin before and after administration of 300 mg/kg glucose via an indwelling catheter. Inserts (bar graphs), Body weight of control (open bar) and prenatal T-treated females (closed bar) at the time of the test. B, Summary bar graphs for the different variables: basal glucose (milligrams per deciliter), basal insulin (microunits per liter), basal I to G (I/G) ratio, cumulative insulin response (Cum. Ins. Resp.; microunits per liter), cumulative I to G ratio and insulin sensitivity (Ins. Sens.) index in control (open bars) and prenatal T-treated females (closed bars). *, Significant differences (P < 0.05).
Study 3: impact of postnatal obesity on insulin sensitivity
As one would expect from overfeeding, the growth rates of OFC and OFT females were higher (P < 0.0001) compared with control and prenatal T-treated females. Puberty, as defined by the first increase in progesterone above 0.5 ng/ml measured in twice-weekly samples, occurred at 27.8 ± 0.4, 27.3 ± 1.3, 27.0 ± 1.0, and 26.5 ± 1.5 wk in control, OFC, T, and OFT groups, respectively, and did not differ between the groups.
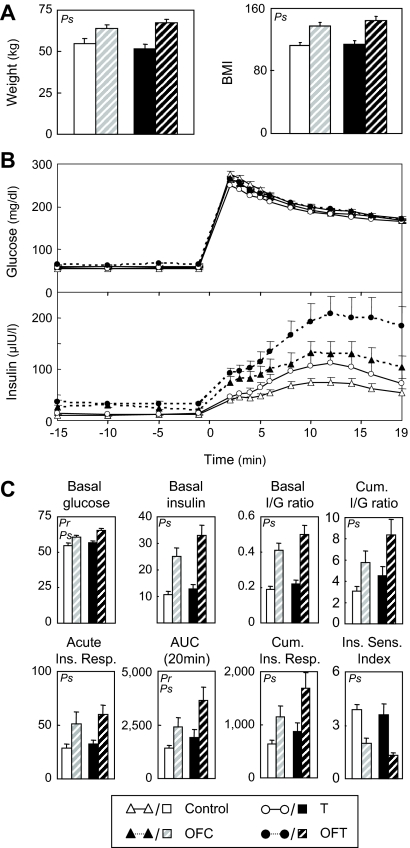
At 20 wk of age (first IVGTT), overfed females were marginally heavier (P < 0.05) (Fig. 3A, insert) compared with the non-overfed females. ANOVA revealed a significant prenatal T effect manifested as increases in cumulative I to G ratio, cumulative insulin response, and AUC and a significant weight gain effect on insulin sensitivity index (Fig. 3B). The body weights and body mass index (BMI) of overfed females at 28 wk were greater than the nonoverfed group (Fig. 4). ANOVA revealed significant prenatal T effects (some variables), significant effects of overfeeding (most variables), but no significant interactions (no overfeeding enhancement of prenatal T effects) (Fig. 4). Basal glucose and AUC of insulin response were higher in prenatal T-treated females compared with controls. Overfeeding increased basal glucose, basal insulin levels, and basal I to G ratios relative to those that were not overfed (Fig. 4). Acute insulin response, cumulative insulin response, AUC, and cumulative I to G ratio were all higher and insulin sensitivity index lower in overfed females compared with those maintained on the original diet.
Figure 3.
Comparison of insulin dynamics of control (n = 8), OFC (n = 7), prenatal T-treated (n = 8), and OFT (n = 7) females at 20 wk of age. These four test groups include the control and prenatal T-treated females used for comparison with DHT females at 11 wk of age (Fig. 2) plus three additional controls. The OFC and OFT females received additional ration starting at 14 wk of age to increase their body weight to about 25% above that of controls. Inset (bar graphs), Body weight at the time of the IVGTT (20 wk). A, Circulating patterns of glucose and insulin before and after administration of 300 mg/kg glucose via an indwelling catheter for the four groups. B, Summary bar graphs of the different variables: basal glucose (milligrams per deciliter), basal insulin (microunits per liter), basal I to G ratio, cumulative I to G ratio, acute insulin response (microunits per liter), AUC of insulin response [(microunits per liter); AUC at 20 min], cumulative insulin response (microunits per liter), and insulin sensitivity index. For each variable, Pr indicates prenatal T treatment effect was significant; Ps indicates postnatal overfeeding effect was significant. There was no significant prenatal/postnatal treatment interaction.
