Abstract
RFamide-related peptide (RFRP), the mammalian homolog of avian gonadotropin-inhibitory hormone, has a pronounced suppressive action on the reproductive axis across species. In mammals, RFRP acts directly on GnRH neurons, and likely at the level of the pituitary, to inhibit gonadotropin secretion. In the present study, we examined whether RFRP might act outside of mammalian brain on reproductive tissues directly. Using RT-PCR and in situ hybridization, we found that both RFRP and its receptors [G protein-coupled receptor (GPR) 147 and GPR74] are expressed in the testis of Syrian hamster. These results were confirmed and extended using double- and triple-label immunohistochemistry. RFRP expression was observed in spermatocytes and in round to early elongated spermatids. Significant expression of RFRP was not seen in Leydig cells. GPR147 protein was observed in myoid cells in all stages of spermatogenesis, pachytene spermatocytes, maturation division spermatocytes, and in round and late elongated spermatids. GPR74 proteins only appeared in late elongated spermatids. Additionally, we found that RFRP and its receptor mRNA are markedly altered by day length and reproductive condition. These findings highlight a possible novel autocrine and/or paracrine role for RFRP in Syrian hamster testis, potentially contributing to the differentiation of spermatids during spermiogenesis.
An inhibitory, reproductive brain peptide is also present in testis and likely participates in the regulation of sperm production.
Reproduction in vertebrates is controlled by several neuropeptides/hormones coordinating the activity of the hypothalamo-pituitary-gonadal axis. At the level of the hypothalamus, numerous lines of investigation have focused on the regulation of GnRH and its control over the secretion of the gonadotropins, LH, and FSH. Steroidogenesis and spermatogenesis are thought to be managed principally by this trickle-down cascade. However, recent evidence suggests that hypothamic neuropeptides regulating the GnRH system play a functionally significant role at the level of the gonad (1,2,3).
Recently, a family of neuropeptides containing an Arg-Phe-NH2 (RFamide) motif at the C-terminal has been shown to have pronounced impact on the reproductive axis (4,5,6,7,8). Among these neuropeptides, avian gonadotropin-inhibitory hormone (GnIH) (8) and its mammalian homolog, RFamide-related peptide (RFRP) (6,7), have been shown to inhibit gonadotropin release (7,8,9,10,11,12,13), synthesis (12,14), and activity of GnRH neurons (15,16). GnIH was first discovered and cloned in avian brain (8,11,17). In birds, GnIH cells are found in the paraventricular nucleus, with widespread projections to hypothalamic loci, including GnRH neurons (9,13) and terminal fibers in the median eminence (8,9,13). GnIH application inhibits gonadotropin release in a dose-dependent manner in cultured quail pituitaries in vitro (8). In vivo, GnIH injections result in a decrease in LH (11) and rapid suppression of female sexual behavior, suggesting a potential neuromodulatory role for this neurochemical (18). In mammals, RFRP also suppresses reproductive axis activity (7,15,19,20) and may participate in the negative feedback effects of gonadal steroids (e.g. estrogen) (7).
In addition to the brain, the expression of rat RFRP and its receptor has been detected in peripheral organs by RT-PCR (6). More recently, the expression of GnIH and its receptor has been characterized in songbird and quail gonads, oviduct, epididymis, and vas deferens (3), suggesting a role for this peptide at multiple levels of the hypothalamo-pituitary-gonadal axis. Chronic delivery of GnIH to male quail by intraperitoneal implanted mini-osmotic pumps not only causes down-regulation of gonadotropin common α and LHß subunits mRNA transcription in the pituitary and reduced LH in plasma, but this treatment also results in lower plasma testosterone concentrations, increased germ cell apoptosis in testis, and reduced spermatogenesis (12). Together, these findings suggest that GnIH/RFRP may act directly at the level of the gonad, in addition to the hypothalamus and pituitary, to participate in hormone production and/or gametogenesis.
At the cellular level, two G protein-coupled receptors (GPRs), GPR147 and GPR74, for GnIH/RFRP have been identified (6,21,22,23,24,25,26). In one study employing in vitro assays using Chinese hamster ovary cells, it was revealed that the GPR147 receptor couples with Gαi3 and Gαs proteins, whereas GPR74 couples with Gαi2, Gαi3, Gαo, and Gαs proteins (26). Both receptors are capable of inhibiting forskolin-stimulated cAMP accumulation, suggesting an activation of Gi/o subunits of G proteins (24,25). GPR74 has been reported to inhibit the transcriptional activity of cAMP response element (25). Together, these findings suggest that both GPR74 and GPR147 can have both inhibitory and, potentially, stimulatory downstream effects on cellular activity.
In mammalian testis, the seminiferous epithelium undergoes a complete series of changes in cell associations culminating in the formation of newly derived sperm (27). The “cycle map” provides a valuable guideline for understanding the complexity of the organization seen in developing tubules (28). Expression of specific genes at certain stages within limited cell types provides important temporal clues for the functions of these genes, and we have applied this logic when evaluating the present results. In this study, we sought to examine and characterize the expression of RFRP and its receptors, GPR147 and GPR74, in testis. Because we found that testicular expression of these genes only occurs in seminiferous tubules of Syrian hamsters, we focused our observations on the seminiferous cycle. As a seasonal reproductive species, male Syrian hamsters respond to artificial shortening of photoperiod with gonadal involution under laboratory conditions (29,30), providing a useful model system for exploring putative regulators of gonadal function. By comparing the expression pattern of RFRP and its receptors in reproductively competent hamsters with animals having regressed testes, important insight can be gained into testicular functioning. Importantly, a subset of hamsters is photoperiodically nonresponsive (NR), ignoring short photoperiods and maintaining an active reproductive system, allowing us to evaluate the impact of photoperiod and/or reproductive status on RFRP and its receptors independently.
Materials and Methods
Animals and materials
Adult (>60 d of age), male Syrian Lakeview Golden hamsters (Mesocricetus auratus) were used in the present experiments. All animals were purchased from Charles River (Wilmington, MA). Animals were housed in translucent propylene cages (48 × 27 × 20 cm), maintained at 23 ± 1 C, and provided with ad libitum access to food and water for the duration of the study. All animals were maintained in a 14-h light, 10-h dark cycle before the onset of any experimental treatment. Six hamsters held in 14-h light, 10-h dark cycle were used for initial RT-PCR, histology and exploration of the pattern of RFRP, GPR147, and GPR74 peptide, and mRNA expression at distinct stages of the seminiferous cycle. An additional 22 hamsters were used for photoperiod experiments [long day (LD), animals 14-h light, 10-h dark cycle, n = 8; short-day responders (SD-R), 10-h light, 14-h dark cycle, n = 6; short-day nonresponders (SD-NR), 10-h light, 14-h dark cycle, n = 8]. LD hamsters remained in 14-h light, 10-h dark cycle, whereas SD animals were transferred to 10-h light, 14-h dark cycle. After 10 wk in respective photoperiods, tissues were collected and SD hamsters were separated into SD-R and SD-NR animals based on testicular mass as described below. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Berkeley.
