Abstract
The cytochrome P450 2A1 (CYP2A1) is a P450 enzyme that catalyzes the metabolism of testosterone. CYP2A1 has been reported to be present in rat testis. However, its developmental changes and function have not been well characterized. The purpose of this study was to measure the abundance of CYP2A1 (Cyp2a1) mRNA in the developing rat testis and Leydig cells and examine the effects of its product, 7α-hydroxytestosterone (7HT), on an important enzyme, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) that interconverts active corticosterone and inactive 11-dehydrocorticosterone. As detected by real-time PCR, Cyp2a1 was found to be present exclusively in the Leydig cell. CYP2A1 activity in adult Leydig cells was 5-fold higher than those in progenitor or immature Leydig cells. 7HT competitively suppressed 11β-HSD1 oxidase and reductase activities in rat testis microsome with inhibitory constant of 1.2 and 2.9 μm, respectively. In intact Leydig cells, 7HT did not inhibit 11β-HSD1 reductase activity, but it stimulated its reductase activity. Thus, at 100 nm and higher concentrations, 7HT significantly switched 11β-HSD1 oxidoreductase activities toward reductase. The present data shows that 7HT, the product formed by CYP2A1 from testosterone, regulates the direction of 11β-HSD1 activity in rat Leydig cells.
7α–Hydroxytestosterone, the product formed by CYP2A1 from testosterone, regulates the direction of 11β-hydroxysteroid dehydrogenase activity in rat Leydig cells.
Leydig cells are primary endocrine cells that produce androgens for males. Excessive glucocorticoids have been linked to the reduced serum testosterone (T) levels and reproductive dysfunction (1,2). This suppression is partially through direct inhibition of T biosynthesis in Leydig cells via a glucocorticoid receptor-mediated mechanism (1). Glucocorticoids have been shown to suppress basal and cAMP-induced gene expression of the cholesterol transport protein acute steroidogenic regulatory protein and T biosynthetic enzymes including P450-dependent side chain cleavage enzyme, thus leading to the reduced T production (3).
Intracellular levels of glucocorticoids are controlled by the metabolizing enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD). Two isoforms of 11β-HSD (11β-HSD1 and 11β-HSD2) have been characterized. 11β-HSD1 is a bidirectional enzyme with a wide tissue distribution and functions as either an oxidase [in rodents, catalyzing corticosterone (CORT), into 11-dehydro-CORT (11DHC)] or a reductase (11DHC into CORT) in several tissues (4). In contrast, 11β-HSD2 is a unidirectional oxidase that specifically localizes in mineralocorticoid receptor targeted tissues and functions in the inactivation of glucocorticoids (5). In glucocorticoid target tissues such as liver, 11β-HSD1 acts predominantly as a reductase to locally regenerate active glucocorticoids from inactive 11-dehydrosteroids (6). It has been hypothesized that production of ample reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) in liver by hexose-6-phosphate dehydrogenase (H6PDH), which uses glucose-6-phosphate (G6P) as substrate and nicotinamide adenine dinucleotide phosphate (NADP+) as cofactor, drives the reductase direction of 11β-HSD1 catalysis (7,8).
Mature rat Leydig cells have 1000-fold higher levels of 11β-HSD1 (Hsd11b1) mRNA than 11β-HSD2 mRNA (Hsd11b2) (9). The reductase activity of the bidirectional 11β-HSD1 might be influenced by other enzymes, such as cytochrome P450 2A1 (CYP2A1), a P450 enzyme that catalyzes the metabolism of T into 7α-hydroxytestosterone (7HT). CYP2A1 has been reported to be present in rat testis and generate 7α-hydroxyl steroids, especially 7HT (10,11). Recently some 7α-hydroxyl steroids such as 7α-hydroxydehydroepiandrosterone have been reported to be substrates of 11β-HSD1 (12). Thus, 7HT could act as the substrate of 11β-HSD1 in rat Leydig cells. However, the developmental change of CYP2A1 gene (Cyp2a1) and the manner in which CYP2A1 affects 11β-HSD1 oxidase and reductase activities remain unclear.
The postnatal development of Leydig cells can be recapitulated by using a Leydig cell-specific toxicant ethane dimethanesulfonate (EDS) that kills only mature Leydig cells in the rat testis (13). After mature Leydig cells disappear, newly formed Leydig cells refill the interstitial space. The progressive expression of Leydig cell-specific genes during regeneration is similar to the gene expression pattern during Leydig cell ontogeny (13). Therefore, in the present study, we investigated whether CYP2A1 expression and activity in EDS-treated rat testis and asked whether the product of T by CYP2A1, 7HT, affects 11β-HSD1 direction in rat Leydig cells.
