Abstract
Aging in rodents and humans is characterized by loss of muscle mass (sarcopenia). Testosterone supplementation increases muscle mass in healthy older men. Here, using a mouse model, we investigated the molecular mechanisms by which testosterone prevents sarcopenia and promotes muscle growth in aging. Aged mice of 22 months of age received a single sc injection of GnRH antagonist every 2 wk to suppress endogenous testosterone production and were implanted subdermally under anesthesia with 0.5 or 1.0 cm testosterone-filled implants for 2 months (n = 15/group). Young and old mice (n = 15/group), of 2 and 22 months of age, respectively, received empty implants and were used as controls. Compared with young animals, a significant (P < 0.05) increase in muscle cell apoptosis coupled with a decrease in gastrocnemius muscles weight (by 16.7%) and muscle fiber cross-sectional area, of both fast and slow fiber types, was noted in old mice. Importantly, such age-related changes were fully reversed by higher dose (1 cm) of testosterone treatment. Testosterone treatment effectively suppressed age-specific increases in oxidative stress, processed myostatin levels, activation of c-Jun NH2-terminal kinase, and cyclin-dependent kinase inhibitor p21 in aged muscles. Furthermore, it restored age-related decreases in glucose-6-phosphate dehydrogenase levels, phospho-Akt, and Notch signaling. These alterations were associated with satellite cell proliferation and differentiation. Collectively these results suggest involvement of multiple signal transduction pathways in sarcopenia. Testosterone reverses sarcopenia through stimulation of cellular metabolism and survival pathway together with inhibition of death pathway.
Testosterone reverses sarcopenia through stimulation of cellular metabolism and survival pathway together with inhibition of death pathway.
Sarcopenia is defined as the progressive decline of skeletal muscle mass and strength, which occurs with aging (1,2). The rate of muscle loss is estimated to be 1–2% per year after the age of 50 yr and can affect even healthy physically active adults. Secondary to loss of skeletal muscle mass, there is a corollary decrease in functional independence and the ability to perform activities of daily living within the elderly population (2). Approximately 25% of people above the age of 70 yr and 40% of those who have reached the age of 80 yr are clinically sarcopenic (2,3). Additionally, aging-associated skeletal muscle loss also leads to an increased risk of falls, fractures, dependency, and all-cause mortality (3,4,5).
Mechanisms that regulate age-related loss of skeletal muscle mass are not well defined, but the pathogenesis is likely multifactorial. With age, in a process similar to that occurring in many other tissues, there is a gradual decline of regenerative potential in skeletal muscle. This may in large part be due to a decline in Notch signaling, which is essential for activation, proliferation, and myogenic progression of satellite cells (6,7,8). Intriguingly, however, the regenerative potential of aged satellite cells can be restored by forced local activation of Notch signaling (6) or exposure to a youthful systemic environment, achieved by heterochronic parabiosis (7). Taken together, this suggests that the intrinsic regenerative capacity of aged satellite cells remains intact.
Apoptosis, or programmed cell death, increases in skeletal muscle cells with aging and may also contribute to aging-associated sarcopenia (9,10,11,12,13). Thus, a combined approach targeting both diminished satellite cell regenerative potential and increased muscle cell apoptosis may present a framework for therapeutic intervention of aging-associated sarcopenia.
Testosterone, through its anabolic effects on muscle, is an important determinant of body composition in humans. Therefore, it is not surprising that testosterone supplementation increases muscle mass in healthy young and old men, healthy hypogonadal men, older men with low testosterone levels, and men with chronic illness and low testosterone levels (14). A recent multicenter study of testosterone therapy in older men further documented significant gains in total and appendicular lean mass, muscle strength, and aerobic endurance with significant reductions in whole-body and trunk fat (15). In addition, we previously demonstrated that such testosterone-induced increase in muscle size in both young and old men is associated with hypertrophy of muscle fibers and significant increases in myonuclear and satellite cell numbers (16,17,18). The mechanisms by which testosterone increases satellite cell number and promotes muscle growth in aging are not well understood.
Recently we have shown that the inactivation of c-jun NH2-terminal kinase (JNK) together with the activation of p38 MAPK is critical for testosterone-induced activation of Notch signaling and, consequently, induction of muscle fiber hypertrophy in young mice (19). Given that JNK signaling constitutes a critical component of apoptotic signaling in skeletal muscles after injury (20) and in aging (13), it is possible that testosterone-mediated inhibition of JNK could lead to suppression of muscle cell apoptosis and cause fiber growth. We hypothesize that testosterone, through stimulation of Notch signaling together with the inhibition of JNK mediated apoptotic signaling, prevents sarcopenia associated with aging. Furthermore, activation of Akt can also promote skeletal muscle hypertrophy (21,22,23) functioning through multiple signaling molecules involved in both survival and apoptotic pathways (24). Given that Akt signaling plays an important role in skeletal muscle growth, we further hypothesize that activation of Akt together with the inhibition of JNK may be critical for testosterone-mediated protection of sarcopenia in aging. To test these hypotheses, in the present study, we examined the repertoire of testosterone-mediated cellular and molecular pathways that improve regenerative potential of satellite cells and suppress muscle cell apoptosis, and hence prevent age-related decline in muscle mass.
Materials and Methods
Animals
C57BL6J mice at various age groups were obtained from Harlan Laboratories (Indianapolis, IN). Animals were housed in a standard animal facility under controlled temperature (22 C) and photoperiod (12-h light, 12-h dark cycle) with food and water ad libitum. To investigate the molecular mechanisms of by which testosterone prevents aging-associated sarcopenia, aged mice of 22 months of age received a single sc injection of GnRH antagonist (GnRH-A) every 2 wk to suppress endogenous testosterone production and were implanted subdermally under anesthesia with 0.5 or 1.0 cm testosterone-filled implants for 2 months (n = 15/group). Young and old mice (n = 15/group), of 2 and 22 months of age, respectively, received empty implants only without GnRH-A and used as controls. The implant lengths were based on the results of a previous study (25), which showed a dose-dependent increase in circulating levels of testosterone in gonadotropin-deficient mice. This study has shown that both 0.5 and 1.0 cm testosterone implants can provide supraphysiological levels of testosterone in gonadotropin-deficient mice. In a recent study, we have also shown that 1.0 cm testosterone implant results in supraphysiological levels of testosterone in GnRH-A-treated young mice (20). Two months after removing the implants, when the mice of the old control and old treated with GnRH-A + testosterone groups were 24 months of age, they were killed. Young controls were 4 months of age at the time of autopsy. Testosterone-filled implants were prepared from polydimethylsiloxane tubing (outer diameter, 1.96 mm; inner diameter, 1.47 mm; Dow Corning, Midland, MI). Acyline was kindly provided by Dr. Richard P. Blye (Contraceptive and Reproductive Health Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD). Animal handling and experimentation were in accordance with the recommendation of the American Veterinary Medical Association and were approved by the Charles Drew University School of Medicine and Science Animal Care and Use Review Committee.