Figure 4.
Comparison of insulin dynamics of control (n = 8), OFC (n = 7), prenatal T-treated (n = 8), and OFT (n = 7) females at 28 wk of age (details in legend of Fig. 3). A, Bar graphs of body weight and BMI at the time of the IVGTT (28 wk). B, Circulating patterns of glucose and insulin before and after administration of 300 mg/kg glucose via an indwelling catheter for the four groups. C, Summary bar graphs for the different variables: basal glucose (milligrams per deciliter), basal insulin (microunits per liter), basal I to G ratio, cumulative I to G ratio, acute insulin response (microunits per liter), AUC of insulin response [(microunits per liter); AUC at 20 min], cumulative insulin response (microunits per liter), and insulin sensitivity index. For each variable, Pr indicates prenatal T treatment effect was significant; Ps indicates postnatal overfeeding effect was significant. There was no significant prenatal/postnatal treatment interaction.
Study 4: insulin dynamics in offspring of overfed females
Birth weights of offspring of OFC females (6.5 ± 0.4 kg) tended to be higher (P = 0.064) than the offspring of controls (4.6 ± 0.6 kg). The anogenital ratio did not differ between groups (offspring of control: 0.07 ± 0.01; offspring of OFC: 0.07 ± 0.01). The growth curves of offspring of both control and OFC dams followed a cubic function, although the trajectories of these two curves differed (Fig. 5A, left panel). The growth rate of the offspring from OFC dams was significantly greater than the offspring of controls (0.37 kg more per week) through the first 20 wk of age (P < 0.0001). At wk 20, the growth rate of the offspring from overfed dams was still significantly higher (0.18 kg more per week) than that in offspring from control dams (P < 0.0001). By wk 40, the rate of growth in offspring of OFC females had slowed and did not differ from the rate of growth of offspring of controls (P = 0.66). Comparison of weight at the time of performance of the two IVGTTs (11 and 60 wk) found that body weights (Fig. 5A, right panel) and BMI (not shown) of offspring of control and OFC dams did not differ. There were no significant differences in any of the variables during the first IVGTT (Fig. 5C). At 60 wk of age, the cumulative insulin response, area under the curve of insulin response, and cumulative I to G ratio of offspring of OFC females were higher (P < 0.05) in offspring of OFC dams compared with the offspring of control dams (Fig. 5C).
Figure 5.
Comparison of growth trajectory and insulin dynamics of offspring of control (n = 3) and OFC (n = 3) females used in study 3 (Figs. 3 and 4). A, Growth trajectory of the two groups of offspring (left). Arrows indicate timing of performance of the two IVGTTs. The weights of offspring of control and OFC females at the time of the performance of the two IVGTT are shown on the right. B, Circulating patterns of glucose and insulin before and after administration of 300 mg/kg glucose during the two IVGTTs. C, Summary bar graphs of for the different variables: basal glucose (milligrams per deciliter), basal insulin (microunits per liter), basal I to G (Basal I/G) ratio, cumulative I to G (Cum. I/G) ratio, acute insulin response (Ins. Resp.; microunits per liter), AUC of insulin response [(microunits per liter); AUC at 20 min], cumulative insulin response (microunits per liter), and insulin sensitivity (Sens.) index. For each variable, the results from both IVGTTs are shown side by side (1 and 2). *, Significant differences (P < 0.05).