Materials and Methods
Chemicals and animals
CORT, 7HT and 11DHC were purchased from Steraloids (Wilton, NH). [1,2-3H]corticosterone (3H-CORT), specific activity 40 Ci/mmol, was purchased from DuPont-NEN Life Science Products (Boston, MA). [1,2-3H]11-dehydrocorticosterone was prepared from labeled 3H-CORT as described earlier (14). Male Sprague Dawley rats (from d 1 to 90 d old) were purchased from Charles River Laboratories (Wilmington, MA). The EDS was a gift from Dr. L. Earl Gray (U.S. Environmental Protection Agency, Research Triangle Park, NC).
Animal treatments
Six normal rats each age group at 1- to 90-d-old postpartum were killed by asphyxiation with CO2 and testes were collected for total RNA extraction. Another 96 adult 90-d-old rats were divided into eight groups of 12 animals. Of these rats, 84 rats were given 75 mg EDS per kilogram body weight, a chemical that specifically kills Leydig cells in adult rat testis without affecting other cell types, in a single ip injection. The other 12 animals received the same volume of vehicle [1:4 (vol/vol) DMSO/water] and were killed 1 h later and served as control. Rats were killed at the fourth, seventh, 14th, 21st, 35th, 56th, and 90th d of EDS treatment by asphyxiation with CO2. Animals in each group were killed similarly, and testes were frozen in liquid nitrogen for RNA extraction. The animal protocol was approved by the Institutional Animal Care and Use Committee of the Rockefeller University.
Cell isolation
Testes were processed for purification of Leydig cells. The progenitor, immature, and adult Leydig cells were purified from rat testes at 21st, 35th, and 90th d postpartum, as described previously (15,16). In brief, 40 21-d-old (for progenitor Leydig cells), 18 35-d-old (for immature Leydig cells), and six 90-d-old (for adult Leydig cells) rats were killed, followed by removal and decapsulation of testes. Then the testes were digested with collagenase, and finally cells were separated by Percoll density gradient centrifugation. Purities of Leydig cell fractions were evaluated by histochemical staining of 3β-hydroxysteroid dehydrogenase, with 0.4 mm etiocholanolone as an enzyme substrate (17).
Preparation of microsomal protein
The microsomes from 90-d-old rat adult Leydig cells were prepared, as described previously (18). In brief, Leydig cells were homogenized in cold 0.01 m PBS (pH 7.2), containing 0.25 m sucrose. Subsequently the homogenates were centrifuged at 700 × g for 30 min, and the supernatants were transferred to other tubes followed by centrifugation at 10,000 × g for 30 min. The resultant supernatants were again centrifuged twice at 105,000 × g for 1 h. The pellets were resuspended and the protein contents were determined by Bio-Rad protein assay kit (catalog no. 500-0006; Bio-Rad, Hercules, CA). Microsomes were used to assay 11β-HSD1 oxidoreductase activities.
Primer selection
All primers in this study were chosen using a sequence analysis software package (Primer 3; Whitehead Institute for Biomedical Research, Cambridge, MA) following guidelines for internal stability. Forward and reverse primers were in different exons to minimize the effects of possible DNA contamination. The primers for Cyp2a1 were: 5′-TGGACACAGGA CTGCTTCTG-3′ (forward); 5′-TGTAGGTAGCCTGTTCGCCT-3′ (reverse). For the internal standard, primers to ribosomal protein S16 (Rps16) were used, as described previously (9).
Real-time PCR
PCR was carried out in a 25-μl volume using a 96-well plate format using the SYBR Green PCR core reagents purchased from PE Applied Biosystems (Foster City, CA). Primer titration was performed and the concentration of 300 nm was selected. Fluorescence was detected in real-time on an ABI 7700 system (Applied Biosystems). Each sample was run in duplicate, in parallel with no template controls. The levels were normalized to Rps16.