All nucleotide primers (Table 1) were obtained from Invitrogen (Carlsbad, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) if not otherwise specified.
Table 1.
Primers used
| Related gene | Amplicon size (position) | GenBank accession no. | Sense primer | Antisense primer |
|---|---|---|---|---|
| Cloning PCR nnd for In situ template | ||||
| RFRP | 407 (8–414) | DQ371799 | 5′-TGCCCCATTGTCACAGCAAAGA-3′ | 5′-AGGACTCTGGATTTCTTGATGCTGGC-3′ |
| GPR147 | 410 (1–410) | GQ452845 | 5′-tgctgggaggcctggcccgaga-3′ | 5′-CGGAAGTTCTCGTTGAAGTAGCCGT-3′ |
| GPR74 | 813 (1–813) | GQ452846 | 5′-TGCACACAGTCACTAATTTTTTCATCTT-3′ | 5′-GAGCTGGGAAAGCTTCTTGGAAACCA-3′ |
| RT-PCR | ||||
| β-Actin | 424 (121–544) | AJ312092 | 5′-CAGGGCGTGATGGTGGGCATGGG-3′ | 5′-CAGCCAGGTCCAGACGCAGGAT-3′ |
| RFRP | 407 (8–414) | DQ371799 | 5′-TGCCCCATTGTCACAGCAAAGA-3′ | 5′-AGGACTCTGGATTTCTTGATGCTGGC-3′ |
| GPR147 | 401 (10–410) | GQ452845 | 5′-GCCTGGCCCGAGAAGGGCATGCG-3′ | 5′-CGGAAGTTCTCGTTGAAGTAGCCGT-3′ |
| GPR74 | 236 (1–236) | GQ452846 | 5′-TGCACACAGTCACTAATTTTTTCATCTT-3′ | 5′-CTTTGGCTTAAAGGGGTAGACGACACA-3′ |
| Real-time PCR | ||||
| β-Actin | 202 (121–322) | AJ312092 | 5′-CAGGGCGTGATGGTGGGCATGGG-3′ | 5′-CCTCAGTGAGCAGCACAGGGT-3′ |
| RFRP | 299 (8–306) | DQ371799 | 5′-TGCCCCATTGTCACAGCAAAGA-3′ | 5′-TCTGGCTGTTGTTCTCCCAAACCT-3′ |
| GPR147 | 401 (10–410) | GQ452845 | 5′-gcctggcccgagaagggcatgcg-3′ | 5′-CGGAAGTTCTCGTTGAAGTAGCCGT-3′ |
| GPR74 | 236 (1–236) | GQ452846 | 5′-TGCACACAGTCACTAATTTTTTCATCTT-3′ | 5′-CTTTGGCTTAAAGGGGTAGACGACACA-3′ |
| KiSS-1 | 226 (68–293) | GU172170 | 5′-gaaccctggacccacaggcca-3′ | 5′-CTCACGCTGCACCAGCACCG-3′ |
| GPR54 | 139 (379–517) | GU172171 | 5′-tcctgctcttcgccgcctgct-3′ | 5′-TTGCTGTAGGACATGCAGTGAGCC-3′ |
| GnRH-1 | 174 (1–174) | MAU91938 | 5′-ccagccagcactggtcctatgg-3′ | 5′-CCAGAACTCCTCGAAGGTCCCT-3′ |
| GnRH-R1 | 130 (185–314) | GU172167 | 5′-tgccactagatggcatgtggaa-3′ | 5′-TCCACGCTAATCACTACCATCATG-3′ |
RT-PCR
Tissues were collected and total RNA was extracted (RNeasy Mini Kit; QIAGEN Inc., Valencia, CA). The 3′-rapid amplification of cDNA ends cDNAs were synthesized by SMART cDNA synthesis kit (CLONTECH Laboratories Inc., Palo Alto, CA). Touch-down PCR was then conducted using specific primers (Table 1) for Syrian hamster RFRP, GPR147, and GPR74: 3 min at 95 C initial denaturing followed by 16 touch-down cycles from 68 to 60 C (annealing temperature, decrease 0.5 C every cycle) and continued for another 25 cycles with a 60 C annealing temperature. Resulting PCR products were detected by running a 1% agarose gel.
Tissue preparation
Hamsters were anesthetized deeply with an inhaled isoflurane/oxygen mixture before they were killed. Flash-frozen tissues were collected onto dry ice. Tissues were embedded in OCT Compound (Tissue-Tek, Torrance, CA), sectioned at 20 μm using a cryostat (Leica CM3050-S; Leica Microsystems Inc., Bannockburn, IL), and thaw mounted onto gelatin-coated slides (Premium frosted; Fisher, Santa Clara, CA). Tissue sections were then stored at −80 C until use. Flash-frozen tissues were rehydrated and fixed in PBS with 4% paraformaldehyde for 15 min before in situ hybridization and immunohistochemistry.
In situ hybridization
The localization of mRNA expression was identified by in situ hybridization as previously described (7) with minor modification. Briefly, fixed slides were hybridized with digoxigenin (DIG)-labeled sense or antisense RNA probes (200 ng/ml) at 50 C overnight followed by a 30-min RNase digestion to reduce background labeling. The DIG-labeled probes were produced by a linearized dual promoter (SP6 and T7) plasmid (Topo-II vector from Invitrogen) containing partial gene sequences (between primers listed in Table 1) for Syrian hamster RFRP, GPR147, and GPR74. Hybridized probes were visualized by an alkaline phosphatase (AP) substrate (NBT/BCIP; Roche, Indianapolis, IN) precipitating reaction using an AP conjugated antidigoxigenin antibody (Anti-Dig-AP; Roche).