Insulin and glucose clearance
Figure 6 summarizes the developmental changes in insulin and glucose clearance in control and prenatal T-treated females (before and after overfeeding) as well as the first-generation offspring of control and overfed dams. Prenatal T treatment had no effect on glucose/insulin clearance at all three ages studied. Overfeeding reduced glucose clearance at 20 wk and insulin clearance at 28 wk of age. No significant changes in glucose clearance were evident in offspring of control and OFC mothers at both time points studied. In contrast, despite the smaller number studied, insulin clearance was reduced in offspring of OFC females as early as 11 wk of age. By 60 wk of age, clearance of insulin had diminished to such an extent that this variable could not be calculated in offspring of OFC females (insulin levels continued to remain high at the end of study). Neither of these variables were affected in prenatal DHT-treated females and the T60-90 females (not shown).
Figure 6.
Comparison of glucose clearance (top panels) and insulin clearance (bottom panels) of control (C) and prenatal T-treated (T) females before weaning (11 wk) and after dietary manipulation (20, 28 wk) are shown in the three left panels (calculated from data presented in Figs. 1, 3, and 4). Same variables for offspring of control and OFC females at 11 and 60 wk of age are shown in the right two panels (calculated from data presented in Fig. 5). *, Significant differences (P < 0.05).
Discussion
Insulin, a major regulator of glucose homeostasis, plays a key stimulatory role in fetal organ growth and development (36). Metabolic defects such as insulin resistance and impaired pancreatic β-cell function are established risk factors in the development of premature onset of type 2 diabetes mellitus in women with PCOS (11). Findings from the present study extend earlier findings in sheep (28) and provide evidence that the programming of insulin resistance is mediated via androgenic actions of T and point to gestational d 60–90 as the critical period for this programming. Furthermore, our findings document the rapid initiation of deleterious effects of neonatal overfeeding on insulin sensitivity within weeks of its initiation and the transfer of traits to the next generation. The results of these findings and their implications for women with PCOS and other metabolic disorders are discussed below.
Effects of prenatal T and DHT excess
Findings that insulin sensitivity was reduced in 11-wk-old (first developmental time point studied) prenatal T-treated females corroborate our previous findings in 5-wk-old females (28). The finding that disruptive effects of prenatal T excess at 28 wk of age was limited to AUC at 20 min may relate to controls also becoming insulin resistant at this age; puberty in this cohort of animals occurred at approximately 27 wk of age. In humans, insulin sensitivity decreased around puberty and increased postpubertally, only to decrease again due to aging (37).
The findings that the effects of prenatal T on insulin sensitivity were mimicked by prenatal DHT treatment supports the premise that the effects of prenatal T excess are mediated by androgenic actions of T. This is consistent with findings from androgen receptor knockout mice documenting a key role for androgen receptor in the spontaneous development of insulin resistance (38). Euglycemic-hyperinsulinemic clamp studies in female rats treated postnatally with T or DHT (within 3 h of birth) also resulted in reduced insulin sensitivity in adulthood (39). Whereas DHT can be metabolized to 3-β-diol and act through the estrogen receptor-β (ERβ /ESR2) (40), this does not appear likely because metabolic perturbations are evident in ERα (ESR1) but not ERβ null mice (41). Our ongoing studies also indicate that prenatal estradiol treatment improves insulin sensitivity (42).