11β-HSD1 assay
11β-HSD1 activity assay tubes contained 25 nm CORT (within the range of physiological levels of CORT). A preliminary experiment has determined velocity of 11β-HSD1 oxidase and reductase activities within the linear range to guarantee that the substrate concentration is maximal. 3H-CORT or [1,2-3H]11-dehydrocorticosterone was used as substrates to measure either 11β-HSD1 oxidase or reductase activity. 11β-HSD1 activity assay was performed as described previously (9). For microsomal preparation, the Leydig cell microsomes were incubated with substrates and NADPH in PBS (pH 7.4) buffer. For intact Leydig cells, substrates were added to reaction mixture (DMEM-F12 buffer) without adding any cofactor. The reactions were stopped by adding 2 ml ice-cold ether. The steroids were extracted, and the organic layer was dried under nitrogen. The steroids were separated chromatographically on thin-layer plates in chloroform and methanol [90:10 (vol/vol)], and the radioactivity was measured using a scanning radiometer (System AR2000; Bioscan Inc., Washington, DC). The percentage conversion of CORT to 11DHC and 11DHC to CORT was calculated by dividing the radioactive counts identified as 11DHC (or CORT, respectively) by the total counts associated with CORT plus 11DHC.
17β-HSD-3 (17β-HSD3) assay
To check whether the regulation of 7HT of 11β-HSD1 is specific, another Leydig cell-specific enzyme 17β-HSD3 that catalyzes conversion of androstenedione (DIONE) into T was measured. 17β-HSD3 activity in Leydig cell microsomes was measured as described previously (19). In brief, 17β-HSD3 activity assay tubes contained 100 nm DIONE and 40,000 dpm [3H]DIONE. The 90-min reactions were initiated by addition of 5 μg rat Leydig cell microsome proteins with 0.2 mm NADPH in presence of different concentrations of 7HT to determine IC50. A preliminary experiment has verified the velocity of 17β-HSD3 to be within the linear range in the above condition. The reactions were stopped by adding 2 ml ice-cold ether. The steroids were extracted, and the organic layer was dried under nitrogen. The steroids were separated chromatographically on thin-layer plates in chloroform and methanol (97:3), and the radioactivity was measured using a scanning radiometer (System AR2000; Bioscan). The percentage conversion of DIONE to T was calculated by dividing the radioactive counts identified as testosterone by the total counts associated with DIONE plus T.
CYP2A1 assay
The CYP2A1 activities of intact progenitor, immature, and adult Leydig cells were assayed by measuring the conversion of [3H]T (substrate) into 7HT, the product. Individual control and experimental tubes were set for each cell type used. Each cell type was incubated with 100 nm [3H]T. The reactions were terminated by adding 2 ml ice-cold ether. The steroids were extracted, and the organic layer was dried under nitrogen. The steroids were separated by thin-layer chromatography. After loading the steroids on TLC plates, the plates were developed in a solvent system made of chloroform and methanol [97:3 (vol/vol)]. The plates were scanned using a scanning radiometer (System AR2000; Bioscan). The T and 7HT corresponding to the cold steroid marker were identified under UV light. The percentage conversion of T to 7HT was calculated by dividing the radioactive counts identified as T or 7HT by the total counts associated with T plus 7HT.
Measurement of NADPH
The measurement of intracellular NADPH levels were performed as described previously (20). In brief, 2 × 106 adult Leydig cells treated with or without 10 μm 7HT in 0.2 ml of 0.1 m NaOH were homogenized. The homogenates were then denatured and centrifuged at 10,000 × g and 4 C for 10 min. Supernatants were neutralized with 0.1 m HCl and centrifuged again in the same conditions. The NADPH assay was performed by spectrophotometric cycling assay using 3-(4,5-dmethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide as the electron acceptor and phenazine ethosulfate as an electron carries. The produced formazan from 3-(4,5-dmethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was detected at 578 nm.
Statistics
Each experiment was repeated three to four times. Data were subjected to analysis by one-way ANOVA followed by Duncan multiple comparisons testing to identify significant differences between groups when three and more groups were calculated or by the t test when two groups were calculated. All data are expressed as means ± sem. Differences were regarded as significant at P < 0.05.
Results
Detection of Cyp2a1 mRNA in rat Leydig cells and testis
The EDS treatment of adult rats led to the selective destruction of Leydig cells. Seven days after treatment, Leydig cells were not detectable in the testis (13). However, by 21 d of treatment, Leydig cells regeneration was found to be in progress and progenitor Leydig cells were formed. Leydig cells were found to be fully regenerated by 56 d of treatment (13).