Blood collection and tissue preparation
All mice were euthanized with a lethal ip injection of sodium pentobarbital (200 mg/kg body weight). The gastrocnemius muscles were removed and weighed. Portions of the tissues from 10 mice in each group were frozen in liquid nitrogen and stored frozen for subsequent analysis by Western blotting and enzyme immunometric assay (EIA). The gastrocnemius muscles from five remaining animals in each group were fixed in 4% paraformaldehyde for histological and immunohistochemical evaluation. The rationale for using gastrocnemius muscles was based on the results of several earlier studies, which show that this muscle exhibits a substantial decline in mass with age (10,13,26,27). Blood samples were collected from each animal by cardiac puncture immediately after death, and serum was separated and stored at −20 C for subsequent testosterone assay.
Hormone assay
Serum testosterone levels were measured by a previously reported RIA (20). The minimal detection limit in the assay was 0.6 ng/dl. The intra- and interassay coefficient of variations were 8.2 and 13.2%, respectively.
Muscle fiber cross-sectional area (CSA)
Muscle fiber CSA was determined in 5-μm paraffin sections of gastrocnemius muscles using the ImagePro Plus, version 5.1 software (Media Cybernetics, Silver Spring, MD) coupled to an Olympus BHS microscope equipped with a view camera converter video camera (Olympus, Center Valley, PA) (13). For each animal at least 100 fibers were measured.
Measurements of kinase activation
Activation of p38 MAPK and JNK in muscle lysates was measured by TiterZyme EIA kit (Assay Designs Inc., Ann Arbor, MI), as described previously by us (19,28).
Immunohistochemical and immunofluorescence analyses
Paraformaldehyde-fixed, paraffin-embedded muscle sections were immunostained as described previously (13,18,19,20). Primary antibodies included rabbit polyclonal Notch 1 (1:200), and mouse monoclonal proliferating cell nuclear antigen (PCNA; 1:50) and myogenin (1:50). All antibodies were obtained from the Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Immunoreactivity was detected using biotinylated antirabbit or antimouse IgG secondary antibody followed by avidin-biotinylated horseradish peroxidase complex and visualized with diaminobenzidine tetrahydrochloride as per the manufacturer’s instructions (VECTASTAIN Elile ABC rabbit or mouse IgG kit, Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin. Negative control was run for every assay and was processed in an identical manner, except the primary antibody was substituted by the rabbit or mouse IgG.
Enumeration of PCNA- and myogenin-positive nuclei was carried out using an American Optical Microscope with a ×40 objective and a pair of ×10 eyepieces, as described before (19). A square grid fitted with one eyepiece provided a reference area of 62,500 μm2. Cell count was expressed as the percentage of PCNA- and myogenin-positive nuclei present within the reference area.
Colocalization of paired box (PAX) proteins 3 and 7 and PCNA was detected by confocal microscopy using double immunostaining as previously described (18,19,20). In brief, after deparaffinization and rehydration, tissue sections were incubated with blocking serum for 20 min at room temperature. Sections were then incubated with a rabbit polyclonal PAX 3/7 antibody (1:50; Santa Cruz Biotechnology) at 4 C overnight, followed by donkey antirabbit fluorescein isothiocyanate secondary antibody for 45 min at room temperature. The sections were then incubated with a mouse monoclonal PCNA (1:50) at 4 C overnight, followed by goat-antimouse Texas Red-labeled secondary antibody for 45 min at room temperature. For controls, sections were treated only with secondary antibody, and no signals were detected. Confocal imaging was performed using a TCS-SP-MP confocal microscope (Leica, Bannockburn, IL) equipped with a 488-nm argon laser for excitation of green fluorophores such as fluorescein isothiocyanate and a 543-nm helium-neon laser for excitation of red flurophores such as Texas Red.
Western blotting
Western blotting was performed using muscle lysates as described previously (13,19,20). In brief, proteins (50–80 μg) were separated on a 4–12% sodium dodecyl sulfate-polyacrylamide gel with 2-(N-morpholine) ethane sulfonic acid or 3[N-morholino]propanesulfonic acid buffer purchased from Invitrogen (Carlsbad, CA) at 200 V. Gel was transferred on an immunoblot polyvinyl difluoride membrane (Bio-Rad, Hercules, CA) overnight at 4 C. Membranes were blocked in blocking solution of 0.3% Tween 20 in Tris-buffered saline and 10% nonfat dry milk (TBS-T) for 1 h at room temperature and then probed using mouse monoclonal 4-hydroxynonenal protein adducts (4-HNE; 1:1000; BD Transduction Laboratories, San Diego, CA), PCNA (1:100), myogenin (1:300), p21 (1:500; Santa Cruz Biotechnology), and rabbit polyclonal myostatin (1:300), glucose-6 phosphate dehydrogenase (G6PDH; 1:500; Abcam, Cambridge, UK), Notch 1 (1:200), and phospho-Akt, which detects Akt only when phosphorylated at threonine 308 (1:500; Santa Cruz Biotechnology) for 1 h at room temperature or overnight at 4 C with constant shaking. After 3× 10-min washes in TBS-T buffer, membranes were then incubated in antirabbit (Amersham Biosciences, Piscataway, NJ) or antimouse IgG-horseradish peroxidase (Santa Cruz Biotechnology) secondary antibodies at a 1:2000 dilution. All antibodies were diluted in blocking buffer. The myostatin antibody was provided by Dr. Nestor Gonzalez-Cadavid (Charles Drew University, Los Angeles, CA). This antibody has been previously reported to detect two predominant protein species with apparent molecular masses of 45–55 and 32 kDa (29,30). The specificity of this antibody was also confirmed in a recent study from our group, which showed both these bands were absent in myostatin knockout mice (30). For immunodetection, membranes were washed three times in TBS-T wash buffer, incubated with enhanced chemiluminescence solutions per the manufacturer’s specifications (Amersham Biosciences), and exposed to Hyper film enhanced chemiluminescence. The membranes were stripped and reprobed with a rabbit polyclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:2000) for normalization of the loading. Band intensities were determined using Quantity One software from Bio-Rad.