The finding of reduced insulin sensitivity at 11 wk of age in the absence of changes in glucose indicates that the defect is likely present at the target tissue level and the pancreas is able to adequately compensate by secreting sufficient insulin to overcome insulin resistance. These findings differ from prenatal T-treated monkeys, which manifest increased insulin sensitivity during infancy (43), suggestive of a hyperresponsive pancreas. Conceivably, prenatal T excess in both monkeys and sheep has an impact at multiple levels, which include insulin target tissues and pancreatic β-cells. The adaptive response of the pancreas is initially to compensate by oversecreting insulin. With progressive increase in severity of insulin resistance, the β-cells may lose the capacity to adequately compensate (44). The differential responses of monkeys (increased insulin sensitivity and secretion in neonates, and glucose intolerance and impaired insulin secretion in older monkeys) and sheep (insulin resistance and ability to compensate during prepubertal life) may represent different time points in this altered trajectory. If so, development of insulin resistance in the prenatal T-treated sheep at 11 wk of age places them at risk of developing glucose intolerance/type 2 diabetes later in life, as suggested by studies with midreproductive-aged monkeys (27,45). The developmental time points at which these changes in trajectory of pathophysiology occur may differ between species, depending on differences in timing of differentiation of organs being reprogrammed and postnatal somatic maturation. It should be recognized that the ages when sheep were assessed in this study (11, 20, and 28 wk) encompass the pre-/peripubertal periods when control animals also become insulin resistant as they approach puberty (46). The finding that prenatal T-treated rats manifest hyperinsulinemia, although IVGTTs reveal no differences in insulin-glucose dynamics (47), may also relate to timing of study relative to development of the phenotype.
The finding of reduced insulin sensitivity in both the 60- to 90- and 30- to 90-d gestational T treatment groups suggests that gestational d 60–90 as the critical period for programming insulin resistance in sheep. In rhesus monkeys, late gestational T treatment reduced insulin sensitivity (27), whereas early gestational T treatment resulted in β-cell defects (27,43), a feature not evident in prepubertal sheep. These differences may relate to the developmental time points when such studies were conducted (peripubertal in sheep vs. mid- to late reproductive years in monkeys) or that other glucose control mechanisms are operational in sheep (48). Whereas both gestational T treatments reduced insulin sensitivity, their effects on ovarian and neuroendocrine programming differed suggestive of differing critical period of susceptibility of various organ systems. T treatment from d 30–90 but not d 60–90 (Padmanabhan, V., and S. Roy, unpublished data) of gestation resulted in early ovarian follicular depletion (49) and profound disruption of neuroendocrine feedback systems and ovulatory frequency (50).
The finding that prenatal T excess leads to hyperinsulinemia (28) suggests that insulin target tissues such as liver, muscle, and adipose tissue are being reprogrammed during development. Whereas glucose intolerance was not evident at the time points studied in the prenatal T-treated females, it is conceivable that the hyperinsulinemic status evident at 11 wk of age will lead to glucose intolerance later in adulthood. Earlier studies have found that elevated insulin during perinatal life, which in itself can be developmentally programmed, increases the risk of obesity and type 2 diabetes in adulthood (51). Although BMI and body weight were similar between control and prenatal T-treated ewes in this study, in the absence of sensitive measurements of visceral fat and fat partitioning, the contributory role of increased abdominal adiposity in mediating insulin resistance cannot be ascertained. Preliminary observations during surgeries indicate an increased amount of abdominal fat in the prenatal T-treated females. Altered secretion of inflammatory and immune mediators such as adipokines, free fatty acid toxicity, and differences in abdominal (visceral) vs. sc fat are implicated in the pathogenesis of obesity-related insulin resistance and increased risk of diabetes (52,53,54).
Effects of overfeeding
Findings of higher basal glucose concentrations and hyperinsulinemia evidenced in overfed prepubertal sheep in this study follow a similar pattern as found in Dorset sheep made obese later in life (55); adult sheep overfed for over 1 yr doubled their weight and were hyperinsulinemic and hyperglycemic. Our studies involving a body weight increase of 30% in prepubertal lambs found a reduction in insulin sensitivity within 6 wk of overfeeding with a higher degree of hyperinsulinemia and higher fasting glucose concentrations at 17 wk of overfeeding. These findings are consistent with human studies that have found a positive relation between hyperinsulinemia and insulin resistance with a greater degree of compensatory hyperinsulinemia accompanying more severe insulin resistance (55).