Immunohistochemistry
Fixed sections were processed as follows. First, sections were rinsed three times in PBS with 0.1% Triton X-100 (PBT) followed by a 30-min incubation in blocking buffer (PBT) supplemented with 5% normal donkey serum. Sections were then incubated in primary antibodies diluted in blocking buffer at 4 C for 48 h [rabbit anti-RFRP, 1:10000 (PAC 123/124) (7); goat anti-GPR147, 1:500 (catalog no. ab45350; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); and goat anti-GPR74, 1:500 (catalog no. sc-46206, Santa Cruz Biotechnology, Inc.)]. To examine the specificity of the antibodies against RFRP, GPR147, and GPR74, we compared labeling seen with the primary antibodies to staining using 1) no primary antibody with all other steps in the protocol followed, 2) primary antibodies (200 ng/ml anti-RFRP, 400 ng/ml anti-GPR147, and 400 ng/ml anti-GPR74) preadsorbed, overnight at 4 C, with corresponding peptides (1000 ng/ml for RFRP peptide, 2000 ng/ml for GPR147 peptide, and 2000 ng/ml for GPR74 peptide), and 3) primary antibodies preadsorbed, overnight at 4 C, with closely related peptides [kisspeptin (KiSS)-1 peptide (1000 ng/ml) for the RFRP antibody, GPR74 peptide (2000 ng/ml) for the GPR147 antibody, and GPR147 peptide (2000 ng/ml) for the GPR74 antibody]. Slides were then washed three times (15 min each) in PBT at room temperature, followed by a 2-h incubation with Cy3 conjugated donkey antigoat or Cy5 conjugated donkey antirabbit secondary antibodies (1:300; Jackson ImmunoResearch Laboratory, West Grove, PA) and 4′,6-diamidino-2-phenylindole (DAPI) (1:1000) for nuclear staining in blocking buffer at room temperature. After incubation, tissue was washed three times (15 min each) in PBT, and coverslips were applied using Fluoromount-G mounting media (Electron Microscopy Sciences, Hatfield, PA).
Microscopy
For bright field images, areas identified as labeled cells were digitally captured in 8-bit grayscale under a Zeiss Z1 microscope (Carl Zeiss, Thornwood, NY). Fluorescence-labeled testis sections were used for confocal scans to confirm results at the conventional microscopy level. Testicular tubules were scanned under 10×/0.3 Plan-NeoFluar objective lens with 3-μm increments or under 100×/1.4 Oil Plan-NeoFluar objective lens with 0.48-μm increments using a Zeiss Axiovert 100TV fluorescence microscope with a Zeiss LSM 510 laser scanning confocal attachment. Stacked images were collected as multitract optical sections. Using the LSM 3.95 software (Zeiss), images in the Cy3, Cy5, and DAPI channels were superimposed. Captured images were examined for triple labeling using Zeiss LSM Browser and Photoshop software. Seminiferous epithelial stages were distinguished by the morphology of the cell nuclear label revealed by DAPI (31,32).
Tissue collection for photoperiodic changes in testis function
Before killing, all animals were weighed to the nearest 0.1 g. Animals were killed by decapitation following deep anesthetization with isoflurane. Testes, epididymis, epididymal white adipose tissue (EWAT), and seminal vesicles were excised and weighed as “wet” mass to the nearest 0.01 g. To correct for body mass, all tissue weights were divided by body mass to obtain relative tissue masses.
mRNA quantification
For all quantitative PCR experiments, 1 μg of RNA from each testis was reverse-transcribed to cDNA by the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Real-time PCR was then performed as previously described (33). Briefly, 0.5 ng/μl cDNA (RNA equivalent) for each sample was used as a template for quantitative PCR using the IQ5 Real-Time PCR Detection System (Bio-Rad Laboratories). PCR parameters were: 5 min at 95 C, 45 cycles of 30 sec at 95 C, 30 sec at 60 C, and 30 sec at 72 C, followed by a melt curve analysis. The raw fluorescent data were analyzed by the Real-Time PCR Miner program (33). The resulting PCR efficiency and fractional cycle number at the threshold were used for gene quantification. β-Actin was used as an internal control to normalize mRNA levels among samples. The primers used in real-time PCR for actin, RFRP, GPR147, GPR74, KiSS-1, GPR54, GnRH-1, and GnRH-R1 are listed in Table 1.
Statistics
Data were analyzed by SPSS and SigmaPlot using a series of one-way ANOVAs. Group differences were assessed using post hoc Tukey tests. Results were considered statistically significant when P < 0.05.
Results
RFRP and its receptors are expressed in Syrian hamster testis
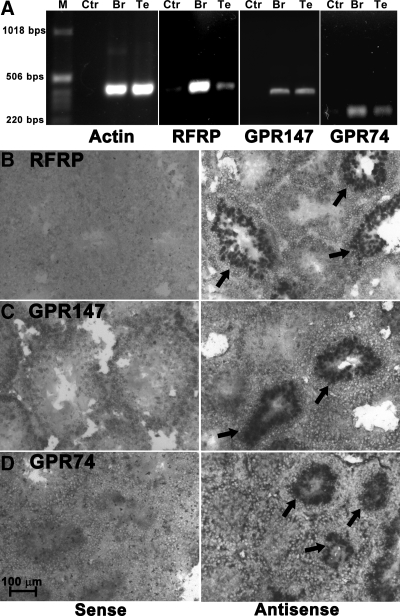
RFRP, GPR147, and GPR74 were partially cloned from Syrian hamster brain using primers designed according to the highly conserved region in the sequences of other mammals, including mouse, rat, and human (Table 1). A 410-bp partial sequence of Syrian hamster GPR147 (GenBank accession no. GQ452845) and 813-bp partial sequence of Syrian hamster GPR74 (GenBank accession no. GQ452846) were sequenced and the blast results of these partial receptor sequences showed high similarity to other mammalian homologs in GenBank (GPR147: homology identities with mouse, rat, and human GPR147 at the protein level are 97, 95, and 86%, respectively; GPR74: homology identities with mouse, rat, and human GPR74 at the protein level are 90, 90, and 81%, respectively). Alignments of the deduced amino acid sequences of the Syrian hamster RFRP precursor, GPR147, and GPR74 with other mammalian orthologs are shown in supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Based on the cloned partial sequence of the GPR147, GPR74, and previously cloned RFRP (GenBank accession no. DQ371799), Syrian hamster specific primer pairs were designed and used in RT-PCR experiments. All genes exhibited a band from testis cDNA with the same molecular weight seen in brain cDNA samples (Fig. 1A). The cloned partial sequences for RFRP, GPR147, and GPR74 were used to generate DIG-labeled probes for in situ hybridization.
Figure 1.
Expression of RFRP, GPR147, and GPR74 in Syrian hamster testis. All three genes, RFRP, GPR147, and GPR74, were found in both brain (Br) and testis (Te) of Syrian hamster by RT-PCR (A). Water (Ctr) was used as a negative control, and β-actin was used as a positive control. In situ hybridization revealed the presence of RFRP (B), GPR147 (C), and GPR74 (D) in hamster testis in a subset of seminiferous tubules. Hybridized probes were visualized by NBT/BCIP (arrows, bright field). Only background signal was observed using sense probes.