Statistical analysis
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Corp., San Rafael, CA). Data are presented as mean ± se unless otherwise indicated. We used one-way ANOVA to compare group differences. If overall ANOVA revealed significant differences, post hoc (pairwise) comparisons were performed using Tukey’s test. Differences were considered significant if P < 0.05.
Results
Body and muscle weights, serum testosterone levels, muscle fiber CSA, and muscle cell apoptosis
Table 1 summarizes the effects of testosterone supplementation on body and gastrocnemius muscle weights, serum testosterone levels, and muscle fiber CSA. The animal body weights did not differ significantly between young and old mice. Testosterone treatment at both doses, however, resulted in a significantly (P < 0.05) increased body weights of the old mice compared with young males. The weight of the gastrocnemius muscles was decreased significantly (P < 0.05) by 16.7% in the aged animals compared with young mice. Treatment with 1 cm testosterone fully reversed age-related decline in muscle mass. Serum testosterone levels (nanograms per milliliter) were higher (0.6 ± 0.3 ng/ml; mean ± sd) in young in comparison with old mice (0.29 ± 0.12). Combined treatment with GnRH-A and testosterone implants of 0.5 and 1 cm resulted in supraphysiological levels of testosterone over the values measured in young animals. There were significant (P < 0.001) reductions in the mean area of both fast and slow fibers in old mice compared with that of young males. Supplementation with 1 cm but not 0.5 cm testosterone resulted in a significant (P < 0.001) increase in the mean CSA of both fiber types when compared with untreated old animals. The observed increase in fiber CSA was comparable with the values measured in young mice. The relative proportion of type I (slow) fiber was not significantly different among various groups, except in 0.5 cm testosterone-treated old mice, which showed a modest but significant (P < 0.05) increase in the proportion of this fiber type compared with young mice (data not shown). In contrast, old mice had a smaller proportion of type II (fast) fibers than young animals (data not shown). Older mice, like older men (18), also had a higher proportion of mixed fibers when compared with young animals. Testosterone treatment at both dose levels significantly (P < 0.05) prevented such changes in the muscle fiber composition associated with aging (data not shown).
Table 1.
Body and gastrocnemius muscle weights, serum testosterone (T) levels, and muscle fiber cross-sectional area
| Treatment | Treatment
|
|||
|---|---|---|---|---|
| Young | Old | Old + 0.5 cm T | Old + 1.0 cm T | |
| Body weight (g) | 26.3 ± 2.8a | 31.0 ± 5.0ab | 32.0 ± 2.8b | 34.4 ± 3.1b |
| Serum T (ng/ml) | 0.61 ± 0.3a | 0.29 ± 0.12b | 5.67 ± 1.49c | 8.01 ± 1.95c |
| Muscle weight (mg) | 156 ± 19a | 130 ± 24b | 150 ± 28ab | 170 ± 14c |
| Fast fiber area (μm2) | 1394 ± 483a | 1067 ± 331b | 1185 ± 462b | 1678 ± 601a |
| Slow fiber area (μm2) | 1408 ± 404a | 1013 ± 271b | 971 ± 309b | 1512 ± 545a |
Values are given as mean ± sd. Means with superscript letters a, b, and c are significantly (P < 0.05) different from each other. Means with superscript letters ab are not significantly different from means with either superscript letters a or b, but are significantly (P < 0.05) different from means with superscript letter c.
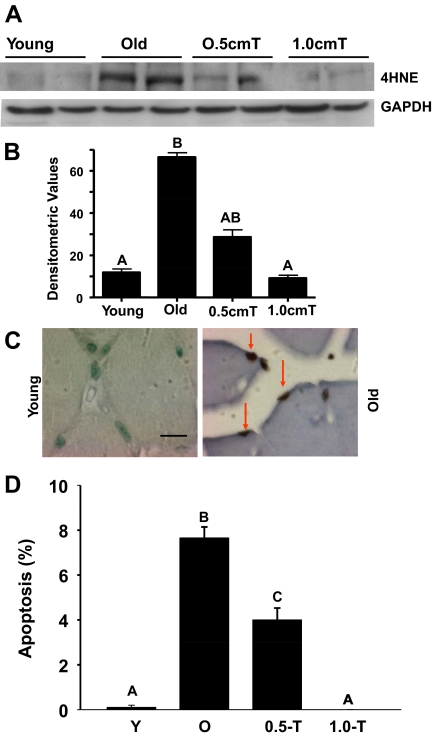
Figure 1, A and B, demonstrate that aging resulted in an increase of 4-HNE, a biomarker of oxidative stress (31,32), as evidenced by immunoblotting of the gastrocnemius muscles in aged mice in comparison with their young counterparts. Testosterone treatment, at both doses, significantly (P < 0.05) reduced this age-associated increase in oxidative stress. Given that oxidative stress plays an important role in muscle cell apoptosis in aging (13), we next analyzed the incidence of muscle cell apoptosis in aged mice with or without testosterone supplementation. Minimal muscle cell apoptosis was observed in young mice (Fig. 1C). Apoptotic index in skeletal muscle of aged mice was about 8% but exhibited a dose-dependent reduction in response to increasing levels of testosterone supplementation (Fig. 1D). Notably, aged mice treated with 1 cm testosterone exhibited little to no skeletal muscle apoptosis, similar to the phenotype observed in young mice (Fig. 1D).
Figure 1.