The rapidity with which overfeeding affected insulin sensitivity (within 6 wk of initiation of overfeeding), and the subsequent development of hyperglycemia at 17 wk from initiation, raises concerns relative to the detrimental effects of continued overfeeding and progressive deterioration of insulin-glucose homeostasis. Our finding of reduced insulin clearance after 17 wk of overfeeding is supportive of hepatic dysfunction in overfed animals. Studies in obese subjects and women with PCOS have found reduced hepatic clearance as a contributing factor in the development of the hyperinsulinemic status (56,57,58). The effects of overfeeding differed from the effects of prenatal T excess in that overfed females manifested fasting hyperglycemia suggestive of failure of β-cells to compensate. Current trends in understanding the mechanisms of type 2 diabetes and its prestages indicate that abnormalities in fatty acid metabolism may lead to lipotoxicity and impairment of normal function (59). Lipotoxicity is thought to originate from lipid spill-over to nonadipose tissues such as liver from overaccumulation of unoxidized long-chain fatty acids in adipose tissue (60).
Interaction between prenatal T excess and postnatal overfeeding
At the prepubertal time points studied, a significant interaction between prenatal T excess and postnatal overfeeding was not apparent. Whether such an interaction would emerge as the animal ages and β-cells fail to compensate remains to be ascertained. Interestingly, the insulin response of OFT females relative to prenatal T-treated females appeared to be numerically higher relative to differences seen between OFC and controls. This, however, did not reach statistical significance due to the small number of animals studied.
F1 offspring of overfed animals
The findings that offspring of overfed animals manifest increased insulin response to IVGTT parallel other animal models of maternal obesity-induced insulin resistance (61,62). The finding that offspring of prenatal T-treated females having low birth weights as well as offspring of overfed animals, which tended to be heavier at birth manifest insulin resistance corroborate the premise that both ends of the spectrum are detrimental to postnatal metabolic homeostasis. Conceivably, the altered metabolic environment during gestation stemming from excess weight gain may have induced defects in peripheral target tissues (liver, pancreas, adipose tissue) contributing to insulin defects seen in the F1 generation. Further studies with a larger sample size are required to dissect out the mediators of this programming. Whether the insulin resistance induced by prenatal T-treated females would also be transferred to the next generation remains to be determined. Studies documenting hyperinsulinemia and insulin resistance in offspring of women with PCOS also support transgenerational transfer of such traits (63).
Relevance
The findings of insulin resistance in prenatal T-treated females and offspring of overweight dams are of translational relevance in the context of small and large for gestational babies as well as postnatal catch up growth (64,65,66). Whereas the extent to which the low birth weight of prenatal T-treated females or their subsequent catch-up growth (29) contribute to the developing insulin resistance is unclear, evidence exits linking low birth weight and/or increased postnatal growth rate to later development of insulin resistance/glucose intolerance/type 2 diabetes (64,65). Interestingly, prenatal T-treated monkeys, which had normal birth weights, showed an increased postnatal growth rate suggesting that this may be a common mediary of development of insulin resistance in the two models (43).
Because the reproductive and metabolic phenotype of prenatal T-treated sheep recapitulates characteristics of women with PCOS (19,22,24), the likelihood of amplification of insulin defects after overfeeding in prenatal T-treated females are of translational relevance to women with PCOS. It is difficult to directly compare the findings in prenatal T-treated sheep studied during the prepubertal period with findings in adult women with PCOS, especially because stratification of PCOS women involves both lean and obese individuals. The trend in early deviation in the degree of hyperinsulinemia between overweight and normal prenatal T-treated females relative to overweight and normal controls within 6 wk of overfeeding suggests that the prenatal T-treated females may be prone to early susceptibility. It is conceivable with advancing age and/or further weight gain an interaction between prenatal T-treatment and postnatal weight gain may emerge. At the prepubertal time points studied, the pancreas of prenatal T-treated sheep appears to be capable of compensating adequately. The early onset of insulin defects with 6 wk of overfeeding in neonatal lambs and its progression to a more severe phenotype (development of hyperglycemia) also highlight concerns relative to the childhood obesity epidemic and the need for early intervention.