Localization of RFRP mRNA and its receptor mRNAs in hamster testis
In situ hybridization confirmed the results from RT-PCR with RFRP and its two receptors being expressed in hamster testis (Fig. 1, B–D). For all three genes, only tubules at specific stages of the seminiferous cycle were positively labeled by the antisense DIG-probe. The strongest labeling for RFRP (Fig. 1B), GPR147 (Fig. 1C), and GPR74 (Fig. 1D) was observed in the inner layers of tubules where spermatocytes and spermatids are located. Strong labeling was not observed in other cell types including spermatogonia and Sertoli cells or in other cells outside of the tubules, i.e. Leydig cells. All sense probes resulted in an absence of staining. Because it is difficult to visualize specific cell types in combination with DAPI labeling, fluorescent histochemistry using specific antibodies for RFRP, GPR147, and GPR74 was applied to subsequent analyses.
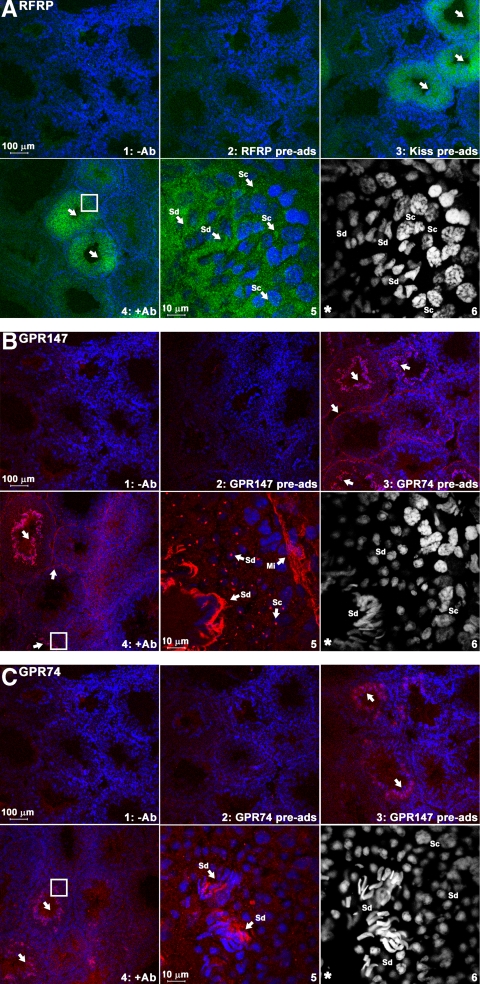
Localization of RFRP peptide and its receptor proteins in hamster testis
All antibodies used in this study produce a specific signal abolished by control procedures (i.e. no primary antibodies or preadsorption with corresponding antigenic peptides). Preadsorption with related peptides did not block staining (KiSS-1 peptide for the RFRP antibody, GPR74 peptide for the GPR147 antibody, GPR147 peptide for the GPR74 antibody) (Fig. 2). In agreement with results seen using in situ hybridization, RFRP ligands were found in both spermatocytes and spermatids (Fig. 2A). GPR147 proteins (Fig. 2B) were observed in myoid cells of all tubules and spermatocytes, round spermatids, and elongated spermatids, whereas GPR74 protein was only observed in elongated spermatids (Fig. 2C). Because we did not see GPR147 mRNA signal in myoid cells using in situ hybridization, it is possible that the mRNA in myoid cells is expressed at relatively low levels or is more unstable than the accumulated receptor proteins, precluding detection by nonradioactive in situ hybridization. It is unlikely that the GPR147 antibody is erroneously labeling a related protein, because all control procedures provide strong evidence supporting its specificity. GPR147 and GPR74 labeling was observed in elongated spermatids, with a very high-density signal concentrated in their elongated tails. GPR147 labeling in round spermatocytes and round spermatids was highly concentrated in an isolated region of the cell body.
Figure 2.
Expression of RFRP peptide and its receptor proteins in seminiferous tubules. Immunohistochemistry using specific antibodies against RFRP (A), GPR147 (B), and GPR74 (C) in hamster testis. RFRP ligand (A, green) was found around the nucleus of spermatocytes (Sc) and spermatids (Sd). GPR147 receptor (B, red) was found in myoid cells (Mi) along the edge of tubules, spermatocytes, and both round and elongated spermatids. GPR74 receptor (C, red) was only found in elongated spermatids. Nuclei were stained by DAPI (blue or white color). For each label, controls are shown as follows: no primary antibody (upper left), preadsorption with corresponding antigenic peptide (upper middle), and preadsorption with related peptides (upper right). Lower left, Primary antibody [RFRP (A), GPR147 (B), or GPR74 (C)]. Lower middle, High-power image of area outlined in lower left. Arrows indicate the positively stained tubules (upper right) or cells (lower middle). The highlighted DAPI channel for cell nuclear staining is shown in lower right.
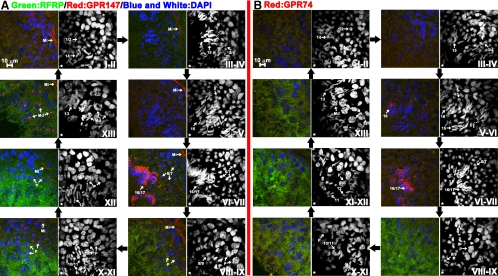
Expression of RFRP and its receptors during the seminiferous epithelial cycle
To understand the temporal and spatial expression pattern of RFRP and its receptors in hamster testis during the seminiferous epithelial cycle, triple-labeled sections were further analyzed using confocal microscopy to allow an examination of the nuclear morphology of spermatids that do/do not express these genes (Fig. 3). Stages were distinguished by the morphology of DAPI-labeled nuclei based on the methods and standards established by Vigodner and his colleagues (32). Both triangular spermatids (steps 8–9) and elongated spermatids (steps 10–13) exhibit strong RFRP labeling in stage VIII to XIII tubules (Fig. 3, A and B). GPR147 receptors were found in myoid cells of all tubules, late pachytene (P) spermatocytes from stage VIII to XII, maturation division (MD) spermatocytes in stage XIII, round spermatids at middle steps 6–7, and elongated spermatids at late steps 16–17 from stage VI to VII (Fig. 3A). GPR74 was only expressed in tubules with elongated spermatids at steps 16–17 in stage VI to VII. Although we did not observe strong RFRP expression in tubules at stage VI-VII, both GPR147 and GPR74 exhibited very strong expression in spermatids in stage VI–VII tubules.
Figure 3.