Testosterone supplementation results in suppression of 4-HNE levels and apoptosis in aged muscles. Panel A, Western blots of muscle lysates from young, old, and old mice treated with testosterone (T) show suppression of age-related increase in 4-HNE levels by both doses of testosterone. The gels are representative of two animals at each group from one of three separate experiments. GAPDH in the immunoblot is shown as a loading control. B, Quantification of band intensities. Values are mean ± sem. Means with unlike letters are significantly (P < 0.05) different. Means with superscript letter A are different from means with letter B. However, means with letters AB are not different from means with either letter A or B. Panel C, In situ detection of muscle cell apoptosis by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL). Compared with young, in which no apoptosis is detected, a distinct increase in the incidence of muscle cell apoptosis is evident in aged muscles. Scale bar, 15 μm. Panel D, Quantitation of muscle cell apoptosis in young, old, and old mice treated with testosterone. Apoptotic rate was expressed as the percentage of TUNEL-positive nuclei per total nuclei (apoptotic plus nonapoptotic nuclei) counted in a unit reference area. Testosterone treatment at both doses significantly prevents age-related increase in muscle cell apoptosis. Values are mean ± sem. Means with unlike letters are significantly (P < 0.001) different.
Testosterone treatment suppresses age-related increase in active myostatin levels and prevents JNK activation
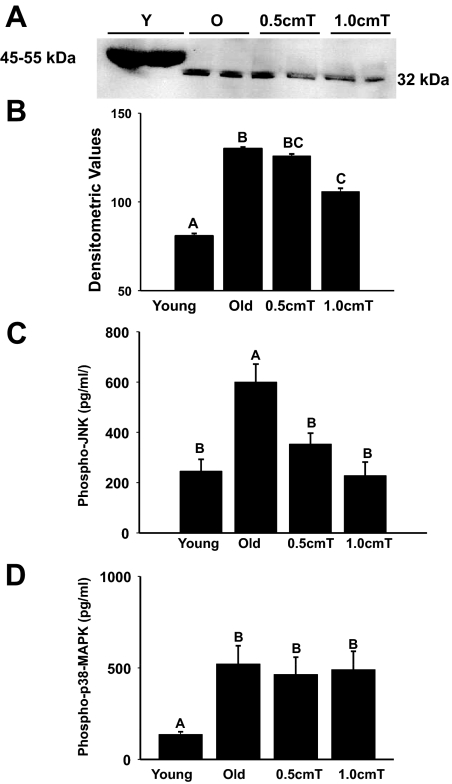
Because testosterone is known to suppress myostatin levels in skeletal muscle (33,34) and inhibition of myostatin promotes muscle growth and reduces sarcopenia (35,36,37), we examined the effects of testosterone supplementation on myostatin levels in the gastrocnemius muscle. Myostatin is synthesized as a zymogen, which requires proteolytic cleavage to undergo activation (38). As shown in Fig. 2A, Western blot analysis reveled the presence of two predominant protein species with apparent molecular masses of 45–55 and 32 kDa, consistent with unprocessed precursor and processed myostatin, respectively (29,30,38). Higher molecular weight bands, representing precursor myostatin, were detected only in the young mice but not the old animals (Fig. 2A). Conversely, the 32-kDa band, corresponding to the processed form, was detected in the aged muscles but not the young muscles. Treatment with 1 cm testosterone effectively suppressed the processed myostatin levels in the aged muscles (Fig. 2A). These findings were further corroborated by densitometry (Fig. 2B).
Figure 2.
Panel A, Western blots of muscle lysates show the presence of unprocessed (45–55 kDa) and the processed (32 kDa) myostatin in young and old mice, respectively. Testosterone (T) effectively suppresses the processed myostatin levels in the aged muscles. The gels are representative of two animals at each group from one of three separate experiments. GAPDH in the immunoblot is shown as a loading control. Panel B, Quantification of band intensities shows significant suppression of the processed myostatin levels after treatment with 1 cm testosterone. Values are mean ± sem. Means with letters BC are not different from means with either superscript letter B or C but are significantly (P < 0.05) different from means with letter A. Panel C, EIA assay reveals a significant increase in phospho-JNK levels in old animals when compared with young animals, which can be significantly prevented by testosterone treatment at both doses. Values are mean ± sem. Means with unlike letters are significantly (P < 0.001) different. Panel D, Phopho-p38 MAPK levels were significantly increased in aged muscles compared with that of young animals. However, testosterone treatment had no effect on aging-associated activation of p38 MAPK. Values are mean ± sem. Means with unlike letters are significantly (P < 0.05) different.
Given that myostatin abrogates muscle growth through activation of JNK together with the inhibition of p38 MAPK (39,40), we examined the contribution of these kinases during testosterone-induced muscle growth in aging. EIA revealed a significant (P < 0.001) increase in phospho-JNK levels in old animals when compared with young animals and that could be significantly prevented by testosterone treatment at both doses (Fig. 2C). Phospho-p38 MAPK levels were significantly (P < 0.05) increased in aged muscles compared with that of young muscles (Fig. 2D). However, testosterone treatment had no effect on p38 MAPK activation in aged muscles (Fig. 2D).