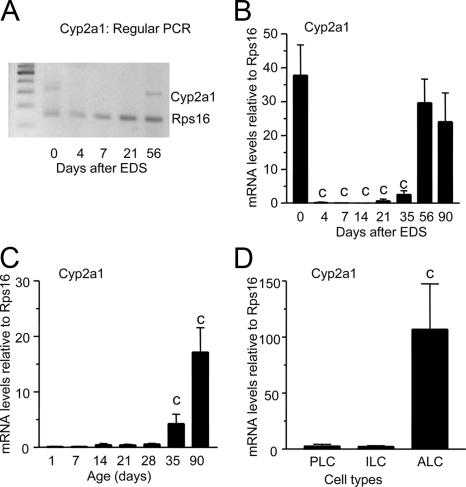
Regular PCR detected an expected 313-bp band of Cyp2a1 cDNA in normal 90-d-old rat testes (0 d after EDS treatment, Fig. 1A), which disappeared on the fourth, seventh, and 21st d of EDS exposure and reappeared on 56th d of treatment. In the adult testes, we found that the amounts of Cyp2a1 mRNA fell when Leydig cells were depleted and rose during the time of Leydig cell regeneration, which started around postnatal day (PND) 21 (Fig. 1B). We measured Cyp2a1 mRNA levels in the developing rat testes (Fig. 1C). The Cyp2a1 expression levels increased at PND 35 and reached adult level at PND 90 (Fig. 1C). The Cyp2a1 mRNAs were measured in purified progenitor Leydig cells (from PND 21), immature Leydig cells (from PND 35), and adult Leydig cells (from PND 90). Only adult Leydig cells expressed higher Cyp2a1 (Fig. 1D). These data show that CYP2A1 is present only in adult Leydig cells and is in parallel with the developmental change of another Leydig cell protein 11β-HSD1 (21).
Figure 1.
RT-PCR and real-time PCR of Cyp2a1 in rat testis and Leydig cells. A, RT-PCR detected single 300-bp band (for Cyp2a1) in 90-d-old rat testis (0 d after EDS), and the band was lower in intensity when Leydig cells were depleted and gained intensity during the 56 d of Leydig cell regeneration after EDS treatment. B, The real-time PCR measured the relative mRNA levels of Cyp2a1 in EDS-treated rat testis. C, Cyp2a1 in normal testis. D, Cyp2a1 in purified progenitor (PLC), immature (ILC), and adult Leydig cells (ALC). Mean ± sem, n = 5–6. A significant difference was shown when compared with the first bar at each panel at a, P < 0.05, and c, P < 0.001.
Cyp2a1 activity
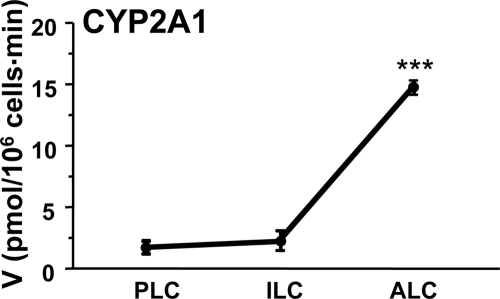
The Cyp2a1 activity was assayed using progenitor, immature and adult Leydig cells by adding radiolabeled T. We found that Cyp2a1 activity was significantly higher in adult Leydig cells than in the progenitor and immature Leydig cells (Fig. 2). These data show that Cyp2a1 was maximally expressed in the adult Leydig cells.
Figure 2.
CYP2A1 activities in progenitor (PLC), immature (ILC), and adult Leydig cells (ALC). CYP2A1 was measured by the conversion of 3H-testosterone (T) (1 μm) into 3H-7HT in 0.2 × 106 cells for 30 min. Mean ± sem, n = 4. A significant difference was shown when compared with the first bar at each panel at ***, P < 0.001. V, Velocity.
Effects of 7HT on 11β-HSD1 activity in Leydig cells
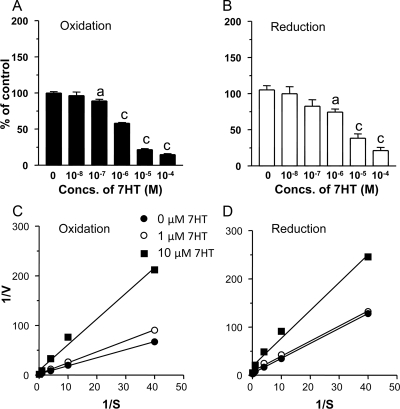
Using Leydig cell microsomes, the effects of 7HT on the oxidoreductase activities of 11β-HSD1 were investigated. 7HT was found to inhibit both oxidase and reductase activities of 11β-HSD1, in a competitive manner (Fig. 3). The Michaelis constant and inhibitory constant for 11β-HSD1 oxidase were determined to be 1.12 and 1.2 μm, respectively. The reductase has Michaelis constant and inhibitory constant of 0.82 and 2.9 μm, respectively. The mode of 7HT in the inhibition of 11β-HSD1 oxidase and reductase is competitive (Fig. 3, C and D).