In summary, findings from this study provide support for androgenic programming of insulin resistance, define 60–90 d of gestation as the window of susceptibility for developing insulin resistance, highlight the deleterious effects of postnatal weight gain on insulin dynamics and glucose homeostasis, and importantly, the transfer of deleterious traits to the subsequent generation. The findings are of translational significance to women with PCOS, the reproductive and metabolic phenotype of whom the prenatal T-treated sheep recapitulate, and to the human obesity epidemic.
Acknowledgments
We thank Douglas Doop for assistance with breeding/lambing, his expert and conscientious animal care, and Sheep Research Facility management. The study, which spanned over 5 yr, would not have been possible without committed participation of the multitude of postdoctoral fellows (Mohan Manikkam, Teresa Steckler, Hiren Sarma, Wen Bo Yan, Shar Kavoussi), undergraduate and graduate students (Anastasia Alekseyev, Olga Astapova, Andrea Bellamy, Gwen Louis, Crystal Rosser, Jodie Woznica), and research staff (James Dell'Orco, Jonathan Flak, James Lee, Pamela Olton) helping with prenatal steroid treatment, weekly weights, performance of IVGTTs, which required a ratio of one person per animal during sampling, and/or glucose and insulin measurements. We thank Dr. Stephen Franks (Imperial College of London) for his help relating the findings to PCOS and advice/encouragement of Dr. Andrea Dunaif (Northwestern University) in initiating the metabolic studies addressing the effects of prenatal T excess.
Footnotes
This work was supported by United States. Public Health Service Grant P01 HD44232 (to V.P.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 4, 2009
Abbreviations: AUC, Area under the curve; BMI, body mass index; CV, coefficient of variation; DHT, dihydrotestosterone; I to G ratio, insulin to glucose ratio; IVGTT, iv glucose tolerance test; PCOS, polycystic ovary syndrome; T, testosterone.
References
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE 2007 Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 92:4546–4556 [DOI] [PubMed] [Google Scholar]
- Lord JM, Flight IH, Norman RJ 2003 Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, d-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev 3:CD003053 [DOI] [PubMed] [Google Scholar]
- Essah PA, Nestler JE 2006 The metabolic syndrome in polycystic ovary syndrome. J Endocrinol Invest 29:270–280 [DOI] [PubMed] [Google Scholar]
- Sam S, Dunaif A 2003 Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab 14:365–370 [DOI] [PubMed] [Google Scholar]
- Barber TM, McCarthy MI, Franks S, Wass JA 2007 Metabolic syndrome in polycystic ovary syndrome. Endokrynol Pol 58:34–41 [PubMed] [Google Scholar]
- Diamanti-Kandarakis E 2007 Role of obesity and adiposity in polycystic ovary syndrome. Int J Obes (Lond) 31(Suppl 2):S8–S13; discussion S31–S32 [DOI] [PubMed] [Google Scholar]
- Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ 4 December 2009 Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 10.1016/j.fertnstert.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Fauser BC, Macklon NS 2008 Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med 26:72–84 [DOI] [PubMed] [Google Scholar]
- MacPhee M 2008 Global childhood obesity: how to curb an epidemic. J Pediatr Nurs 23:1–4 [DOI] [PubMed] [Google Scholar]
- Hart R, Norman R 2006 Polycystic ovarian syndrome—prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol 2006 20:751–778 [DOI] [PubMed] [Google Scholar]
- Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss 3rd JF, Dunaif A, Spielman RS 2006 Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab 91:4112–4117 [DOI] [PubMed] [Google Scholar]
- Barker DJP 1994 Programming the baby. In: Mothers, babies, and disease in later life. London: BMJ Publishing Group; 14–36 [Google Scholar]
- Nijland MJ, Ford SP, Nathanielsz PW 2008 Prenatal origins of adult disease. Curr Opin Obstet Gynecol 20:132–138 [DOI] [PubMed] [Google Scholar]
- Devaskar SU, Thamotharan M 2007 Metabolic programming in the pathogenesis of insulin resistance. Rev Endocr Metab Disord 8:105–113 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN 2007 Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res 51:912–917 [DOI] [PubMed] [Google Scholar]
- Apter D 1998 Endocrine and metabolic abnormalities in adolescents with a PCOS-like condition: consequences for adult reproduction. Trends Endocrinol Metab 9:58–61 [DOI] [PubMed] [Google Scholar]