RFRP peptide and its receptor proteins are expressed at specific stages during the seminiferous cycle. Triple-label immunohistochemistry staining for RFRP/GPR147/DAPI (A) and RFRP/GPR74/DAPI (B) in hamster testis reveals a temporal expression pattern during the seminiferous cycle. Spermatids at various steps of spermiogenesis are assigned numbers 1–17 as described in the text. RFRP ligand (A and B, green) is expressed in tubules from stages VIII to XIII surrounding the nucleus of both spermatocytes and spermatids during steps 8–13. GPR147 receptors (A, red) were found in myoid cells (Mi) of all tubules, P primary spermatocytes from stages VIII to XII, the MD spermatocytes in stage XIII, round spermatids a during steps 6–7 and elongated spermatids at during steps 16–17 from stages VI to VII. GPR74 receptors (B, red) were only found in elongated spermatids at late steps 16–17 from stages VI to VII. Cell nuclei were stained by DAPI (blue or white). The DAPI channel is shown in the right panel. An asterisk indicates the lumen side of the tubule.
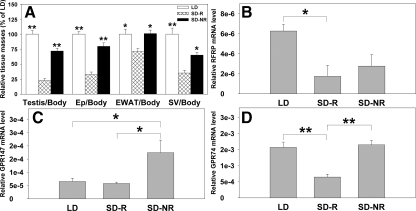
Organ masses in animals held in different photoperiodic conditions
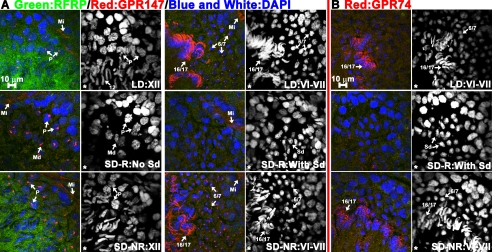
To further understand the role of the RFRP system in testicular function, hamsters were exposed to either stimulatory (14-h light, 10-h dark cycle) or inhibitory (10-h light, 14-h dark cycle) day lengths. Hamsters responding to SD lengths with reproductive inhibition (SD-R) had significantly smaller reproductive tissues, i.e. paired testes, epididymis, EWAT, and seminal vesicles than LD and SD-NR animals (P < 0.05 in all cases; Fig. 4A). Testes of SD-R animals had atrophied tubules and did not exhibit elongated spermatids and mature spermatozoa as expected (Fig. 5).
Figure 4.
RFRP mRNA and its receptors are altered by photoperiod and reproductive condition. Mean (±sem) relative paired testis, epididymis (Ep), EWAT, and seminal vesicles (SV) masses in animals held in either LD or SD. SD animals were classified as R or NR based on paired testis masses (A). Mean (±sem) RFRP (B), GPR147 (C), and GPR74 (D) mRNA levels in the testes of LD, SD-R, and SD-NR animals were quantified by real-time PCR. Data are presented relative to β-actin. Significant differences among animal groups were found for RFRP, GPR147, and GPR74 transcription but not the other genes (i.e. GnRH1, Kiss-1, and their receptors; supplemental Fig. S2) (*, P < 0.05; **, P < 0.01).
Figure 5.
RFRP peptide and its receptor proteins are suppressed in SD-R animals. Triple-label immunohistochemistry staining for RFRP/GPR147/DAPI (A) and RFRP/GPR74/DAPI (B) in the testes of LD, SD-R, and SD-NR hamsters. For all genes, SD-NR animals showed a similar expression pattern to LD animals. Minimal RFRP expression (A and B, green) was seen in SD-R animals. GPR147 receptor (A, red) can be found in myoid cells (Mi) of all tubules in LD, SD-R, and SD-NR animals. In SD-R animals, P, primary spermatocytes, and MD spermatocytes exhibited GPR147 expression. GPR174 expression was not seen in round spermatids (Sd) in SD-R animals. GPR74 expression was found in not observed in SD-R animals. Spermatids at different steps of spermiogenesis were assigned values of 1–17. Cell nuclei were stained by DAPI (blue or white). The DAPI channel (white) is shown in the right panel. An asterisk indicates the lumen side of the tubule.
Expression of RFRP and its receptors are impacted by photoperiod and reproductive condition
Real-time PCR was conducted to compare the transcriptional profiles of RFRP, KiSS-1, GnRH-1, and their receptors in testes among LD, SD-R, and SD-NR animals (Fig. 4, B–D). RFRP mRNA was significantly decreased in the testes of SD-R hamsters relative to LD animals (P < 0.05). A similar, non significant trend was observed in the testes of SD-NR hamsters (P = 0.064; Fig. 4B). In contrast to findings for RFRP expression, GPR147 mRNA levels were markedly increased in SD-NR animals relative to both SD-R and LD hamsters (P < 0.05 in each case; Fig. 4C), whereas GPR74 mRNA was significantly reduced in SD-R hamsters compared with LD and SD-NR animals (P < 0.05 in each case; Fig. 4D). Unlike the changes seen with RFRP and its receptors with regard to photoperiod and reproductive condition, no changes were observed in KiSS-1 (supplemental Fig. S1A) and its receptor, GPR54 (supplemental Fig. S1B), or GnRH-1 (supplemental Fig. S1C) and its receptor, GnRH-R1 (supplemental Fig. S1D). At the tissue level, LD and SD-NR animals shared a similar expression pattern for all three genes (Fig. 5). In tubules containing round spermatids from SD-R animals, RFRP and GPR147 protein were not detected (Fig. 5A). However, P primary spermatocytes and the MD spermatocytes in SD-R tubules expressed GPR147 proteins similar to that seen in LD or SD-NR VIII–XIII tubules (Fig. 5A). Because elongated spermatids are not seen in SD-R animals, GPR74 protein was not observed (Fig. 5B).
Discussion
The present findings reveal that RFRP and its receptors are expressed in hamster gonadal tissue with a pattern of expression strongly associated with specific stages of spermatogenesis. Furthermore, gonadal expression of RFRP and its receptors is markedly altered by photoperiod-driven changes in reproductive status, further suggesting an important autocrine and/or paracrine role for RFRP in testis. The presence of RFRP in the gonads of hamsters, along with results for GnIH seen in the gonads of avian species (3), suggests a long-standing evolutionarily conserved role for RFRP/GnIH in this gland.
The concept of local regulation by traditional reproductive brain neuropeptides is not new. For example, GnRH is found in human ovaries and is thought to have an autocrine/paracrine function (34). GnRH can induce ovulation in hypophysectomized rats, presumably by acting directly on ovarian tissue (35,36). GnRH receptor is also found in rat ovary and fish gonads (37,38,39,40). In addition to GnRH, a host of other neuropeptides including neuropeptide Y (41,42,43,44,45), galanin (46), and KiSS-1 (1) are present in vertebrate gonads. The gonadal neuropeptide Y receptor (47), galanin receptor (48), vasoactive intestinal peptide receptor (49,50,51,52,53), corticotropin-releasing factor receptor (54,55,56), urocortin receptor (57), and KiSS receptor (1) have also been reported.