Figure 3.
Effects of 7HT on 11β-HSD1 oxidase and reductase activities in rat testis microsome preparations. A and C are 11β-HSD1 oxidase, and B and D are reductase. A and B show the percentage of activities at different concentrations (Concs.) of 7HT. C and D are Lineweaver-Burk plots of kinetic data, showing the competitive inhibition of 11β-HSD1 oxidase and reductase by 7HT. The experiments were repeated twice in a duplicate assay. A significant difference was shown when compared with the first bar at each panel at a, P < 0.05, and c, P < 0.001. S, Substrate concentration; V, velocity.
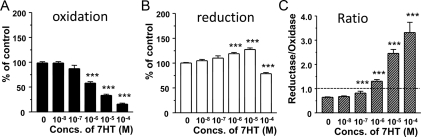
The effects of 7HT on 11β-HSD1 oxidase and reductase activities were further investigated in intact adult Leydig cells. 7HT was found to inhibit 11β-HSD1 oxidase activity with an IC50 of 0.8 μm (Fig. 4). Surprisingly, it stimulated 11β-HSD1 reductase activity at 1 and 10 μm but inhibited the activity at 100 μm. Thus, 7HT significantly switched the oxidoreductase activities toward reductase side started at 100 nm (Fig. 4C) as one was set as equivalent balance of reductase and oxidase. We further measured the change of intracellular NADPH level and NADPH to NADP+ ratio after 1 μm 7HT treatment. 7HT at 1 μm significantly increased the NADPH to NADP+ ratio by 3-fold (the intracellular NADPH concentrations were 74.5 ± 12.5 μm in the control and 215.4. ± 43.7 μm in 7HT-treated sample, mean ± sem, n = 6).
Figure 4.
Effects of 7HT on 11β-HSD1 oxidase and reductase activities in intact adult Leydig cells. 11β-HSD1 oxidase (A), reductase (B), and the reductase to oxidase ratio (C) after incubation with different concentrations (Concs.) of 7HT are shown. One is set for the equivalent balance of reductase and oxidase. Mean ± sem, n = 4. ***, P < 0.001.
Effects of 7HT on 17β-HSD3 activity in Leydig cells
To see whether the effect of 7HT on 11β-HSD1 is specific, the effects of 7HT on the oxidoreductase activities of another important NADP+/NADPH-dependent steroidogenic enzyme 17β-HSD3 were investigated by adding various concentrations (10−8 to 10−4 m) of 7HT. 7HT did not significantly affect the activities of 17β-HSD3 (Table 1).
Table 1.
Effects of 7HT on 17β-HSD3 activity
| Concentrations of 7HT (m)
|
||||||
|---|---|---|---|---|---|---|
| 0 | 10−8 | 10−7 | 10−6 | 10−5 | 10−4 | |
| Conversion rate (%)a | 66.53 ± 0.82 | 63.42 ± 1.48 | 67.01 ± 1.30 | 65.41 ± 0.77 | 66.23 ± 0.95 | 69.58 ± 0.39 |
17β-HSD3 activity was measured by incubating 100 nm androstenedione with 5 μg rat Leydig cell microsome and 0.2 mm NADPH.
Discussion
The present study demonstrated that CYP2A1, which catalyzes the formation of 7HT from T, and its mRNA were specifically present in adult Leydig cells. 7HT can interfere with Leydig cell function by inhibiting 11β-HSD1 oxidase activity but increasing reductase activity via increasing intracellular NADPH level.
11β-HSD1 oxidase and reductase activities have been reported to rely on concentrations of pyridine nucleotide cofactor NADP+/NADPH pool in the smooth endoplasmic reticulum luminal pool in liver cells. In liver cells, 11β-HSD1 and H6PDH are colocalized inside the lumen of smooth endoplasmic reticulum (7,8). H6PDH uses NADP+ as a cofactor and G6P as a substrate. It is well established that pyridine nucleotides are impermeable to smooth endoplasmic reticulum membrane and that G6P can be transported into smooth endoplasmic reticulum lumen by G6P transporting protein (7,8). This generates NADPH from NADP+ increasing NADPH to NADP+ ratio, which drives 11β-HSD1 to function as a reductase. Although cytosolic glucose-6-phosphase dehydrogenase accounts for 95% of NADPH generated by the whole cell, this enzyme does not seem to affect the direction of 11βHSD1 catalysis. This is supported by the fact that depletion of this enzyme by RNA silencing does not change the direction of 11βHSD1 catalysis (22). In contrast, H6PDH, which accounts for only 5% of NADPH production, causes 11β-HSD1 to act as a reductase in liver cells because the enzymes and the cofactors are compartmentalized in the lumen of smooth endoplasmic reticulum. Therefore, when H6PDH is absent in mice by null mutation, 11βHSD1 has oxidase activity in the liver (23).