Testosterone-induced muscle fiber hypertrophy is associated with stimulation of Notch signaling and suppression of age-specific induction of cyclin-dependent kinase (CDK) inhibitor p21
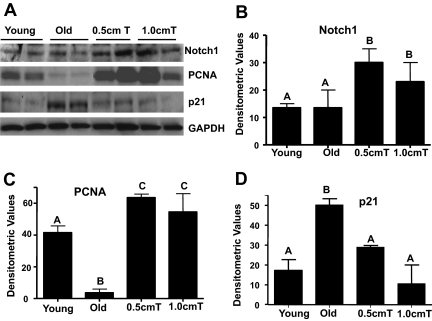
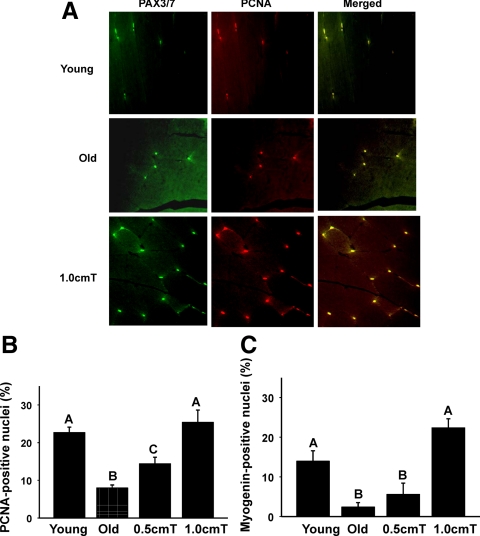
Notch signaling is essential for activation, proliferation, and myogenic progression of satellite cells necessary for muscle growth (6,7,8). We used immunoblot analysis to examine the participation of the Notch signaling in testosterone-induced muscle fiber hypertrophy. Compared with both untreated young and old mice, in which a modest expression of Notch 1 was detected, we found increased expression Notch 1 in muscle lysates from old animals after testosterone treatment (Fig. 3A). Activation of Notch signaling after testosterone treatment was associated with cell proliferation as demonstrated by increased expression of PCNA in muscle lysates (Fig. 3A). Because the activation of Notch is also known to attenuate age-specific induction of multiple CDK inhibitors, including p21 (41), which interferes satellite cell-regenerative capacity (42,43,44), we examined the effect of testosterone supplementation on p21 expression. Compared with young mice, in which minimal p21 expression was detected, a significant age-associated increase was observed in aged mice by immunoblotting (Fig. 3A). Testosterone treatment at both doses effectively reversed the age-associated increase in p21 expression (Fig. 3A). These findings were substantiated by densitometric evaluation (Fig. 3, B–D). Costaining for PCNA and PAX3/7 confirmed increased expression of PCNA after testosterone treatment in satellite cells (Fig. 4A). There were significant reductions in the numbers of PCNA and myogenin nuclei in old mice compared with that of young males (Fig. 4, B and C). The numbers of PCNA- and myogenin-positive nuclei were significantly (P < 0.001) higher after testosterone treatment in aged muscles (Fig. 4, B and C). In fact, no significant differences in the number of these nuclei were noted between young controls and 1 cm testosterone-treated old mice (Fig. 4, B and C).
Figure 3.
Testosterone-induced muscle fiber hypertrophy in old mice is associated with stimulation of Notch signaling and suppression of age-specific induction of CDK inhibitor p21. Panel A, Western blots of muscle lysates show increased levels of Notch 1 in muscle lysates from old animals after testosterone (T) treatment when compared with both untreated young and old mice, in which a modest expression of Notch is detected. Activation of Notch signaling after testosterone treatment is associated with increased expression of PCNA in muscle lysates. Compared with young animals, in which little or no expression is detected, a distinct age-related increase in p21 expression is detected in aged mice. Testosterone treatment at both doses effectively up-regulates both Notch 1 and PCNA but attenuates age-specific increase in p21 levels. Panels B–D, Quantification of band intensities shows robust up-regulation of Notch 1 and PCNA and complete restoration of p21 to its young levels in aged mice after testosterone treatment. Values are mean ± sem. Means with unlike letters are significantly (P < 0.05) different.
Figure 4.
A, Double-immunofluorescence staining for PAX3/7 (green) and PCNA (red) from young (upper panels), old (middle panels), and old+testosterone-treated gastrocnemius muscles (lower panels) shows colocalization of PAX3/7 and PCNA (yellow). Scale bar, 50 μm. Panels B and C, Quantitation of the numbers of PCNA (B)- and myogenin-positive (Panel C) shows a significant increase in the number of these cells in aged muscles after testosterone treatment. Values are mean ± sem. Means with unlike letters are significantly (P < 0.05) different.
Testosterone treatment stimulates Akt signaling and restores G6PDH levels in aged skeletal muscles
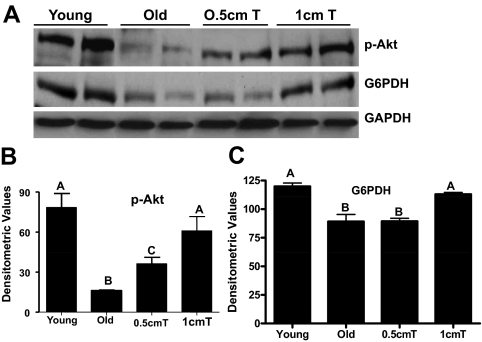
As shown in Fig. 5, A and B, there was a substantial reduction in phospho-Akt levels in gastrocnemius muscles from old mice when compared with young animals. Testosterone treatment at both doses effectively prevented such age-related decrease in phospho-Akt levels (Fig. 5, A and B). Notably, the 1-cm testosterone implant restored Akt signaling in aged skeletal muscles to levels comparable with that seen in young muscle (Fig. 5, A and B).
Figure 5.
Panel A, Western blot analysis shows decreased phospho-Akt and G6PDH levels in aged gastrocnemius muscles compared with that of young muscles. Supplementation with 1 cm testosterone (T) prevents such age-related decrease in phospho-Akt and G6PDH levels. The gels are representative of two animals at each group from one of three separate experiments. GAPDH in the immunoblot is shown as a loading control. Panels B and C, Quantification of band intensities shows complete restoration of phospho-Akt and G6PDH, respectively, to their young levels after treatment with 1 cm testosterone. Values are mean ± sem. Means with unlike letters are significantly (P < 0.05) different.
Given that G6PDH levels are decreased in aged gastrocnemius muscles (13), which can trigger apoptosis in various cell types (45,46,47), we additionally examined the effects of testosterone supplementation on G6PDH expression. As expected, G6PDH, the first and rate-limiting enzyme of the pentose phosphate pathway (48), is reduced in skeletal muscle with aging (Fig. 5, A and C). Supplementation with 1 cm testosterone fully prevented such age-related decrease in G6PDH levels (Fig. 5, A and C).
Discussion
In this study, using a murine model, we elucidated molecular mechanisms by which testosterone both improves regenerative potential of aged-satellite cells and suppresses skeletal muscle apoptosis and, in turn, reverses some of the biological features of aging-associated sarcopenia. Testosterone supplementation induces remarkable gains in muscle mass and strength in both young and older men (14,15). We previously demonstrated that such testosterone-induced increase in muscle size in both young and old men is associated with hypertrophy of muscle fibers and significant increases in myonuclear and satellite cell numbers (16,17,18). In concert with previous findings, here we show that testosterone treatment significantly enhances muscle growth in old mice as evidenced by increases in muscle mass and hypertrophy of both types I and II fibers. We further demonstrate that testosterone treatment is capable of preventing age-related increase in oxidative stress and muscle cell apoptosis, a key contributor of aging-associated sarcopenia (10,11,12,13). Thus, testosterone can promote muscle growth in aging by not only augmenting muscle regeneration but also suppressing skeletal muscle cell apoptosis. We further show that testosterone-induced muscle fiber hypertrophy in aging is associated with suppression of myostatin and p21 expression, inhibition of JNK signaling, stimulation of Akt and Notch signaling, and restoration of G6PDH levels.