In mammals, the distribution and functional significance of RFRP have been principally investigated in the central nervous system. Few studies have focused on the function of RFRP in the periphery, although mRNA transcripts for rat RFRP and its receptor have been noted from RT-PCR experiment using whole tissue homogenates (6). Recently, Bentley et al. (3) reported the presence of GnIH and its receptor in both male and female songbird and quail reproductive tissues. As mentioned previously, additional work by this group indicated that chronic, peripheral administration of GnIH leads to testicular involution and reduced spermatogenesis (12), suggesting an inhibitory role for GnIH on the reproductive axis, potentially at the level of the testis. In the present study, we provide the first report detailing the expression pattern of RFRP and two of its receptors, GPR147 and GPR74, in the seminiferous epithelium in a mammal. Our findings showing that RFRP is robustly expressed during particular stages of the seminiferous cycle, along with its suppression during testicular quiescence, suggests that RFRP might serve a positive role in hamster sperm production. Alternatively, as discussed further below, it is possible that RFRP modulation is necessary to balance the actions of other positive regulators when sperm is being produced. Future, comparative studies using similar manipulations and measures are necessary to assess the role of GnIH/RFRP in the gonads across species.
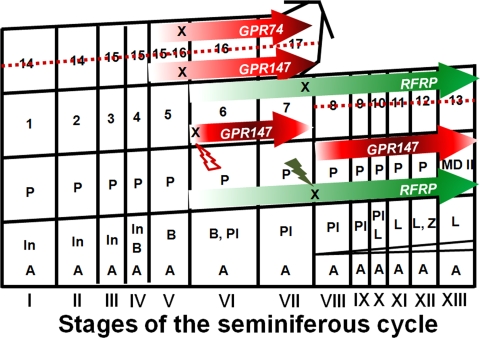
In situ hybridization (Fig. 1) and immunochemical labeling (Figs. 2 and 3) indicate that spermatocytes and spermatids are the major cell types expressing RFRP and its receptors. The temporal expression pattern of RFRP and its receptors provide important insight into the role that RFRP may play in mammalian spermatogenesis. RFRP expression increases markedly in tubules during stages VII–XIII, the differentiation period of late spermatocytes (late P primary spermatocytes and the MD spermatocytes; Fig. 2) to early round spermatids. GPR147 expression increases in late spermatocytes, implying the participation of RFRP signaling through GPR147 during this time. The RFRP signal (at both the ligand and receptor levels) is absent once new early round spermatids (steps 1–5) are produced from stage I until stage VI. In stage VI/VII, robust expression of both GPR147 and GPR74 receptors is seen in late elongated spermatids (steps 16–17). Together, these finding suggest that both RFRP receptors might be involved in the final maturation of spermatids, potentially necessary to counterbalance the influence of positive regulatory factors at this time. We also found that GPR147 receptor appears as bright “dots” in late round spermatids at steps 6–7 in stages VI-VII but disappears once the elongated spermatids start to differentiate in stage VIII (steps 8–9) and remains absent until stage V (step 15 spermatids) in next cycle, suggesting that GPR147 receptors might also be necessary in the transition of late round spermatids (steps 6–7) to triangular spermatids (steps 8–9). In myoid cells, constitutive expression of GPR147 receptor was observed. A summary of these observations mapped onto the seminiferous epithelium cycle map previous described by Clermont and Trott (27) is shown in Fig. 6.
Figure 6.
Diagram of the expression pattern of RFRP and its receptors pattern during the seminiferous epithelium cycle in Syrian hamsters. The map of the Syrian hamster seminiferous epithelium cycle was previous described by Clermont and Trott (27). The expression of RFRP, GPR147, and GPR74 are indicated by colored arrows. The density of the arrows represents the relative expression level of each gene in specific cell types across stages. Type A (A), intermediate (In), and Type B (B) spermatogonia; preleptotene primary spermatocytes (Pl), leptotene (L), zygotene (Z), P primary spermatocytes; MD spermatocytes; spermatids at various steps of spermiogenesis (1–17). The cell types that are absent in SD-R animals are indicated by a dashed line. Down-regulation of RFRP, GPR147, and GPR74 in SD-R animals is noted by X. Lightning bolts indicate times of down-regulation for RFRP and GPR147.
To examine the pattern of RFRP expression in reproductively quiescent and reproductively competent hamsters, we used a photoperiodic model to induce gonadal involution in a subset of animals. Animals with regressed gonads exhibited low expression of RFRP, GPR147, and GPR74 mRNA, providing additional support for a role for this peptide in spermatogenesis. Alternatively, it is possible that testicular regression resulted in fewer germ (and other) cells, thereby leading to a decrease in RFRP and its receptors in testicular tissue. Future studies monitoring the progression of RFRP changes throughout the process of testicular involution are required to select between these possibilities. Although the GPR147 receptor was reported to have a higher binding affinity for RFRP than GPR74, it has a lower intrinsic efficacy to RFRP (26). As a result, we expected to observe greater GPR147 expression in SD-NR animals relative to LD animals to compensate for the decrease in RFRP expression in SD-NR animals (Fig. 4, B and C). Although GPR147 receptors were still found in late spermatocytes (i.e. P and MD spermatocytes) in SD-R animals, RFRP was absent (Figs. 5 and 6), indicating down-regulation of this peptide in SD-R hamsters. Although late spermatocytes in SD animals differentiate into early round spermatids, later round spermatids (steps 6–7) fail to express GPR147 (Figs. 5A and 6) and elongated spermatids are not observed. In combination with the findings in reproductively competent animals, this observation suggests that GPR147 might be involved the differentiation to late round spermatids (Fig. 6). The fact that GPR147 and GPR74 bind differentially to various G proteins subtypes (26) implies the potential for both stimulatory and inhibitory biological functions of these two receptors through changes in adenylate cyclase and cAMP response element-mediated transcriptional regulation depending on cellular context.