Although 11β-HSD1 functioned primarily as an oxidase in adult Leydig cells, in which the oxidase to reductase ratio was 1.56 ± 0.05 (Fig. 4), there was some reductase activity. In the present study, we showed that 7HT can affect the reductase to oxidase ratio. CYP2A1 has been reported at very high levels in the liver and at modest levels in the testis (24). Whether 7HT can reach a sufficient level to interfere with 11β-HSD1 in liver will require further investigation because normal circulating T is around 2–3 ng/ml (∼10 nm). CYP2A1 has been reported to be present in rat testis (10), generating 7α-hydroxyl steroid, especially 7HT. 7α-Hydroxylase activities in mature rat testis can produce equivalent amounts of T and 7HT (11). T can reach the level of about 100 ng/ml (∼300 nm) in rat testis interstitial fluids (25); thus, 7HT will reach levels greater than 150 nm, a level that has significantly increased the 11β-HSD1 reductase to oxidase ratio (Fig. 4C). The exact testicular 7HT levels in vivo are not known in humans. However, 7HT has been reported to be formed in human testis by 7α-hydroxylase (26). Whether the 7α-hydroxylase activity is catalyzed by CYP2A1 or another enzyme is unclear because CYP2A1 has not been cloned in humans yet. 7HT form in human testis could influence human testicular 11β-HSD1 that is abundant in human testis (27).
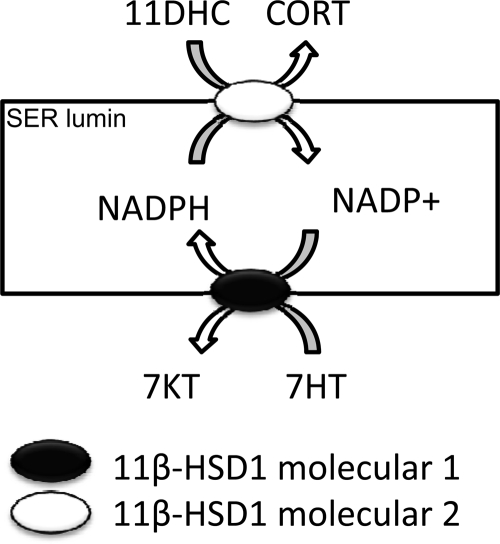
Although 7HT has been shown to significantly inhibit 11β-HSD1 reductase in Leydig cell microsomes via a competitive mode, it did not affect the reductase activity in intact Leydig cells except at very higher concentrations greater than 100 μm. Instead, it increased 11β-HSD1 reductase activity in the intact Leydig cells at 1 and 10 μm. It has been shown that another 7α-hydroxysteroid, 7α-hydroxydeepiandrosterone, is the substrate of human 11β-HSD1, and is converted to 7-oxodehydroepiandrosterone (28,29). In the present study, we also observed that 7HT increased the intracellular NADPH level. Therefore, we hypothesize that 7HT is the substrate of 11β-HSD1 oxidase, which converts NADP+ into NADPH, and the latter drives 11β-HSD1 toward reductase activity (Fig. 5). Although, it competitively inhibited 11β-HSD1 reductase, the increased NADPH can offset its direct inhibition except at higher concentrations greater than 100 μm.
Figure 5.
Working hypothesis for the activation of 11β-HSD1 reductase activity by 7HT. 11β-HSD1 is located in smooth endoplasmic reticulum (SER) lumin. One molecule of 11β-HSD1 catalyzes 7HT to 7-ketotestosterone (7KT) generating one molecule of NADPH, which is used by the second 11β-HSD1 to catalyze 11DHC and CORT as a reductase.
7HT has also been reported to inhibit other steroidogenic enzymes in the testis including 5α-reductase and 3α-hydroxysteroid dehydrogenase (26). However, in the present study, we did not observe any effects of 7HT on 17β-HSD3 in Leydig cells when concentrations up to 100 μm were assayed. 7HT also did not affect 3β-hydroxysteroid dehydrogenase (26). Thus, the effect of 7HT on steroidogenic enzymes in testis might be limited to the testosterone and glucocorticoid metabolizing enzyme.