Skeletal muscle regeneration is largely dependent on a small population of self-renewing committed stem cells, or satellite cells (6,7,8,49,50), which play an important role in mediating the anabolic response of testosterone leading to muscle fiber hypertrophy (17,18). When activated, satellite cells undergo proliferation and differentiation and commit to a myoblast cell fate. These cells then either join preexisting fibers causing hypertrophy or migrate and make new fibers (51). The regenerative potential of skeletal muscle declines with age, which is largely due to a decline in satellite cell functionality (6,7,8). Myostatin is an endogenous inhibitor of muscle growth in diverse species (38,52,53,54), and accumulating evidence indicates that myostatin regulates myogenesis through inhibition of satellite cell activation, proliferation, and differentiation (35,36,37,44). Loss of myostatin further enhances muscle regeneration during sarcopenia (35,36,37). Our data here indicate that testosterone indeed down-regulates processed myostatin expression in aged muscles. This is consistent with earlier works indicating that testosterone negatively regulates myostatin levels in skeletal muscles of rats and mice (33,34). Collectively, these data suggest that testosterone may promote skeletal muscle growth in old mice by suppressing processed myostatin levels.
Recently we have also shown that the inactivation of JNK together with the activation of p38 MAPK is critical for testosterone-induced activation of Notch signaling and muscle fiber hypertrophy in young mice (19). However, unlike in young animals, we found that testosterone supplementation clearly suppresses aging-associated activation of JNK in skeletal muscle but exerts no substantial effect on p38 MAPK activation. These observations led us to believe that the signaling mediating muscle hypertrophy can vary with age. This is consistent with earlier reports indicating that the regulation of cellular homeostasis by MAPKs is more complex and varies depending on age, tissue type, and nature of the stimulus (55,56,57). Mechanisms by which testosterone suppresses activation of JNK are not known. One intriguing possibility is that testosterone could suppress JNK activation through inactivation of myostatin. Indeed, in the present study, we found down-regulation of myostatin expression by testosterone. Also, the possibility that testosterone may inhibit JNK signaling through suppression of oxidative stress cannot be excluded (58). The observed increase in oxidative stress, as evidenced by an increase in 4-HNE levels in aged muscle and its mitigation by testosterone supplementation is consistent with this view. Aged muscles can undergo wasting due to a loss of fibers and atrophy of existing fibers (59). Additionally, loss of muscle cell nuclei through increased apoptosis likely contributes to fiber atrophy and, in turn, sarcopenia (12). We previously demonstrated that the JNK signaling pathway constitutes a critical component of apoptotic signaling in skeletal muscles after injury (20) or in aging (13). Therefore, it is possible that testosterone-mediated inhibition of JNK could lead to suppression of muscle cell apoptosis and a corollary increase in fiber growth. It is also possible that suppression of JNK could activate cellular proliferation and, in turn, muscle growth by attenuating myostatin-induced up-regulation of p21 (40,60), a potent inhibitor of various CDK activities (61). In this context, it is pertinent to note here that blocking JNK signaling pathway by pretreatment with SP600126 effectively attenuated myostatin-induced up-regulation of p21 and abolished the growth inhibitor role of myostatin in C2C12 cells (40).
We are also intrigued by the observation that testosterone is capable of restoring Akt signaling in aged skeletal muscle. Activation of Akt signaling promotes skeletal muscle hypertrophy (21,22,23), functioning through multiple signaling proteins involved in both survival and apoptotic pathways in skeletal muscle (24). Akt also protects cells from apoptosis by stimulating glucose transport in the cells and the recruitment of hexokinases to mitochondria (62). There have also been in vitro as well as in vivo studies indicating that myostatin is a negative regulator of Akt signaling in cardiac myocytes and skeletal muscles (39,63). Thus, the restoration of Akt signaling in aged muscles possibly emanates from testosterone-induced suppression of myostatin levels. These results indicate that the inactivation of JNK together with the activation of Akt may be critical for testosterone-induced muscle fiber hypertrophy in aging.
Notch signaling pathway is essential for the activation, proliferation and myogenic progression of satellite cells necessary for muscle regeneration and repair (6,7,8,49). Our recent studies, in both elderly men (18) and young mice (19), indicate the involvement of Notch signaling in testosterone-mediated muscle cell proliferation. In the present study, consistent with a role for Notch signaling in muscle growth, we found increased expression of Notch 1 in aged skeletal muscle after testosterone treatment. We also found that activation of Notch signaling is associated with muscle cell proliferation and differentiation as demonstrated by increased expression of PCNA and myogenin levels. Importantly, we show colocalization of PCNA and PAX3/7, suggesting increase in satellite cell number as well as differentiation (51,64). These results indicate that Notch signaling, as in humans (18), also plays an important role in testosterone-induced muscle fiber hypertrophy in mice. It is pertinent to note here that old skeletal muscle inhibits its own repair and growth by shifting balance from active Notch to pSmad3, which up-regulates various CDK inhibitors, including p21 (41), and diminishes satellite cell regenerative capacity (42,43,44). Consistent with this notion, here we show that testosterone attenuates the age-specific up-regulation of p21. Collectively, these data indicate that testosterone supplementation could restore the systemic environment to its youthful state and promote muscle growth by rescuing the satellite cell regenerative potential and suppressing muscle cell apoptosis in aged mice.