- Davies MJ, Norman RJ 2002 Programming and reproductive functioning. Trends Endocrinol Metab 13:386–392 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V 2007 Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW 1998 Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab 9:62–67 [DOI] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM 1994 Ovarian hyperandrogenism as a result of congenital adrenal virilizing disorders: evidence for prenatal masculanization of neuroendocrine function in women. J Clin Endocrinol Metab 79:1328–1333 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V 2006 Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, eds. Contemporary endocrinology: androgen excess disorders in women: polycystic ovary syndrome and other disorders. 2nd ed. Totowa, New Jersey: Humana Press Inc.; 259–272 [Google Scholar]
- Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ 1991 Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common α-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab 73:525–532 [DOI] [PubMed] [Google Scholar]
- Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V 2009 Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity—implication to PCOS. Endocrinology 150:1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH 2007 Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol 23:438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Herkimer C, Alekseyev A, Padmanabhan V, Developmental programming: rosiglitazone, an insulin sensitizer, improves insulin sensitivity and reproductive cycles in prenatal testosterone-treated female sheep. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008, p 51 (Abstract P2-495) [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH 2000 Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab 85:1206–1210 [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T 2005 Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol (Endocrinol Metab) 289:801–806 [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V 2004 Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 145:790–798 [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V 2007 Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology 148:3532–3540 [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch K, MohanKumar PS, Padmanabhan V 2009 Developmental programming: impact of prenatal testosterone excess on maternal and fetal steroid milieu. Biol Reprod 81:84 [Google Scholar]
- Huopio H, Jääskeläinen J, Komulainen J, Miettinen R, Kärkkäinen P, Laakso M, Tapanainen P, Voutilainen R, Otonkoski T 2002 Acute insulin response tests for the differential diagnosis of congenital hyperinsulinism. J Clin Endocrinol Metab 87:4502–4507 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Kaneko JJ 1989 Carbohydrate metabolism and its diseases. In: Kaneko JJ, ed. Clinical biochemistry of domestic animals. 4th ed. San Diego: Academic Press; 65–68 [Google Scholar]
- Wood RI, Foster DL 1998 Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod 3:130–140 [DOI] [PubMed] [Google Scholar]
- Ballard PL 2005 Hormonal influences on fetal development. In: Taeusch HW, Ballard RA, Gleason CA, Avery ME, eds. Avery’s diseases of the newborn. 8th ed. Philadelphia: Elsevier Saunders; 46–56 [Google Scholar]
- Goran MI, Gower BA 2001 Longitudinal studies on pubertal insulin resistance. Diabetes 50:2444–2450 [DOI] [PubMed] [Google Scholar]
- Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C 2005 Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes 54:1717–1725 [DOI] [PubMed] [Google Scholar]
- Alexanderson C, Eriksson E, Stener-Victorin E, Lystig T, Gabrielsson B, Lönn M, Holmäng A 2007 Postnatal testosterone exposure results in insulin resistance, enlarged mesenteric adipocytes, and an atherogenic lipid profile in adult female rats: comparisons with estradiol and dihydrotestosterone. Endocrinology 148:5369–5376 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L 2008 An alternate pathway for androgen regulation of brain function: activation of estrogen receptor β by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav 53:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A 2006 Evidence that oestrogen receptor α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49:588–597 [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Padmanabhan V, Developmental programming: opposing effects of prenatal testosterone and estradiol excess on insulin sensitivity and reproductive cyclicity in sheep and modulation by postnatal estradiol. Program of the 91st Annual Meeting of The Endocrine Society, Washington, DC, 2009 (Abstract P1-276) [Google Scholar]