In summary, CYP2A1 is exclusively located in rat adult Leydig cells and catalyzes the conversion of T to 7HT, and 7HT alters the direction of 11β-HSD1 catalysis by directly inhibiting 11β-HSD1 oxidase and indirectly stimulating its reductase.
Acknowledgments
We thank Ms. Chantal M. Sottas for excellent technical support.
Footnotes
This work was supported by in part by National Institutes of Health Grant HD050570 (to R.-S.G).
Disclosure Summary: G.-X.H., P.V.P., N.K., R.-S.G., B.-B.C., Q.-Q.L., and Z.-Q.Z. have nothing to declare.
First Published Online December 16, 2009
Abbreviations: CORT, Corticosterone; CYP2A1, cytochrome P450 2A1; 11DHC, 11-dehydro-CORT; DIONE, androstenedione; EDS, ethane dimethanesulfonate; G6P, glucose-6-phosphate; 3H-CORT, [1,2-3H]corticosterone; H6PDH, hexose-6-phosphate dehydrogenase; 11β-HSD, 11β-hydroxysteroid dehydrogenase; 17β-HSD3, 17β-HSD-3; 7HT, 7α-hydroxytestosterone; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate; PND, postnatal day; T, testosterone.
References
- Smals AG, Kloppenborg PW, Benraad TJ 1977 Plasma testosterone profiles in Cushing’s syndrome. J Clin Endocrinol Metab 45:240–245 [DOI] [PubMed] [Google Scholar]
- Monder C, Miroff Y, Marandici A, Hardy MP 1994 11β-Hydroxysteroid dehydrogenase alleviates glucocorticoid-mediated inhibition of steroidogenesis in rat Leydig cells. Endocrinology 134:1199–1204 [DOI] [PubMed] [Google Scholar]
- Martin LJ, Tremblay JJ 2009 The nuclear receptors NUR77 and SF1 play additive roles with c-JUN through distinct elements on the mouse Star promoter. J Mol Endocrinol 42:119–129 [DOI] [PubMed] [Google Scholar]
- Monder C 1993 The forms and functions of 11β-hydroxysteroid dehydrogenase. J Steroid Biochem Mol Biol 45:161–165 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Mune T, Monder C, White PC 1994 NAD(+)-dependent isoform of 11β-hydroxysteroid dehydrogenase. Cloning and characterization of cDNA from sheep kidney. J Biol Chem 269:25959–25962 [PubMed] [Google Scholar]
- Holmes MC, Kotelevtsev Y, Mullins JJ, Seckl JR 2001 Phenotypic analysis of mice bearing targeted deletions of 11β-hydroxysteroid dehydrogenases 1 and 2 genes. Mol Cell Endocrinol 171:15–20 [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Nashev LG, Schweizer RA, Frick C, Odermatt A 2004 Hexose-6-phosphate dehydrogenase determines the reaction direction of 11β-hydroxysteroid dehydrogenase type 1 as an oxoreductase. FEBS Lett 571:129–133 [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Csala M, Benedetti A 2009 Hexose-6-phosphate dehydrogenase: linking endocrinology and metabolism in the endoplasmic reticulum. J Mol Endocrinol 42:283–289 [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Niu EM, Sottas CM, Hardy DO, Catterall JF, Latif SA, Morris DJ, Hardy MP 2005 11β-Hydroxysteroid dehydrogenase 2 in rat Leydig cells: its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology 146:2657–2664 [DOI] [PubMed] [Google Scholar]
- Seng JE, Leakey JE, Arlotto MP, Parkinson A, Gandy J 1991 Cellular localization of cytochrome P450IIA1 in testes of mature Sprague-Dawley rats. Biol Reprod 45:876–882 [DOI] [PubMed] [Google Scholar]
- Eechaute W, Lacroix E, Leusen I 1974 The conversion of testosterone to 7α-hydroxy-testosterone by incubated rat testes. Steroids 24:753–764 [DOI] [PubMed] [Google Scholar]
- Muller C, Pompon D, Urban P, Morfin R 2006 Inter-conversion of 7α- and 7β-hydroxy-dehydroepiandrosterone by the human 11β-hydroxysteroid dehydrogenase type 1. J Steroid Biochem Mol Biol 99:215–222 [DOI] [PubMed] [Google Scholar]
- Teerds K, Rijntjes E 2007 Dynamics of Leydig cell regeneration after EDS. In: Payne AH, Hardy MP, eds. The Leydig cell in health and disease. Totowa, NJ: Humana Press; 91–116 [Google Scholar]