Because elongated spermatids are not seen in SD-R animals, minimal GPR74 receptor expression was detectable (Figs. 5B and 6), whereas in SD-NR animals with normal elongated spermatids, both mRNA (Fig. 4D) and protein (Fig. 5B) levels of GPR74 are similar to that seen in LD animals. Because both GPR147 and GPR74 are highly expressed during the late stages of spermatogenesis, RFRP signaling may be part of the mechanism allowing for the maintenance of reproductive function in SD-NR animals. Alternatively, it is possible that RFRP is responsible for balancing the actions of positive regulators of spermatogenesis as suggested previously. Consequently, at times when sperm are not being produced, negative regulation by RFRP is not required and energetic resources can be conserved by down-regulating the activity of this system. Importantly, two other hypothalamic peptides, KiSS-1 and GnRH-1, and their receptors did not exhibit changes associated with photoperiod or reproductive condition. This finding suggests a role for RFRP in testicular function rather than a general down-regulation of reproductive peptides in regressed testis.
The findings presented suggest an important role for RFRP as a local regulator of testicular function. The fact that RFRP and its receptors are restricted to seminiferous tubules and developing spermatids argues for its involvement in spermatogenesis in Syrian hamsters. RFRP expression was markedly impacted by photoperiod and reproductive condition, suggesting that changes in testicular RFRP are part of the events leading to reproductive inhibition. Together with findings in avian species (3,12), the present results suggest a highly conserved local regulatory role for RFRP/GnIH in gonadal and peripheral reproductive tissues, although the specific local function may differ across species.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant HD050470 (to L.J.K.) and National Science Foundation Grant IOB-0641188 (to G.E.B.).
Disclosure Summary: The authors have nothing to declare.
First Published Online January 5, 2010
Abbreviations: AP, Alkaline phosphatase; DAPI, 4′,6-diamidino-2-phenylindole; DIG, digoxigenin; EWAT, epididymal white adipose tissue; GnIH, gonadotropin-inhibitory hormone; GPR, G protein-coupled receptor; KiSS, kisspeptin; LD, long day; MD, maturation division; P, pachytene; RFRP, RFamide-related peptide; PBT, PBS plus 0.1% Triton X-100; SD-R, short-day responder; SD-NR, short-day nonresponder.
References
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sánchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, Tena-Sempere M 2006 Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology 147:4852–4862 [DOI] [PubMed] [Google Scholar]
- Maddineni SR, Ocón-Grove OM, Krzysik-Walker SM, Hendricks 3rd GL, Ramachandran R 2008 Gonadotropin-inhibitory hormone (GnIH) receptor gene is expressed in the chicken ovary: potential role of GnIH in follicular maturation. Reproduction 135:267–274 [DOI] [PubMed] [Google Scholar]
- Bentley GE, Ubuka T, McGuire NL, Chowdhury VS, Morita Y, Yano T, Hasunuma I, Binns M, Wingfield JC, Tsutsui K 2008 Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol 156:34–43 [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM 2005 Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M 2000 New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol 2:703–708 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R 2006 Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103:2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ 2000 A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 275:661–667 [DOI] [PubMed] [Google Scholar]
- Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC 2003 Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol 15:794–802 [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS 2007 Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Bentley GE, O'Brien S, Moore IT, Wingfield JC, Tsutsui K 2004 Gonadotropin-inhibitory hormone in Gambel’s white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol 182:33–42 [DOI] [PubMed] [Google Scholar]
- Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K 2006 Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology 147:1187–1194 [DOI] [PubMed] [Google Scholar]
- Ukena K, Ubuka T, Tsutsui K 2003 Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res 312:73–79 [DOI] [PubMed] [Google Scholar]
- Ciccone NA, Dunn IC, Boswell T, Tsutsui K, Ubuka T, Ukena K, Sharp PJ 2004 Gonadotrophin inhibitory hormone depresses gonadotrophin α and follicle-stimulating hormone β subunit expression in the pituitary of the domestic chicken. J Neuroendocrinol 16:999–1006 [DOI] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE 2009 RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 150:2799–2804 [DOI] [PubMed] [Google Scholar]
- Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M 2009 Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol 587:1401–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake H, Hisada M, Kawada T, Minakata H, Ukena K, Tsutsui K 2001 Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. Biochem J 354:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC 2006 Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH). Horm Behav 49:550–555 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ 2006 Driving reproduction: RFamide peptides behind the wheel. Horm Behav 50:655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizwan MZ, Porteous R, Herbison AE, Anderson GM 2009 Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology 150:1413–1420 [DOI] [PubMed] [Google Scholar]
- Parker RM, Copeland NG, Eyre HJ, Liu M, Gilbert DJ, Crawford J, Couzens M, Sutherland GR, Jenkins NA, Herzog H 2000 Molecular cloning and characterisation of GPR74 a novel G-protein coupled receptor closest related to the Y-receptor family. Brain Res Mol Brain Res 77:199–208 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S 2003 Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta 1593:151–157 [DOI] [PubMed] [Google Scholar]
- Yin H, Ukena K, Ubuka T, Tsutsui K 2005 A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J Endocrinol 184:257–266 [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mazarguil H, Marcus D, Quelven I, Kotani M, Lannoy V, Dumont Y, Quirion R, Detheux M, Parmentier M, Zajac JM 2002 Pharmacological characterization of human NPFF(1) and NPFF(2) receptors expressed in CHO cells by using NPY Y(1) receptor antagonists. Eur J Pharmacol 451:245–256 [DOI] [PubMed] [Google Scholar]
- Elshourbagy NA, Ames RS, Fitzgerald LR, Foley JJ, Chambers JK, Szekeres PG, Evans NA, Schmidt DB, Buckley PT, Dytko GM, Murdock PR, Milligan G, Groarke DA, Tan KB, Shabon U, Nuthulaganti P, Wang DY, Wilson S, Bergsma DJ, Sarau HM 2000 Receptor for the pain modulatory neuropeptides FF and AF is an orphan G protein-coupled receptor. J Biol Chem 275:25965–25971 [DOI] [PubMed] [Google Scholar]