Our studies also provide preliminary evidence of improved cellular metabolism indicated by restoration of Akt signaling and G6PDH levels in old mice after testosterone treatment. Numerous links have now been established between glucose metabolism and cell fate (47,62). Akt not only decreases skeletal muscle cell apoptosis but can also promote cell survival by directly regulating cellular glucose uptake by inducing expression of the glucose transporter, Glut 1, at the plasma membrane, targeting hexokinase activity to the mitochondria, and suppressing the caspase-2-mediated death pathway (62,65), the key signaling pathway in muscle cell apoptosis (13,20). Indeed, in the present study, we found complete restoration of Akt signaling in aged muscles comparable with that seen in young muscles after testosterone treatment. Thus, Akt may also play a role in testosterone-mediated muscle growth in aging by improving cellular metabolism.
In summary, we have provided new insights into the molecular mechanisms by which testosterone may induce skeletal muscle fiber hypertrophy (Fig. 6). Testosterone appears to be working by both increasing muscle regeneration and attenuating skeletal muscle cell loss. These findings point toward novel venues for restoration of the systemic environment that supports muscle growth. By reversing aging-associated changes of multiple pathways, perhaps testosterone ultimately restores the systemic environment to its youthful state, which may even be important as an adjunct to stem-cell based therapies (66). A deeper understanding of the mechanistic pathways that mediate testosterone-induced muscle fiber hypertrophy may unveil novel targets for the development of anabolic therapies in aging and various neuromuscular disorders.
Figure 6.
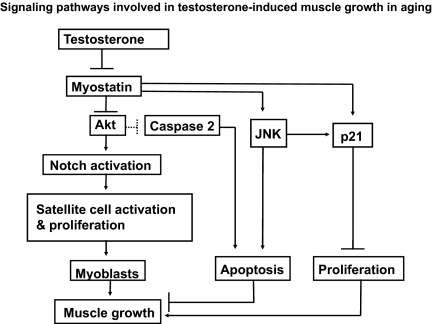
Key signaling pathways involved in testosterone-mediated mitigation of sarcopenia in aging. Testosterone, though suppression myostatin, inhibits JNK but stimulates Akt signaling. Suppression of JNK promotes muscle growth by not only inhibiting muscle cell apoptosis but also stimulating cellular proliferation by attenuating myostatin-induced up-regulation of p21. Akt can promote muscle growth through direct activation of Notch signaling (67) as well as through modulation of multiple signaling molecules involved in both apoptotic and survival pathways in muscle remodeling (24). Akt can also restrain caspase-2-mediated death pathway and promote muscle growth by stimulation of cellular metabolism (47). Thus, testosterone through stimulation of survival pathway together with the inhibition of death pathway possibly restores the microenvironment and promotes muscle growth in aging.
Footnotes
This work was supported by a grant from the American Federation of Aging Research (to I.S.-H.). Additional support was provided by National Institutes of Health Minority Biomedical Research Support Grant 5SO6 GM068510 (to I.S.-H.), National Institutes of Health Grant F32 AG034703 (to I.S.), and National Institutes of Health Research Center in Minority Institutions Grant 3P20RR011145 (to Charles Drew University).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 18, 2009
Abbreviations: CDK, Cyclin-dependent kinase; CSA, cross-sectional area; EIA, enzyme immunometric assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GnRH-A, GnRH antagonist; G6PDH, glucose-6 phosphate dehydrogenase; 4-HNE, 4-hydroxynonenal protein adducts; JNK, c-jun NH2-terminal kinase; PAX, paired box; PCNA, proliferating cell nuclear antigen; TBS-T, Tween 20 in Tris-buffered saline and nonfat dry milk.
References
- Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA 2002 Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76:473–481 [DOI] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C 2006 Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerentol 41:1234–1238 [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD 1998 Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763 [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Hughes VA 2000 Sarcopenia: current concepts. J. Gerentol A Biol Sci Med Sci 55:M716–M724 [DOI] [PubMed] [Google Scholar]
- Melton 3rd LJ, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL 2000 Epidemiology of sarcopenia. J Am Geriatr Soc 48:625–630 [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA 2003 Notch-mediated restoration of regenerative potential to aged muscle. Science 302:1575–1577 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA 2005 Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TM 2005Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 43:407–410 [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE 2005 Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40:473–481 [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA 2005 Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309:481–484 [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Alway SE 2005 Apoptotic responses to hind limb suspension in gastrocnemius muscle from adult and aged rats. Am J Physiol Regul Integr Comp Physiol 289:R1015–R1026 [DOI] [PubMed] [Google Scholar]
- Alway SE, Siu PM 2008 Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev 36:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, Gonzalez-Cadavid NF, Sinha-Hikim I 2008 Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis 13:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, Montori VM, Gao W, Dalton JT 2006 Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab 2:146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheki KE, Ulloor J, Colletti P, Roubenoff R, Azen SP 2009 Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S 2002 Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283:E154–E164 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Roth SM, Lee MI, Bhasin S 2003 Testosterone induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285:E197–E205 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S 2006 Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community dwelling, older men. J Clin Endocrinol Metab 91:3024–3033 [DOI] [PubMed] [Google Scholar]
- Brown D, Hikim AP, Kovacheva EL, Sinha-Hikim I 2009 Mouse model of testosterone-induced muscle fiber hypertrophy: involvement of p38 mitogen-activated protein kinase-mediated Notch signaling. J Endocrinol 201:129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Braga M, Shen R, Sinha Hikim AP 2007 Involvement of c-Jun NH2-terminal kinase and nitric oxide-mediated mitochondria-dependent intrinsic pathway signaling in cardiotoxin-induced muscle cell death: role of testosterone. Apoptosis 12:1965–1978 [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrmgeour A, Lawrence JC, Glass DJ, Yancopoulos GD 2001 Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019 [DOI] [PubMed] [Google Scholar]
- Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ 2004 Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24:9295–9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurpur PB, Liu J, Burkin DJ, Kaufman SJ 2009 Valproic acid activates the PI3K/Akt/mTOR pathway in muscle and ameliorates pathology in a mouse model of Duchenne muscular dystrophy. Am J Pathol 174:999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN 2006 Signaling pathway in skeletal muscle remodeling. Annu Rev Biochem 75:19–37 [DOI] [PubMed] [Google Scholar]