- Lakshmi V, Monder C 1985 Extraction of 11β-hydroxysteroid dehydrogenase from rat liver microsomes by detergents. J Steroid Biochem 22:331–340 [DOI] [PubMed] [Google Scholar]
- Salva A, Klinefelter GR, Hardy MP 2001 Purification of rat Leydig cells: increased yields after unit-gravity sedimentation of collagenase-dispersed interstitial cells. J Androl 22:665–671 [PubMed] [Google Scholar]
- Shan LX, Hardy MP 1992 Developmental changes in levels of luteinizing hormone receptor and androgen receptor in rat Leydig cells. Endocrinology 131:1107–1114 [DOI] [PubMed] [Google Scholar]
- Payne AH, Wong KL, Vega MM 1980 Differential effects of single and repeated administrations of gonadotropins on luteinizing hormone receptors and testosterone synthesis in two populations of Leydig cells. J Biol Chem 255:7118–7122 [PubMed] [Google Scholar]
- Ge RS, Gao HB, Nacharaju VL, Gunsalus GL, Hardy MP 1997 Identification of a kinetically distinct activity of 11β-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology 138: 2435–2442 [DOI] [PubMed] [Google Scholar]
- Hu GX, Zhou HY, Li XW, Chen BB, Xiao YC, Lian QQ, Liang G, Kim HH, Zheng ZQ, Hardy DO, Ge RS 2009 The (+)- and (−)-gossypols potently inhibit both 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase 3 in human and rat testes. J Steroid Biochem Mol Biol 115:14–19 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Larher F 1997 Cycling assay for nicotinamide adenine dinucleotides: NaCl precipitation and ethanol solubilization of the reduced tetrazolium. Anal Biochem 251:153–157 [DOI] [PubMed] [Google Scholar]
- Phillips DM, Lakshmi V, Monder C 1989 Corticosteroid 11β-dehydrogenase in rat testis. Endocrinology 125:209–216 [DOI] [PubMed] [Google Scholar]
- Hewitt KN, Walker EA, Stewart PM 2005 Minireview: hexose-6-phosphate dehydrogenase and redox control of 11β-hydroxysteroid dehydrogenase type 1 activity. Endocrinology 146: 2539–2543 [DOI] [PubMed] [Google Scholar]
- Lavery GG, Walker EA, Turan N, Rogoff D, Ryder JW, Shelton JM, Richardson JA, Falciani F, White PC, Stewart PM, Parker KL, McMillan DR 2008 Deletion of hexose-6-phosphate dehydrogenase activates the unfolded protein response pathway and induces skeletal myopathy. J Biol Chem 283:8453–8461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaoka S, Hashizume T, Funae Y 2005 Localization of rat cytochrome P450 in various tissues and comparison of arachidonic acid metabolism by rat P450 with that by human P450 orthologs. Drug Metab Pharmacokinet 20:478–484 [DOI] [PubMed] [Google Scholar]
- Sriraman V, Niu E, Matias JR, Donahoe PK, MacLaughlin DT, Hardy MP, Lee MM 2001 Mullerian inhibiting substance inhibits testosterone synthesis in adult rats. J Androl 22:750–758 [PubMed] [Google Scholar]
- Rosness PA, Sunde A, Eik-Nes KB 1977 Production and effects of 7α-hydroxytestosterone on testosterone and dihydrotestosterone metabolism in rat testis. Biochim Biophys Acta 488:55–68 [DOI] [PubMed] [Google Scholar]
- Tannin GM, Agarwal AK, Monder C, New MI, White PC 1991 The human gene for 11β-hydroxysteroid dehydrogenase. Structure, tissue distribution, and chromosomal localization. J Biol Chem 266:16653–16658 [PubMed] [Google Scholar]
- Hennebert O, Pernelle C, Ferroud C, Morfin R 2007 7α- and 7β-hydroxy-epiandrosterone as substrates and inhibitors for the human 11β-hydroxysteroid dehydrogenase type 1. J Steroid Biochem Mol Biol 105:159–165 [DOI] [PubMed] [Google Scholar]
- Hennebert O, Chalbot S, Alran S, Morfin R 2007 Dehydroepiandrosterone 7α-hydroxylation in human tissues: possible interference with type 1 11β-hydroxysteroid dehydrogenase-mediated processes. J Steroid Biochem Mol Biol 104:326–333 [DOI] [PubMed] [Google Scholar]