- Abbott DH, Goodfriend TL, Dunaif A, Muller SJ, Dumesic DA, Increased body weight and enhanced insulin sensitivity in infant female rhesus monkeys exposed to androgen excess during early gestation. Program of the 89th Annual Meeting of The Endocrine Society, Toronto, Canada, 2007, p 417 (Abstract P2-348) [Google Scholar]
- Bergman RN 1989 Lilly lecture 1989: toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 38:1512–1527 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Abbott DH 2005 Early origins of polycystic ovary syndrome. Reprod Fertil Dev 17:349–360 [DOI] [PubMed] [Google Scholar]
- Gatford KL, De Blasio MJ, Thavaneswaran P, Robinson JS, McMillen IC, Owens JA 2004 Postnatal ontogeny of glucose homeostasis and insulin action in sheep. Am J Physiol Endocrinol Metab 286:E1050–E1059 [DOI] [PubMed] [Google Scholar]
- Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE 2008 Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab 295:E262–E268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell GH 1983 Hormonal control of glucose homoeostasis in ruminants. Proc Nutr Soc 42:149–167 [DOI] [PubMed] [Google Scholar]
- Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V 2009 Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment and depletion of follicular reserve. Biol Reprod 80:726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DL, Jackson LM, Padmanabhan V 2006 Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinol 254–255:109–119 [DOI] [PubMed] [Google Scholar]
- Plagemann A 2008 A matter of insulin: developmental programming of body weight regulation. J Matern Fetal Neonatal Med 21:143–148 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M 2007 Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 120(2 Suppl 1):S3–S8; discussion S29–S532 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y 2008 The role of fat topology in the risk of disease. Int J Obes (Lond) 32(Suppl 7):S83–S92 [DOI] [PubMed] [Google Scholar]
- Phillips LK, Prins JB 2008 The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep 10:156–164 [DOI] [PubMed] [Google Scholar]
- Bergman EN, Reulein SS, Corlett RE 1989 Effects of obesity on insulin sensitivity and responsiveness in sheep. Am J Physiol 257:E772–E781 [DOI] [PubMed] [Google Scholar]
- Rossell R, Gomis R, Casamitjana R, Segura R, Vilardell E, Rivera F 1983 Reduced hepatic insulin extraction in obesity: relation with plasma insulin levels. J Clin Endocrinol Metab 56:608–611 [DOI] [PubMed] [Google Scholar]
- Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C, Galloway JA, Frank BH, Karrison T, Van Cauter E 1988 Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest 81:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampelli M, Fulghesu AM, Cucinelli F, Pavone V, Caruso A, Mancuso S, Lanzone A 1997 Heterogeneity in β cell activity, hepatic insulin clearance and peripheral insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod 12:1897–1901 [DOI] [PubMed] [Google Scholar]
- McGarry JD 2002 Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18 [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE 2009 Diabetes and apoptosis: lipotoxicity. Apoptosis 14:1484–1495 [DOI] [PubMed] [Google Scholar]
- Mühlhausler BS 2009 Nutritional models of type 2 diabetes mellitus. Methods Mol Biol 560:19–36 [DOI] [PubMed] [Google Scholar]
- Mingrone G, Manco M, Mora ME, Guidone C, Iaconelli A, Gniuli D, Leccesi L, Chiellini C, Ghirlanda G 2008 Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 31:1872–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Pérez V, Echiburú B, MaliqueoM, Ladrón de Guevara AL, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S 2009 Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 94:1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman PL, Cutfield WS 2006 Insulin sensitivity in people born preterm, with low or very low birth weight and small for gestational age. J Endocrinol Invest 29(Suppl 1):2–8 [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR 2005 Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115:e290–e296 [DOI] [PubMed] [Google Scholar]
- Dulloo AG 2008 Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab 22:155–171 [DOI] [PubMed] [Google Scholar]