- Gouardères C, Mazarguil H, Mollereau C, Chartrel N, Leprince J, Vaudry H, Zajac JM 2007 Functional differences between NPFF1 and NPFF2 receptor coupling: high intrinsic activities of RFamide-related peptides on stimulation of [35S]GTPγS binding. Neuropharmacology 52:376–386 [DOI] [PubMed] [Google Scholar]
- Clermont Y, Trott M 1969 Duration of the cycle of the seminiferous epithelium in the mouse and hamster determined by means of 3H-thymidine and radioautography. Fertil Steril 20:805–817 [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED 1990 Histological and histopathological evaluation of the testis. 1st ed. Clearwater, FL: Cache River Press [Google Scholar]
- Sinha Hikim AP, Bartke A, Russell LD 1988 Morphometric studies on hamster testes in gonadally active and inactive states: light microscope findings. Biol Reprod 39:1225–1237 [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Bartke AJ, Russell LD 1988 The seasonal breeding hamster as a model to study structure-function relationships in the testis. Tissue Cell 20:63–78 [DOI] [PubMed] [Google Scholar]
- Mizuno M, Harris CL, Suzuki N, Matsuo S, Morgan BP 2005 Expression of CD46 in developing rat spermatozoa: ultrastructural localization and utility as a marker of the various stages of the seminiferous tubuli. Biol Reprod 72:908–915 [DOI] [PubMed] [Google Scholar]
- Vigodner M, Lewin LM, Glaser T, Shochat L, Mittelman L, Golan R 2002 Use of confocal microscopy for the study of spermatogenesis. Methods Cell Sci 24:169–180 [DOI] [PubMed] [Google Scholar]
- Zhao S, Fernald RD 2005 Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol 12:1047–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aten RF, Polan ML, Bayless R, Behrman HR 1987 A gonadotropin-releasing hormone (GnRH)-like protein in human ovaries: similarity to the GnRH-like ovarian protein of the rat. J Clin Endocrinol Metab 64:1288–1293 [DOI] [PubMed] [Google Scholar]
- Corbin A, Bex FJ 1981 Luteinizing hormone releasing hormone agonists induce ovulation in hypophysectomized proestrous rats: direct ovarian effect. Life Sci 29:185–192 [DOI] [PubMed] [Google Scholar]
- Ekholm C, Hillensjö T, Isaksson O 1981 Gonadotropin releasing hormone agonists stimulate oocyte meiosis and ovulation in hypophysectomized rats. Endocrinology 108:2022–2024 [DOI] [PubMed] [Google Scholar]
- Bogerd J, Diepenbroek WB, Hund E, van Oosterhout F, Teves AC, Leurs R, Blomenröhr M 2002 Two gonadotropin-releasing hormone receptors in the African catfish: no differences in ligand selectivity, but differences in tissue distribution. Endocrinology 143:4673–4682 [DOI] [PubMed] [Google Scholar]
- Schirman-Hildesheim TD, Bar T, Ben-Aroya N, Koch Y 2005 Differential gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acid expression patterns in different tissues of the female rat across the estrous cycle. Endocrinology 146:3401–3408 [DOI] [PubMed] [Google Scholar]
- Whitelaw PF, Eidne KA, Sellar R, Smyth CD, Hillier SG 1995 Gonadotropin-releasing hormone receptor messenger ribonucleic acid expression in rat ovary. Endocrinology 136:172–179 [DOI] [PubMed] [Google Scholar]
- Jones PB, Conn PM, Marian J, Hsueh AJ 1980 Binding of gonadotropin releasing hormone agonist to rat ovarian granulosa cells. Life Sci 27:2125–2132 [DOI] [PubMed] [Google Scholar]
- Achi MV, Figueroa JM, González Nicolini V, Villar MJ, Tramezzani JH 1995 NPY- and CGRP-like immunoreactive nerve fibers in the testis and mesorchium of the toad (Bufo arenarum). Cell Tissue Res 281:375–378 [DOI] [PubMed] [Google Scholar]
- Adrian TE, Gu J, Allen JM, Tatemoto K, Polak JM, Bloom SR 1984 Neuropeptide Y in the human male genital tract. Life Sci 35:2643–2648 [DOI] [PubMed] [Google Scholar]
- Jørgensen JC, Giwercman A, Ottesen B 1996 Neuropeptide Y in the human prenatal and mature gonads. Neuropeptides 30:293–301 [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Fujisawa M, Okuda Y, Okada H, Arakawa S, Kamidono S 1996 Expression and regulation of neuropeptide Y messenger ribonucleic acid in cultured immature rat Leydig and Sertoli cells. Endocrinology 137:1249–1257 [DOI] [PubMed] [Google Scholar]
- Terado M, Nomura M, Mineta K, Fujimoto N, Matsumoto T 2006 Expression of neuropeptide Y gene in mouse testes during testicular development. Asian J Androl 8:443–449 [DOI] [PubMed] [Google Scholar]
- Fox MD, Hyde JF, Muse KN, Keeble SC, Howard G, London SN, Curry Jr TE 1994 Galanin: a novel intraovarian regulatory peptide. Endocrinology 135:636–641 [DOI] [PubMed] [Google Scholar]
- Kopp J, Zhang X, Hökfelt T 1997 Neuropeptide Y1 receptors in the rat genital tract. Regul Pept 70:149–160 [DOI] [PubMed] [Google Scholar]
- Romanelli F, Fillo S, Isidori A, Gaudino S, Conte D 1998 Galanin stimulates steroidogenesis in rat Leydig cells. Life Sci 63:255–263 [DOI] [PubMed] [Google Scholar]
- Johnson AL, Tilly JL 1988 Effects of vasoactive intestinal peptide on steroid secretion and plasminogen activator activity in granulosa cells of the hen. Biol Reprod 38:296–303 [DOI] [PubMed] [Google Scholar]
- El-Gehani F, Tena-Sempere M, Huhtaniemi I 1998 Vasoactive intestinal peptide is an important endocrine regulatory factor of fetal rat testicular steroidogenesis. Endocrinology 139:1474–1480 [DOI] [PubMed] [Google Scholar]
- Kawashima M, Takahashi T, Yasuoka T, Kamiyoshi M, Tanaka K 1995 A vasoactive intestinal peptide binding component in hen granulosa cells. Proc Soc Exp Biol Med 209:387–391 [DOI] [PubMed] [Google Scholar]
- Bajo AM, Juarranz MG, Valenzuela P, Martínez P, Prieto JC, Guijarro LG 2000 Expression of vasoactive intestinal peptide (VIP) receptors in human uterus. Peptides 21:1383–1388 [DOI] [PubMed] [Google Scholar]
- Vaccari S, Latini S, Barberi M, Teti A, Stefanini M, Canipari R 2006 Characterization and expression of different pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptors in rat ovarian follicles. J Endocrinol 191:287–299 [DOI] [PubMed] [Google Scholar]
- Heinrich N, Meyer MR, Furkert J, Sasse A, Beyermann M, Bönigk W, Berger H 1998 Corticotropin-releasing factor (CRF) agonists stimulate testosterone production in mouse leydig cells through CRF receptor-1. Endocrinology 139:651–658 [DOI] [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W 1995 Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA 92:2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser B, Rehmann R, Rivier J, Vale W, Reubi JC 2006 CRF receptors in the rodent and human cardiovascular systems: species differences. Peptides 27:3029–3038 [DOI] [PubMed] [Google Scholar]
- Florio P, Vale W, Petraglia F 2004 Urocortins in human reproduction. Peptides 25:1751–1757 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.