- Singh J, O'Neill C, Handelsman DJ 1995 Induction of spermatogenesis by androgens in gonadotropin deficient (hpg) mice. Endocrinology 136:5311–5321 [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Horton MJ, Zyman Y, Pascoe G 2001 Differential effects of diminished oestrogen and androgen levels on development of skeletal muscle fibers in hypogonadal mice. Acta Physiol Scand 172:179–187 [DOI] [PubMed] [Google Scholar]
- Martin C, Dubouchaud H, Mosoni L, Chardigny JM, Oudot A, Fontaine E, Vergely C, Keriel C, Rochette L, Leverve X, Demaison L 2007 Abnormalities of mitochondrial function can partly explain the metabolic disorders encountered in sarcopenic gastrocnemius. Aging Cell 6:165–177 [DOI] [PubMed] [Google Scholar]
- Jia Y, Castellanos J, Wang C, Sinha-Hikim I, Lue Y, Swerdloff RS, Sinha-Hikim AP 2009 Mitogen-activated protein kinase signaling in male germ cell apoptosis. Biol Reprod 80:771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S 1998 Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA 95:14938–14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaza JN, Reisz-Porszasz S, Dow JS, Kloner RA, Tsao J, Bhasin S, Gonzalez-Cadavid NF 2007 Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrinol 194:63–76 [DOI] [PubMed] [Google Scholar]
- Kohen R, Nysks A 2002 Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reaction, and methods for their quantification. Toxicol Pathol 30:620–650 [DOI] [PubMed] [Google Scholar]
- Tam NN, Gao Y, Leung YK, Ho SM 2003 Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidase and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol 163:2513–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada S, Okuno M, Ishii N 2006 Testosterone causes decrease in the content of skeletal muscle myostatin. Int J Sports Health Sci 4:44–48 [Google Scholar]
- Mendler L, Baka Z, Kovács-Simon A, Dux L 2007 Androgens negatively regulate myostatin expression in an androgen-dependent skeletal muscle. Biochem Biophys Res Commun 361:237–242 [DOI] [PubMed] [Google Scholar]
- Wagner KR, Liu X, Chang X, Allen RE 2005 Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA 102:2519–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriett V, Platt L, Salerno MS, Ling N, Kambadur R, Sharma M 2006 Prolonged absence of myostatin reduces sarcopenia. J Cell Physiol 209:866–873 [DOI] [PubMed] [Google Scholar]
- Siriett V, Salerno MS, Berry C, Nicholas G, Bower R, Kambadur R, Sharma M 2007 Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther 15:1463–1470 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ 1997 Regulation of skeletal muscle mass by a new TGF-β superfamily member. Nature 387:83–90 [DOI] [PubMed] [Google Scholar]
- Morissette MR, Cook SA, Foo SY, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenweig A 2006 Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Chen D, Zhang K, Yu B, Chen X, Meng J 2007 Regulation of myostatin signaling by c-Jun N-terminal kinase in C2C12 cells. Cell Signal 19:2286–2295 [DOI] [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM 2008 Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 454:528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R 2003 Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasarathy S, Dodig M, Muc SM, Kalhan SC, McCullough AJ 2004 Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastomosis rat. Am J Physiol Gastrointest Liver Physiol 287:G1124–G1130 [DOI] [PubMed] [Google Scholar]
- Li Y, Li J, Zhu J, Sun B, Branca M, Tang Y, Foster W, Xiao X, Huard J 2007 Decorin gene transfer promotes muscle differentiation and muscle regeneration. Mol Ther 15:1616–1622 [DOI] [PubMed] [Google Scholar]
- Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X, Stanton RC 1999 Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol 276:C1121–C1131 [DOI] [PubMed] [Google Scholar]
- Tuttle S, Stamato T, Perez ML, Biaglow J 2000 Glucose-6-phosphate dehydrogenase and the oxidative pentose phosphate cycle protect cells against apoptosis induced by low doses ionizing radiation. Radiat Res 153:781–787 [DOI] [PubMed] [Google Scholar]
- Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S 2005 Metabolic regulation of oocyte cell death through CaMKII-mediated phosphorylation of caspase 2. Cell 123:89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros LG, Cascante M, Lee WN 2002 Metabolic profiling of cell growth and death in cancer: application in drug discovery. Drug Discov Today 7:364–372 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA 2002 The regulation of notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3:397–409 [DOI] [PubMed] [Google Scholar]
- Morgan JE, Partridge TA 2003 Cells in focus: muscle satellite cells. Int J Biochem Cell Biol 35:1151–1156 [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Conboy IM 2005 Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell 122:659–667 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ 1997 Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ 2004 Myostatin mutations associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688 [DOI] [PubMed] [Google Scholar]
- Dominique JE, Gérard C 2006 Myostain regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res 312:2401–2414 [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Rosenblatt JI, Papaconstantinou J 2003 Age-associated changes in SAPK/JNK and p38 MAPK signaling in response to the generation of ROS by 3-nitropropionic acid. Mech Ageing Dev 124:733–746 [DOI] [PubMed] [Google Scholar]
- Lin A, Dibling B 2002 The true face of JNK activation in apoptosis. Aging Cell 1:112–116 [DOI] [PubMed] [Google Scholar]
- Wada T, Penninger JM 2004 Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23:2838–2849 [DOI] [PubMed] [Google Scholar]
- Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O 2003 H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activation JNKs and p38 MAPK. Exp Neurol 180:144–155 [DOI] [PubMed] [Google Scholar]
- Alnaqeeb MA, Goldspink G 1987 Changes in fiber type, number and diameter in developing and ageing skeletal muscle. J Anat 153:31–45 [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R 2000 Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243 [DOI] [PubMed] [Google Scholar]
- Child ES, Mann DJ 2006 The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle 5:1313–1319 [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N 2006 Mitochondria hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25:4683–4696 [DOI] [PubMed] [Google Scholar]
- Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, Bigard X, Peinnequin A, Freyssenet D 2009 Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 150:286–294 [DOI] [PubMed] [Google Scholar]
- Hyatt JP, McCall GE, Kander EM, Zhong H, Roy RR, Huey KA 2008 Pax3/7 expression coincides with myoD during chronic skeletal muscle overload. Muscle Nerve 38:861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman PS, Fox CJ, Thompson CB 2004 Beginnings of a signal transduction pathway for bioenergetic control of cell survival. Trends Biochem Sci 29:586–592 [DOI] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA 2008 The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med 14:82–91 [DOI] [PubMed] [Google Scholar]
- Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB 2008 Notch 1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest 118:3660–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]